Transcriptome profiling of peanut (Arachis hypogaea) gynophores in gravitropic response
Hai-fen Li A , Xiao-Ping Chen A B , Fang-he Zhu A , Hai-Yan Liu A , Yan-Bin Hong A and Xuan-Qiang Liang A BA Crops Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, China.
B Corresponding authors. Emails: xpchen1011@gmail.com; liang-804@163.com
This paper originates from a presentation at the ‘VI International Conference on Legume Genetics and Genomics (ICLGG)’ Hyderabad, India, 2–7 October 2012.
Functional Plant Biology 40(12) 1249-1260 https://doi.org/10.1071/FP13075
Submitted: 29 March 2013 Accepted: 18 July 2013 Published: 21 August 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Peanut (Arachis hypogaea L.) produces flowers aerially, but the fruit develops underground. This process is mediated by the gynophore, which always grows vertically downwards. The genetic basis underlying gravitropic bending of gynophores is not well understood. To identify genes related to gynophore gravitropism, gene expression profiles of gynophores cultured in vitro with tip pointing upward (gravitropic stimulation sample) and downward (control) at both 6 and 12 h were compared through a high-density peanut microarray. After gravitropic stimulation, there were 174 differentially expressed genes, including 91 upregulated and 83 downregulated genes at 6 h, and 491 differentially expressed genes including 129 upregulated and 362 downregulated genes at 12 h. The differentially expressed genes identified were assigned to 24 functional categories. Twenty pathways including carbon fixation, aminoacyl-tRNA biosynthesis, pentose phosphate pathway, starch and sucrose metabolism were identified. The quantitative real-time PCR analysis was performed for validation of microarray results. Our study paves the way to better understand the molecular mechanisms underlying the peanut gynophore gravitropism.
Additional keywords: gravitropism, peanut gynophore, transcriptome profiling.
Introduction
Many plants have remarkable abilities to perceive and respond to a variety of environmental stimuli like light, moisture and gravity, and adapt their physiological development to obtain successful growth. Tropism – the directional growth response – is the most obvious adaptable response, and includes phototropism and gravitropism. This adaptive response allows shoots to grow upwards and makes roots grow downwards. Therefore, shoots can have better light perception for photosynthesis and roots can easily acquire water and minerals in the soil. Among the various stimuli, gravity plays the most important and persistent role in plant growth. Gravitropism is a morphological and developmental process by which plants perceive and respond to gravity. It is a well coordinated regulation process including several sequential steps: gravity perception, molecular signal production, signal transduction, and asymmetric organ growth (Fukaki and Tasaka 1999; Haswell 2003). Several genetic studies have shown that a lack of an endodermal cell layer resulted in gravitropism defects in Arabidopsis (Benfey et al. 1993; van den Berg et al. 1995; Di Laurenzio et al. 1996). The results implied the endodermis is the gravity response site. Some models have been proposed to explain the sequential processes in plant gravitropism. First, the starch-statolith theory (Sack 1997) is used to explain gravity perception: according to this theory, starch-filled amyloplasts act as statoliths to sediment in the direction of gravity for plant gravity perception (Weise and Kiss 1999; Kiss 2000). In support of this theory, amyloplast endodermal sedimentation responding to gravity stimulation was determined in flowering shoots of snapdragon (Friedman et al. 1998; Philosoph-Hadas et al. 2001). Following the gravity perception, the Cholodny-Went hypothesis, was proposed to explain the signal transduction processes. Auxin, typically indole-3-acetic acid (IAA), has been defined as the signal molecule in gravitropism. An asymmetry in auxin distribution leads to unequal cell elongation rate on opposite sides of organ, thus, results in organ bending (Goldsmith 1977; Muday 2008). Auxin gradients identified by radiolabelled auxin and reporter gene systems provided a strong support for this hypothesis (Muday and DeLong 2001; Blancaflor and Masson 2003; Morita and Tasaka 2004).
The genetic basis of plant response to gravity induction has been reviewed (Blancaflor and Masson 2003; Hashiguchi et al. 2013). However, only a few studies have attempted to analyse gravity-induced changes at the transcriptional level in a large scale level (Moseyko et al. 2002). To date, a few of genes, especially auxin-related genes, have been found to be associated with gravitropism (Philippar et al. 1999; Kato et al. 2002; Boccalandro et al. 2008; Koprivova et al. 2010; Nakamura et al. 2011; Band et al. 2012). Actin disruption with MDR (multidrug resistance)-like genes knockout or latrunculin B drug treatment enhanced gravitropic response, possibly through auxin transporter targeting. Due to auxin’s role as a signal molecule in gravitropism, auxin influx carriers AUX1 family and auxin efflux carriers AGR/PIN family, specific MDR-like proteins all contribute to plant gravity responses. For instance, disruption of AUX1 and AGR1/EIR1/PIN2/WAV6 leads to severe defects in root gravitropism, suggesting the essential function of auxin transport during gravitropism. Furthermore, the function of ARG1, ARL2, SGR2, SGR3, and ZIG/SGR4 in early phases of gravity signal transduction in shoots has also been characterised. Because of defect to develop a curvature under gravity stimulations, the gps (gravity persistence signal) gene was identified for plant gravitropism. The effort to find auxin receptors led to the discovery of auxin response factors (ARFs) and Aux/IAA genes, mutations of which result in gravitropism defects.
Peanut (Arachis hypogaea L.) displays a unique growth process called geocarpy during its life cycle. The unique process is that the peanut produces flowers aerially, and subsequently buries the fertilised ovules into the soil for the fruit and seeds to develop and mature underground (Smith 1950; Shushu 1990). The specialised downward growth organ that carries and sows the young seeds into the soil is known as the gynophore. The morphology and anatomy of peanut gynophore have been well established (Ziv 1975; Periasamy 1984; Pattee 1987). Following fertilisation, the gynophore begins to form from an intercalary meristem into a pointed stalk-like structure. Embryo development tentatively stops at this time and the gynophore elongates and bends downward to the ground. When its tip goes into the soil, the gynophore stops elongation and its tip containing the developing embryo begins to swell and elongate on the dorsal side. Finally, the tip forms mature peanut seed in the horizontal orientation.
Gravitropism plays an essential role in the successful completion of peanut gynophore function, and the gravitropic behaviour of peanut gynophore is distinctive (Schwuchow et al. 1990). Although the peanut gynophore has structural and anatomical features similar to a typical shoot, it responds to gravity like a root, growing vertically downwards but not like the shoot growing upwards (Jacobs 1947). The positively gravitropic response plays a crucial role in peanut development: the embryo will not develop further unless it is buried underground. The unique gravitropic behaviour of peanut gynophores and its importance in peanut maturation have led many groups to study the underlying mechanisms that regulate the gynophore gravitropism. Auxin, especially IAA, has been well studied in relation to gynophore gravity responses. In 1951, Jacobs has found that IAA located at the distal 10 mm in vertically growing gynophores (Jacobs 1951). Shushu (1990) showed that IAA together with gibberellic acid (GA) near the tip promoted the vertical growth of gynophores. However, the molecular mechanism, especially the genes and pathway related to gravitropic response in peanut gynophores is still unknown.
In this study, a customised NimblerGen oligonucleotide microarray was used to survey the transcriptome profiling of peanut gynophores in gravitropic response for understanding of the molecular mechanism underlying the gynophore gravitropism. The objectives of the present study were to: (1) compare gene expression profiles between peanut gynophores cultured in vitro with tips pointing upward and downward; and (2) identify potential genes and corresponding regulation pathways that are involved in gynophore response to gravity stimuli.
Materials and methods
Plant material
A peanut (Arachis hypogaea L.) cultivar Yueyou7, provided by Crops Research Institute, Guangdong Academy of Agricultural Sciences (GAAS, Guangzhou, China), was grown normally in experiment field in the spring season of 2010. Healthy peanut plants were selected 12–15 days after flowering. Similar size (4–5 cm length) gynophores from the 2nd or 3rd joints of the plants were cut, rinsed with water, sterilised with 70% ethanol for 5 s and 0.1% hydrargyrum solution for 10 min then rinsed three times, each for a few seconds in sterilised ddH2O. Gynophore tips of 1 cm in length were cut and vertically inserted in MS medium with downward or upward tipping, and then cultured in the dark at 28°C. After 6 and 12 h, the 6–8 mm fragments from gynophore tips were cut, immediately frozen in liquid nitrogen and stored at −80°C for RNA extraction.
Total RNA extraction
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. To minimise biological and technological variance, for each experiment, RNA samples were extracted and pooled from a total of 100–150 gynophores of each treatment, and each treatment had two biological replicates. RNA samples were extracted from the two biological replicates individually in each treatment and were treated with RNase-free DNase I (Takara) then cleaned up with RNeasy Cleanup Kit (Qiagen, Shanghai, China). The concentration and quality were assessed using Nano Drop ND-100 spectrophotometer (Nano Drop Technologies Inc., Wilmington, DE, USA) and electrophoresis on a 1% agarose gel. The resulting total RNA was stored at −80°C until further analysis.
Microarray hybridisation and data acquisition
To measure gene expression in peanut, a customised NimblerGen (Nimblegen Systems, Inc., Madison, WI, USA) oligonucleotide microarray (4 × 72 K) has been developed. This microarray with 60-mer oligonucleotide probes contains 36 155 peanut unique transcripts (Chen et al. 2012). The microarray was used in this study and the hybridisation procedure was performed as described previously by Wang et al. (2013). Total RNA (5 μg) was used to synthesise double-stranded cDNA (ds-cDNA) using an Invitrogen SuperScript ds-cDNA synthesis kit. Resultant ds-cDNA was cleaned and fluorescently labelled using Klenow enzyme in accordance with the NimbleGen gene expression analysis protocol. Microarrays were hybridised, stained and conducted at CapitalBio Corporation (Beijing, China) using Roche (Shanghai, China) NimbleGen systems. All microarrays were scanned with a LuxScan 10 KA scanner using LUXSCAN 4.0 software (CapitalBio, Beijing, China). Fluorescence data were processed with SpotData software at CapitalBio Corporation as described previously (Graubert et al. 2007). Faint spots were removed with signal intensity less than 400 units after subtraction of the background and the data normalisation was performed at CapitalBio Corporation based on a robust multichip analysis (RMA, CapitalBio). The microarray analysis was employed to evaluate global gene expression gynophores cultured in vitro with tip pointing upward and downward at 6 and 12 h, respectively, then comparatively analysed differentially expressed genes (DEGs) between gynophores were cultured in vitro with tip pointing upward and downward at the same time. DEGs were defined as the probe sets having a P-value < 0.01 and >2-fold changes in at least one of the comparisons.
Gene identification and function analysis
The gene annotation was performed according to the method described by Shi et al. (2006). The DEGs were mapped to gene ontology (GO) terms using the molecule function annotation system (MAS, http://bioinfo.capitalbio.com/mas, accessed 20 December 2012) to organise genes into hierarchical categories on the basis of biological process and molecular function.
Quantitative real-time RT–PCR
All qRT–PCRs were performed as described previously (Wang et al. 2010). Four microgram of total RNA was reverse transcribed to cDNA using PrimeScript II 1st strand cDNA synthesis kit (Takara, Dalian, China) according to the manufacturer’s protocols. Quantitative real-time RT–PCR was performed with SYBR Premix Ex Taq II kit (Takara) in LightCycler 480 instrument (Roche) equipped with Light-Cycler Software ver. 1.5 (Roche) according to the manufacturer’s instructions (Alos et al. 2008). All the primers specific for selected peanut genes were designed using the Primer ver. 6.0 (Premier Biosoft International, Palo Alto, CA, USA). Standard curves for each genes and actin were generated based on serial dilutions of cDNAs reverse-transcribed from one sample in which target genes were expressed at an appropriate level. The actin gene was used as an internal control for calculating relative transcript abundance. The same RNA samples for the microarry analysis were used for real-time PCR analsysis with three technical replicates for each sample. The relative quantification of RNA expression was calibrated using formula 2−ΔΔCt method (Livak and Schmittgen 2001).
Results
In vitro culture system of peanut gynophore explants responding to gravity stimulation
Gynophores of similar size from the 2nd or 3rd joints of peanut plants 12–15 days after flowering were cut, sterilised and inserted into MS agar medium for culture in darkness. As shown in Fig. 1, the gynophores with tip pointing downward kept their original growth direction for 12 h. Conversely, the tips of gynophores with tip pointing upward began to bend down at 6 h, and the angle of the bend reaching 90° at 12 h. The observation implies that the peanut gynophores in vitro culture system can respond to gravity stimulation in the same way as in peanut plant seedlings, and is valuable for use in studying the gravitropic responses in peanut gynophores.
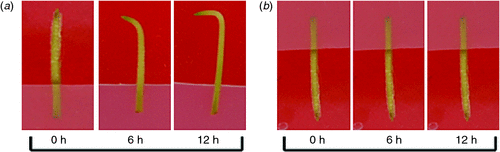
|
Identification of potential gravitropic response genes in peanut gynophore transcriptome
To identify differentially expressed genes related to gynophore gravitropic response, a customised NimblerGen onligonucleotide microarray assay was performed to analyse the mRNA expression profiles of gynophores cultured in vitro with tip pointing upward (gravitropic stimulation sample) and downward (natural growth control). The results showed that 28 029 genes, accounting for 77.52% of all genes in the microarray, were expressed. The remaining 8129 genes (22.48%) were not expressed in all samples – these were excluded in subsequent analyses.
Scatter plots could be used to show the gene expression difference between the two samples in microarray data analysis. In Fig. 2, x-axis and y-axis represent the hybridisation signal intensity of gynophores cultured in vitro with tip pointing downward (control) and upward (gravitropic stimulation sample) respectively. Each point in the figure indicates the hybridisation signal value of one gene. The red and green points represent upregulated expressed genes (ratio >2) and downregulated expressed genes (ratio <0.5) respectively. The black points represent genes that were not differentially expressed (ratio >0.5 and ratio <2). Both red and green points represent significantly differentially expressed genes that were further analysed. The results showed that there was a substantial number of differentially expressed genes between the control gynophores and the gravitropic stimulated gynophores (Fig. 2). In gravitropic stimulated gynophores (with tip pointing downward), the number of upregulated genes was close the number of downregulated genes at 6 h, whereas the number of upregulated genes was more than that of the downregulated genes at 12 h (Fig. 2). According to the 2.0 : 0.5 (ratio >2.0 or ratio <0.5) screening standard, there were 174 differentially expressed genes including 91 upregulated genes and 83 downregulated ones in gravitropic stimulated gynophores after in vitro culture for 6 h (Figs 2, 3). After in vitro culturing for 12 h, there were 491 differentially expressed genes including 129 upregulated genes and 362 downregulated ones in gravitropic stimulated gynophores (Figs 2, 3). Among them, one gene (AHTC1034254, Peroxidase 4-like protein from Glycine max) was upregulated and 4(AHTC1006412, AHTC1010360, AHTC1036262 and AHTC1036618) downregulated at both 6 and 12 h in gravitropic stimulated gynophores after in vitro culture (Fig. 3; Table 1). In gravitropic stimulated gynophores, four genes (AHTC1003533, AHTC1004729, AHTC1012222 and AHTC1034957) were upregulated after in vitro culture for 6 h then downregulated after in vitro culture for 12 h (Fig. 3; Table 1). And one gene (AHTC1014746) was downregulated after in vitro culture for 6 h then upregulated after 12 h of in vitro culture (Fig. 3; Table 1). As shown in Fig. 3, the overall analysis of microarray data revealed that there were 655 differentially expressed genes including 219 upregulated genes and 441 downregulated ones in gravitropic stimulated gynophores after in vitro culture for 6 or 12 h. All upregulated and downregulated genes of gynophores grown in vitro with tip pointing upward at 6 and 12 h were listed in Tables S1 and S2 (available as Supplementary Material to this paper) respectively. The top 20 differently expressed genes of gynophores grown in vitro with tip pointing upward at 6 and 12 h were listed in Tables 2 and 3 respectively.
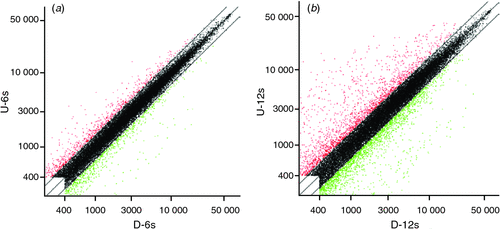
|
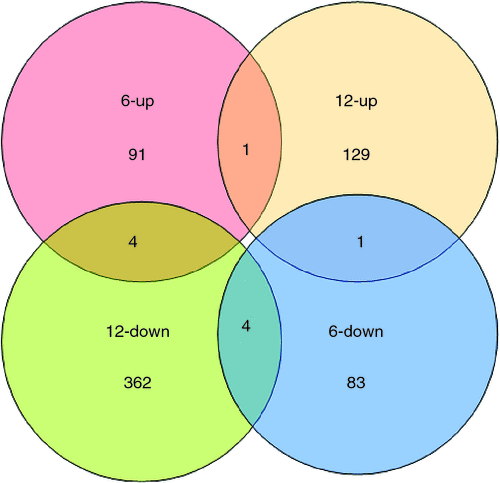
|

|

|

|
Validation of the microarray results using quantitative RT–PCR
To confirm the microarray results, five differentially expressed genes were randomly selected for real-time RT–PCR analysis. The primers were designed on the basis of expressed sequence tags (ESTs) corresponding to the microarray hybridisation probes (Table 4). The expression level of actin gene was used as an internal control. All real-time PCR reactions were repeated biologically two times and repeated technologically three times. The changes of gene expression level among gynophores cultured in vitro with tip pointing upward and downward both at 6 and 12 h were examined. The results showed that, with the exception of AHTC1004450 (auxin efflux carrier), the remaining four genes showed similar patterns with real-time RT–PCR and microarray analyses. Although the extent of expression was slightly different between microarray and real-time RT–PCR, the expression trends were the same between two analysing systems (Table 4).

|
Functional classification of potential gravitropic response genes in peanut gynophores
To identify biological process of differentially expressed genes, gene ontology (GO) analysis was conducted using molecule annotation system (MAS, http://bioinfo.capitalbio.com/mas3, accessed 20 December 2012). The GO analysis obtained with the annotation procedure through homology analysis generated a concise functional annotation. As shown in Fig. 4, the known differentially expressed genes were classified into 24 functional categories (Fig. 4). These differentially expressed genes were mainly distributed in cellular process (13.5%), physiological process (13.5%), catalytic activity (10.6%), metabolism (8.7%), cell (6.4%), cell part (6.4%), response to stimulus (5.7%); other items (4.7%), binding (4.6%) and organelle (4.3%) (Fig. 4). To identify the metabolic pathways of these potential gravitropic genes, the MAS was employed to assign the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of the differential expression genes. Twenty top pathways (all q-values <0.05) are listed in Table 5, which includes carbon fixation, aminoacyl-tRNA biosynthesis, pentose phosphate pathway, and starch and sucrose metabolism.
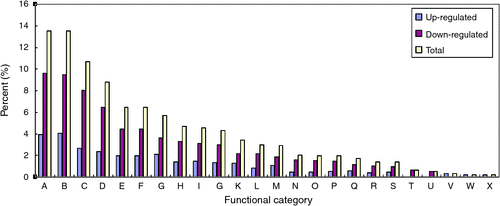
|
Discussion
In this study, a high-density peanut gene microarray was employed to compare the expression profiles of peanut gynophores cultured in vitro with tip pointing upward and downward at both 6 and 12 h. The collection of gravity responsive genes provides a valuable resource for understanding the molecular mechanism underlying the peanut gynophore gravitropism. The differentially expressed genes under gravity stimuli were determined and further confirmed for some of them by quantitative RT–PCR. Potential gravitropic genes and their putative functions presented in this study can help our understanding of the biological processes involved in gravitropism. The microarray data also provided clues for identification of potential molecular markers in peanut gynophores, especially in their gravity perception and response. The MAS assignments demonstrate that except aminoacyl-tRNA biosynthesis, and ribosome 2 translation pathways, all other pathways are metabolic pathways, which potentially functioned in the gynophore gravitropism. Among the metabolism pathways identified, carbohydrate metabolism and amino acid metabolism are the two major pathways, following with energy metabolism, biosynthesis of other secondary metabolites, metabolism of terpenoids and polyketides, glycan biosynthesis and metabolism, and nucleotide metabolism. Similar to our results, transcriptome analysis in Arabidopsis exposed to gravity stimulation also revealed that metabolism is one of major gene groups responding to gravitropism (Kimbrough et al. 2004; Kittang et al. 2004) Among the metabolism pathways identified in this study, energy metabolism was also found to function in plants under gravitropic stimulation according to proteomic analysis (Azri et al. 2009; Herrera et al. 2010). In Arabidopsis, secondary metabolism genes were altered by magnetically induced hyper- and microgravity at both transcription and translation levels (Manzano et al. 2012; Herranz et al. 2013). Proteome analysis of poplar stems exposed to gravitropic stimulation has led to determination of several pathways also identified in our study, including nucleotide metabolism, carbohydrate metabolism, photosynthesis and primary metabolism (energy) (Azri et al. 2009). Millar and Kiss (2013) also showed the metabolic profile of Arabidopsis thalianna during gravitropism and phototropism, and identified carbohydrate metabolism, secondary metabolism and amino acid biosynthesis as the three main responding pathways. They are all included in identified pathways responding to gravitropism in the present study. Consistent with previous results, carbohydrate metabolism, energy metabolism, biosynthesis of other secondary metabolites, and nucleotide metabolism were also identified in our results. Additionally, several novel pathways including aminoacyl-tRNA biosynthesis, ribosome, amino acid metabolism, metabolism of terpenoids and polyketides, glycan biosynthesis and metabolism were identified for the first time to function in gravitropic responses.
Carbohydrate metabolism in peanut gynophore gravitropism
Five carbohydrate metabolism pathways, including pentose phosphate pathway (PPP), pyruvate metabolism, starch and sucrose metabolism, glyoxylate and dicarboxylate metabolism, fructose and mannose metabolism, were discovered in peanut gynophores in response to gravity in our microarray assay. Metabolism of glucose through the pentose phosphate pathway is one of the main antioxidant cellular defence systems (Riganti et al. 2012). It supplies cells nucleic acid and lipid biosynthesis with NADPH, ribose 5-phosphate, and glycolytic intermediates for defence against oxidative stress. Recently, the PPP was determined to also play roles in cell cycle, apoptosis, invasion, angiogenesis, and respond to anti-tumour therapy (Riganti et al. 2012). Pentose level is responsive to gravitropism in cell walls of wheat coleoptiles (Wakabayashi et al. 2003). Pyruvate is the major product of glycolysis. Metabolism of pyruvate is complicated and involves various cycles to generate NADPH, acetyl-CoA (Sugden and Holness 2011). It has a broad application in food industry. Disorder of the pathway is associated with human diseases. Starch and sucrose metabolism contains two major pathways. First, sucrose is converted to starch for carbohydrate storage in the amyloplasts (Czyzewska and Marczewski 2009). Second, starch may be degraded into glucose and maltose either hydrolytically or phosphorolytically for energy within the plant (Zeeman et al. 2007). In cereal grass shoots, starch statoliths function as the gravisensors (Song et al. 1988, and in excised wheat leaves, alteration of gravity dramatically influence carbohydrate metabolism by reducing synthesis of fructose, sucrose, starch and fructan (Obenland and Brown 1994). In plant gravitropism, the widely accepted model is the starch-statolith hypothesis: that sediment of starch filled amyloplasts converts gravity into subsequent cellular signalling for plant growth (Sack 1997). The mutant experiments in roots, hypocotyls and stems showing reduced gravitropism provide supporting evidence for the starch-statolith theory (Kiss et al. 1996, 1997; Weise and Kiss 1999). Glyoxylate and dicarboxylate metabolism refers to the set of glyoxylate or dicarboxylates involving reactions. Among these, the glyoxylate cycle functions in the biosynthesis of carbohydrates from lipids. Fructose-2, 6-bisphosphate regulates the balance between glycolysis and gluconeogenesis (Hampp et al. 1997). In etiolated corn and pea, mannose can increase gravitropic bending by 2-deoxy-D-glucose, an inhibitor of protein glycosylation, and callose deposition may be a biochemical component of gravitropism in plant shoots (Jaffe and Leopold 1984).
Other metabolism in peanut gynophore gravitropism
Other metabolism pathways identified in our study include energy metabolism, biosynthesis of other secondary metabolites, metabolism of terpenoids and polyketides, glycan biosynthesis and metabolism, and nucleotide metabolism. Carbon fixation is one of the major energy metabolism pathways. Plant photosynthesis is the typical carbon fixation process, which serves to transform energy from sunlight into chemical compound (Zelitch 1975; Ducat and Silver 2012). Ortiz et al. (2000) revealed that hypergravity inhibited CO2 fixation during photosynthesis through decreasing chlorophyll a/b ratios. Carbon fixation increased under microgravity environment (Salisbury 1984; Adamchuk et al. 1999). In addition to energy production, the mitochondrial oxidative phosphorylation system also plays important roles in free radical generation and apoptosis (Hüttemann et al. 2007). Altered gravity changes the mitochondrial structure and increases both of the size and activity of mitochondria in cells (Hüttemann et al. 2007). Flavonoid biosynthesis and phenylpropanoid biosynthesis are the two pathways found in the biosynthesis of other secondary metabolites. Flavonoids are the major pigments of red, blue, and purple in plants and constitute a diverse family including the chalcones, flavones, flavonols, flavandiols, anthocyanins, and condensed tannins (Winkel-Shirley 2001). Both microgravity exposure in spaceflight and clinorotation conditions resulted in enhanced accumulation of flavonoids and isoflavone glycosides in soybean seedlings (Levine et al. 2001). Gravity stimuli induced flavonoid accumulation in the site of gravity perception, i.e. columella cells (Levine et al. 2001). In addition, deficiency of flavonoid resulted in delayed gravitropism in Arabidopsis thaliana (Buer and Muday 2004). Aminocyclopropane carboxylic acid reduced flavonoid synthesis thereby inhibited gravity response in A. thaliana (Buer et al. 2006). These results suggest that flavonoid is one of the key components in the plant gravitropic regulation signalling. The way of flavonoid functions in gravity response was determined through regulation of auxin fluxes in roots (Santelia et al. 2008).
Gene expression in peanut gynophore gravitropism
Among the 10 differentially expressed genes at both 6 and 12 h in this study, three encode uncharacterised proteins and one is not found in the database based on sequence similarity analysis using BLASTX algorithm. Here, we will focus on other six proteins identified. Beta-amyrin synthase is a key enzyme in the plant signal pathway for secondary metabolites triterpenoid saponins production (Yendo et al. 2010), which is widely used pharmacological medicine for antiplatelets, antitumorals and immunoadjuvants. In Glycyrriza uralensis, beta-amyrin synthase was found only to be expressed in underground parts, where its expression level in the root tip is much higher than in the rootstock. Since the root tip is the responding part to gravity stimulation, its expression in this part indicates its possible role in gravitropism (Liu and Liu 2012). The upregulated expression at 6 h and downregulated expression at 12 h of beta-amyrin synthase indicates its potential role in responding to gravitropic signals at the early phase. In plants, amine oxidase includes diamine oxidases (DAO) and FAD-binding polyamine oxidases (PAO), which oxidatively catabolised polyamines and function in plant development, stress defence (Wimalasekera et al. 2011). As early as 1965, plant amine oxidase has been demonstrated to catalyse the oxidation of tryptamine and produce 3-indolylacetaldehyde (IAc), which may function as the precursor of 3-indolylacetic acid (IAA) (Clarke and Mann 1957). This may explain how amine oxidase plays a role in gravitropism. Further, it has been reported that the expression of animal-derived semicarbazide-sensitive amine oxidase (SSAO; E.C.1.4.3.6.) in Escherichia coli BL21 is dramatically influenced by simulated microgravity (Zhang et al. 2012). The result indicates amine oxidase’s possible roles in responding to gravity. The expression alteration of amine oxidase in our study suggests that amine oxidase may be involved in the early stage of gravitropism signalling. diphosphonucleotide phosphatase 1 (PPD1) was first identified from yellow lupin (Olczak et al. 2000). It belongs to a novel group of metallophosphatases comprising PPD1, PPD2, PPD3 and PPD4. PPD1 can specifically hydrolyse diphosphonucleotides and phosphodiesters (Olczak et al. 2009). The mRNA expression of PPD1 accumulates at stems and leaves in yellow lupin (Olczak and Olczak 2002). However, so far the function of PPD1 in plant response to gravity has not been reported. The findings in our study suggest that PPD1 may be involved in plant gravitropic regulation. Laccases are another type of oxidase found in our study. They are copper-containing phenolic compounds metalloxidases (Jeon et al. 2012). Laccases are involved in fungal spore pigmentation, tobacco protoplasts regeneration, and the formation and degradation of organic substances like lignin (Mayer and Staples 2002; Claus 2003). Both auxin and laccase may be involved in root meristem formation in Medicago truncatula (Eyles et al. 2013). In white-rot fungus Funalia trogii (Trametes trogii), the production of auxin IAA and the activity of peroxidase-laccase were revealed to have a negative correlation (Ünyayar et al. 2001). The results in this study suggest that peroxidase and laccase may degrade IAA, and IAA may, in turn, repress activities of peroxidase and laccase. The expression alteration of laccase in different time points during gynophore gravitropism indicates that it may respond to gravity stimulation through its regulation of auxin concentration. Ethylene is well known as the inducer of fruit ripening. Moreover, it regulates a variety of metabolic and developmental processes including seed germination, organ senescence in plants (Bleecker and Kende 2000). Ethylene-responsive transcription factor belongs to the ethylene-responsive element binding factors (ERFs), which are characterised by the ERF domain, a highly conserved DNA binding domain (Fujimoto et al. 2000). It has been proposed that ethylene signalling-induced activation of the ethylene response factor 1 (ERF1) gene is associated to the inhibition of auxin signal response (Díaz and Alvarez-Buylla 2006). The downregulation of ethylene-responsive transcription factor at both 6 and 12 h indicates the increasing auxin response, which will enhance the gravitropism of peanut gynophore cultured in vitro with tip pointing upward. RD22-like protein is one subfamily of the BURP-domain protein family, which featured as the conserved BURP domain at the C-terminal. RD22-like protein is a known stress response gene in plants. Drought treatment induced RD22 expression in Arabidopsis, and then RD22 has often been used as drought stress marker (Yamaguchi-Shinozaki and Shinozaki 1993). Also, abscisic acid (ABA) and salt stress can induce RD22 expression (Iwasaki et al. 1995; Abe et al. 1997). Recently, it has been reported that transgenic expression of a RD22-like protein (GmRD22) can reduce salinity and osmotic stress in plants (Wang et al. 2012). Both downregulated expression of RD22-like protein at 6 and 12 h in the gynophores with pointing upward indicate its opposite role in gravitropism, but how the RD22-like protein functions in gravitropism needs further investigation.
Acknowledgements
This research was funded by grants from National Natural Science Foundation of China (No. 31200155 and 31271767), Pearl River Science and Technology Nova of Guangzhou (No. 2011J2200035), Science and Technology Planning Project of Guangdong Province (No. 2011B010500019, 2012B050700007), and supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-14) and foundation of CRI/GDAAS (2012–2). The founders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We declare no conflict of interests.
References
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. The Plant Cell 9, 1859–1868.Adamchuk NI, Mikhaylenko NF, Zolotareva EK, Hilaire E, Guikema JA (1999) Spaceflight effects on structural and some biochemical parameters of Brassica rapa photosynthetic apparatus. Journal of Gravitational Physiology 6, 95–96.
Alos E, Roca M, Iglesias DJ, Minguez-Mosquera MI, Damasceno CM, Thannhauser T.W., Rose J.K., Talon M., Cercos M. (2008) An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiology 147, 1300–1315.
| An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslyisLk%3D&md5=18ee846ac86d2ae2f79f8fa0a42316e1CAS | 18467459PubMed |
Azri W, Chambon C, Herbette S, Brunel N, Coutand C, Leplé JC, Ben Rejeb I, Ammar S, Julien JL, Roeckel-Drevet P (2009) Proteome analysis of apical and basal regions of poplar stem under gravitropic stimulation. Physiologia Plantarum 136, 193–208.
| Proteome analysis of apical and basal regions of poplar stem under gravitropic stimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntlehsb8%3D&md5=8e917ccd29a5526e33d0a2fafb860de7CAS | 19453506PubMed |
Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peret B, Oliva M, Swarup R, Sairanen I, Parry G, Ljung K, Beeckman T, Garibaldi JM, Estelle M, Owen MR, Vissenberg K, Hodgman TC, Pridmore TP, King JR, Vernoux T, Bennett MJ (2012) Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proceedings of the National Academy of Sciences of the United States of America 109, 4668–4673.
| Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvFClsLw%3D&md5=eec3582135d3e9ff0c09e18871faaf20CAS | 22393022PubMed |
Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119, 57–70.
Blancaflor EB, Masson PH (2003) Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiology 133, 1677–1690.
| Plant gravitropism. Unraveling the ups and downs of a complex process.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvFCj&md5=e1e07288d6a3ae4162510f7d51104b8cCAS | 14681531PubMed |
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biology 16, 1–18.
| Ethylene: a gaseous signal molecule in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXpvFOj&md5=25610023b8a85a85d6c1b9debc6b5facCAS | 11031228PubMed |
Boccalandro HE, De Simone SN, Bergmann-Honsberger A, Schepens I, Fankhauser C, Casal JJ (2008) PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiology 146, 108–115.
| PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtFCms7c%3D&md5=a8bbbcc207a3cbb40b43fec69fd53efaCAS | 18024556PubMed |
Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. The Plant Cell 16, 1191–1205.
| The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXktlOqsbw%3D&md5=3d9fc3e699788564cf76a2ae86e04019CAS | 15100399PubMed |
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiology 140, 1384–1396.
| Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjsl2jsrw%3D&md5=c03d7c61bc1822e9bbc49775efeaef6bCAS | 16489132PubMed |
Chen XP, Hong YB, Zhang EH, Liu HY, Zhou GY, Li SX, Zhu FH, Guo BZ, Yu JJ, Liang XQ (2012) Comparison of gene expression profiles in cultivated peanut (Arachis hypogaea) under strong artificial selection. Plant Breeding 131, 620–630.
| Comparison of gene expression profiles in cultivated peanut (Arachis hypogaea) under strong artificial selection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVeitLzO&md5=0cf8ff0d11dc9f1e33e5259f9d2153c6CAS |
Clarke AJ, Mann PJ (1957) The oxidation of tryptamine to 3-indolylacetaldehyde by plant amine oxidase. Biochemical Journal 65, 763–774.
Claus H (2003) Laccases and their occurrence in prokaryotes. Archives of Microbiology 179, 145–150.
Czyzewska D, Marczewski W (2009) Starch metabolism in potato tubers. Postepy Biochemii 55, 441–446.
Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433.
| The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XltVSksLw%3D&md5=5ac92e2c6b1ec7a32eb5982f4ac2262bCAS | 8756724PubMed |
Díaz J, Alvarez-Buylla ER (2006) A model of the ethylene signaling pathway and its gene response in Arabidopsis thaliana: pathway cross-talk and noise-filtering properties. Chaos 16, 023112
| A model of the ethylene signaling pathway and its gene response in Arabidopsis thaliana: pathway cross-talk and noise-filtering properties.Crossref | GoogleScholarGoogle Scholar | 16822015PubMed |
Ducat DC, Silver PA (2012) Improving carbon fixation pathways. Current Opinion in Chemical Biology 16, 337–344.
| Improving carbon fixation pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnslKrtLs%3D&md5=296fcfc3e6222ab256a439a608cf5a81CAS | 22647231PubMed |
Eyles RP, Williams PH, Ohms SJ, Weiller GF, Ogilvie HA, Djordjevic MA, Imin N (2013) MicroRNA profiling of root tissues and root forming explant cultures in Medicago truncatula. Planta 238, 91–105.
| MicroRNA profiling of root tissues and root forming explant cultures in Medicago truncatula.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVWhtrnN&md5=899dd0c015c8653c590164084c8ca97aCAS | 23572382PubMed |
Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, Philosoph-Hadas S (1998) Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiology 118, 483–492.
| Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmslyqt7g%3D&md5=a885f08dbc5810ac246ea5844496e0e3CAS | 9765533PubMed |
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. The Plant Cell 12, 393–404.
Fukaki H, Tasaka M (1999) Gravity perception and gravitropic response of inflorescence stems in Arabidopsis thaliana. Advances in Space Research 24, 763–770.
| Gravity perception and gravitropic response of inflorescence stems in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnmtFWqtQ%3D%3D&md5=6cdbe062bbd86afdc74a23936ac8bac7CAS | 11542620PubMed |
Goldsmith M (1977) The polar transport of auxin. Annual Review of Plant Physiology 28, 439–478.
| The polar transport of auxin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXksFSitrw%3D&md5=214f4c3ac54e3ae1ed2ff196a6410b5aCAS |
Graubert TA, Cahan P, Edwin D, Selzer RR, Richmond TA, Eis PS, Shannon WD, Li X, McLeod HL, Cheverud JM, Ley TJ (2007) A high-resolution map of segmental DNA copy number variation in the mouse genome. PLOS Genetics 3, e3
| A high-resolution map of segmental DNA copy number variation in the mouse genome.Crossref | GoogleScholarGoogle Scholar | 17206864PubMed |
Hampp R, Hoffmann E, Schonherr K, Johann P, De Filippis L (1997) Fusion and metabolism of plant cells as affected by microgravity. Planta 203, S42–S53.
| Fusion and metabolism of plant cells as affected by microgravity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvVyjsbo%3D&md5=c862b97bbaec31405b12951ed23ad359CAS | 9299795PubMed |
Hashiguchi Y, Tasaka M, Morita MT (2013) Mechanism of higher plant gravity sensing. American Journal of Botany 100, 91–100.
| Mechanism of higher plant gravity sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVKltbc%3D&md5=1c812d00da61c6bc9a409b7fe92ed62fCAS | 23115136PubMed |
Haswell ES (2003) Gravity perception: how plants stand up for themselves. Current Biology 13, R761–R763.
| Gravity perception: how plants stand up for themselves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVOmtrw%3D&md5=aa866b4346ecd667fe4da393e50f3132CAS | 14521853PubMed |
Herranz R, Manzano AI, van Loon JJ, Christianen PC, Medina FJ (2013) Proteomic signature of Arabidopsis cell cultures exposed to magnetically induced hyper- and microgravity environments. Astrobiology 13, 217–224.
| Proteomic signature of Arabidopsis cell cultures exposed to magnetically induced hyper- and microgravity environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVOltbc%3D&md5=8d50b80e189ac6cd06100e9c53de4f50CAS | 23510084PubMed |
Herrera R, Krier C, Lalanne C, Ba el HM, Stokes A, Salin F, Fourcaud T, Claverol S, Plomion C (2010) (Not) Keeping the stem straight: proteomic analysis of maritime pine seedlings undergoing phototropism and gravitropism. BMC Plant Biology 10, 217
| (Not) Keeping the stem straight: proteomic analysis of maritime pine seedlings undergoing phototropism and gravitropism.Crossref | GoogleScholarGoogle Scholar | 20925929PubMed |
Hüttemann M, Lee I, Samavati L, Yu H, Doan JW (2007) Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochimica et Biophysica Acta 1773, 1701–1720.
| Regulation of mitochondrial oxidative phosphorylation through cell signaling.Crossref | GoogleScholarGoogle Scholar | 18240421PubMed |
Iwasaki T, Yamaguchi-Shinozaki K, Shinozaki K (1995) Identification of a cis-regulatory region of a gene in Arabidopsis thaliana whose induction by dehydration is mediated by abscisic acid and requires protein synthesis. Molecular & General Genetics 247, 391–398.
| Identification of a cis-regulatory region of a gene in Arabidopsis thaliana whose induction by dehydration is mediated by abscisic acid and requires protein synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmsVahsLk%3D&md5=2587a0806085641af3c29054e3b43205CAS |
Jacobs W (1947) The development of the gynophore of the peanut plant, Arachis hypogaea L. I. The distribution of mitoses, the region of greatest elongation, and the maintenance of vascular continuity in the intercalary meristem. American Journal of Botany 34, 361–370.
| The development of the gynophore of the peanut plant, Arachis hypogaea L. I. The distribution of mitoses, the region of greatest elongation, and the maintenance of vascular continuity in the intercalary meristem.Crossref | GoogleScholarGoogle Scholar |
Jacobs W (1951) Auxin relationships in an intercalary meristem: further studies on the gynophore of Arachis hypogaea. American Journal of Botany 38, 307–310.
Jaffe MJ, Leopold AC (1984) Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta 161, 20–26.
| Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXkt1SktL4%3D&md5=6224f8054d429f35c20b03fd0f8046c5CAS | 11541962PubMed |
Jeon JR, Baldrian P, Murugesan K, Chang YS (2012) Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microbial Biotechnology 5, 318–332.
| Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVaksL3K&md5=0b12a04d3275358575d3143c5e290876CAS | 21791030PubMed |
Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. The Plant Cell 14, 33–46.
| SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1Srsb0%3D&md5=0b4c149d4db9ba9b1a4f9c3ecd8de4d4CAS | 11826297PubMed |
Kimbrough JM, Salinas-Mondragon R, Boss WF, Brown CS, Sederoff HW (2004) The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiology 136, 2790–2805.
| The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvFOqsrs%3D&md5=95d951c73bfacf8669643b5e500f68b9CAS | 15347791PubMed |
Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. Critical Reviews in Plant Sciences 19, 551–573.
| Mechanisms of the early phases of plant gravitropism.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FotVarsg%3D%3D&md5=a515bf500bd48052bbafe8be5d139013CAS | 11806421PubMed |
Kiss JZ, Wright JB, Caspar T (1996) Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiologia Plantarum 97, 237–244.
| Gravitropism in roots of intermediate-starch mutants of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xjslyhtr4%3D&md5=365ea011f30a241f0e54b33567b503bcCAS | 11539189PubMed |
Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS (1997) Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant & Cell Physiology 38, 518–525.
| Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtl2ktbo%3D&md5=61ff621ec7a52bf7c187d7c8aa4ab184CAS |
Kittang AI, van Loon JJ, Vorst O, Hall RD, Fossum K, Iversen TH (2004) Ground based studies of gene expression in Arabidopsis exposed to gravity stresses. Journal of Gravitational Physiology 11, 223–224.
Koprivova A, Mugford ST, Kopriva S (2010) Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Reports 29, 1157–1167.
| Arabidopsis root growth dependence on glutathione is linked to auxin transport.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGgs73M&md5=021c4bb6bb89dea868bbd01bf922934bCAS | 20669021PubMed |
Levine LH, Levine HG, Stryjewski EC, Prima V, Piastuch WC (2001) Effect of spaceflight on isoflavonoid accumulation in etiolated soybean seedlings. Journal of Gravitational Physiology 8, 21–27.
Liu Y, Liu CS (2012) Study on the spatial and temporal expression of beta-AS gene of Glycyrriza uralensis. Zhong Yao Cai 35, 528–531. [In Chinese]
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔ CT method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔ CT method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFelt7s%3D&md5=cda22da73d6034ad2e4c74827f633baaCAS | 11846609PubMed |
Manzano AI, van Loon JJ, Christianen PC, Gonzalez-Rubio JM, Medina FJ, Herranz R (2012) Gravitational and magnetic field variations synergize to cause subtle variations in the global transcriptional state of Arabidopsis in vitro callus cultures. BMC Genomics 13, 105
| Gravitational and magnetic field variations synergize to cause subtle variations in the global transcriptional state of Arabidopsis in vitro callus cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslamurfE&md5=ea35cb0d456f109aa1cd6a56c4a9bebaCAS | 22435851PubMed |
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60, 551–565.
| Laccase: new functions for an old enzyme.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xlt1Ois7w%3D&md5=422f50d0fb8b629ca57b5a0fce125d0cCAS | 12126701PubMed |
Millar KD, Kiss JZ (2013) Analyses of tropistic responses using metabolomics. American Journal of Botany 100, 79–90.
| Analyses of tropistic responses using metabolomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVKltbk%3D&md5=19a2c3b6ee6f686d8c1cec99b698f1acCAS | 23196394PubMed |
Morita MT, Tasaka M (2004) Gravity sensing and signaling. Current Opinion in Plant Biology 7, 712–718.
| Gravity sensing and signaling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXos1Chtrc%3D&md5=e5f5ea5af9b11f05fd7c21914a0b9384CAS | 15491921PubMed |
Moseyko N, Zhu T, Chang HS, Wang X, Feldman LJ (2002) Transcription profiling of the early gravitropic response in Arabidopsis using high-density oligonucleotide probe microarrays. Plant Physiology 130, 720–728.
| Transcription profiling of the early gravitropic response in Arabidopsis using high-density oligonucleotide probe microarrays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotVKntLw%3D&md5=af5e26e9bed5ffb2d89364097b59ce8aCAS | 12376639PubMed |
Muday GKRA (2008) Auxin transport and the integration of gravitropic growth. In ‘Plant tropisms’. (Eds S Gilroy, PH Masson) pp. 47–78. (Blackwell Publishing: Oxford)
Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Science 6, 535–542.
| Polar auxin transport: controlling where and how much.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFGksLc%3D&md5=26a3e0f5bb5f641bc78ee0fad0e355acCAS |
Nakamura M, Toyota M, Tasaka M, Morita MT (2011) An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and F-actin in gravity sensing. The Plant Cell 23, 1830–1848.
| An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and F-actin in gravity sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWqsL0%3D&md5=5f49c7a0c738c6c3b244229bf13ae61fCAS | 21602290PubMed |
Obenland DM, Brown CS (1994) The influence of altered gravity on carbohydrate metabolism in excised wheat leaves. Journal of Plant Physiology 144, 696–699.
| The influence of altered gravity on carbohydrate metabolism in excised wheat leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXisFKns7Y%3D&md5=300df31828207de04438ba19e86cfd50CAS | 11541755PubMed |
Olczak M, Olczak T (2002) Diphosphonucleotide phosphatase/phosphodiesterase from yellow lupin (Lupinus luteus L.) belongs to a novel group of specific metallophosphatases. FEBS Letters 519, 159–163.
| Diphosphonucleotide phosphatase/phosphodiesterase from yellow lupin (Lupinus luteus L.) belongs to a novel group of specific metallophosphatases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjvVSnu78%3D&md5=d19b62762934f6adf28c4cc8a79e90ccCAS | 12023036PubMed |
Olczak M, Kobiałka M, Watorek W (2000) Characterization of diphosphonucleotide phosphatase/phosphodiesterase from yellow lupin (Lupinus luteus) seeds. Biochimica et Biophysica Acta 1478, 239–247.
| Characterization of diphosphonucleotide phosphatase/phosphodiesterase from yellow lupin (Lupinus luteus) seeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsVGhtrs%3D&md5=ac84d3474afa14f5b5ae1b8f57abfb5bCAS | 10825535PubMed |
Olczak M, Ciuraszkiewicz J, Wójtowicz H, Maszczak D, Olczak T (2009) Diphosphonucleotide phosphatase/phosphodiesterase (PPD1) from yellow lupin (Lupinus luteus L.) contains an iron-manganese center. FEBS Letters 583, 3280–3284.
| Diphosphonucleotide phosphatase/phosphodiesterase (PPD1) from yellow lupin (Lupinus luteus L.) contains an iron-manganese center.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ais77P&md5=3158dfeabb8454d9b2cf3d88fc85a766CAS | 19755125PubMed |
Ortiz W, Wignarajah K, Smith JD (2000) Inhibitory effect of hypergravity on photosynthetic carbon dioxide fixation in Euglena gracilis. Journal of Plant Physiology 157, 231–234.
| Inhibitory effect of hypergravity on photosynthetic carbon dioxide fixation in Euglena gracilis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXms1ygtL8%3D&md5=6c16b121940c4e840b5a443466f19831CAS | 11543574PubMed |
Pattee HEMS (1987) Anatomical changes during ontogeny of the peanut (Arachis hypogaea L.) fruit: mature megagametophyte through heart-shaped embryo. Botanical Gazette 148, 156–164.
| Anatomical changes during ontogeny of the peanut (Arachis hypogaea L.) fruit: mature megagametophyte through heart-shaped embryo.Crossref | GoogleScholarGoogle Scholar |
Periasamy KSC (1984) The morphology and anatomy of ovule and fruit development in Arachis hypogaea L. Annals of Botany 53, 3
Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M, Becker D, Hedrich R (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proceedings of the National Academy of Sciences of the United States of America 96, 12 186–12 191.
| Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmvVGjtL4%3D&md5=6b27641abaf44a59967847bdfc5eae9dCAS |
Philosoph-Hadas S, Friedman H, Meir S, Berkovitz-SimanTov R, Rosenberger I, Halevy AH, Kaufman PB, Balk P, Woltering EJ (2001) Gravitropism in cut flower stalks of snapdragon. Advances in Space Research 27, 921–932.
| Gravitropism in cut flower stalks of snapdragon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvFKlurk%3D&md5=eb27085bb85425c0a910398641f797f9CAS | 11596635PubMed |
Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D (2012) The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radical Biology and Medicine 53, 421–436.
| The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtV2mtL3K&md5=b4025b02e7c5452f1b183f70e6ac9922CAS | 22580150PubMed |
Sack FD (1997) Plastids and gravitropic sensing. Planta 203, S63–S68.
| Plastids and gravitropic sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvVyjsbg%3D&md5=1651220be9f9bebd05f9d3c3407a81bdCAS | 11540330PubMed |
Salisbury FB (1984) Achieving maximum plant yield in a weightless, bioregenerative system for a space craft. The Physiologist 27, S31–S34.
Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, Martinoia E (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. Journal of Biological Chemistry 283, 31 218–31 226.
| Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlWjtLjO&md5=d6453680f9959f072377ba9938e55bacCAS |
Schwuchow J, Sack FD, Hartmann E (1990) Microtubule distribution in gravitropic protonemata of the moss Ceratodon. Protoplasma 159, 60–69.
| Microtubule distribution in gravitropic protonemata of the moss Ceratodon.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnlsFKhsA%3D%3D&md5=725c2d6d0aca5cec5fd6c6387bcb1d47CAS | 11537091PubMed |
Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, Cheng J, Wei LP, Wang ZY, Zhu YX (2006) Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. The Plant Cell 18, 651–664.
| Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XisFahur8%3D&md5=002e3a87dddc5e4f6e616d654395c3b8CAS | 16461577PubMed |
Shushu DDCE (1990) Growth of the gynophore of the peanut Arachis hypogaea. 2. Regulation of growth. Canadian Journal of Botany 68, 14
Smith BW (1950) Arachis hypogaea. Aerial flower and subterranean fruit. American Journal of Botany 37, 802–815.
| Arachis hypogaea. Aerial flower and subterranean fruit.Crossref | GoogleScholarGoogle Scholar |
Song I, Lu CR, Brock TG, Kaufman PB (1988) Do starch statoliths act as the gravisensors in cereal grass pulvini? Plant Physiology 86, 1155–1162.
| Do starch statoliths act as the gravisensors in cereal grass pulvini?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnls1Oktw%3D%3D&md5=c2671a0cd785a54e63d795d998244f68CAS | 11538229PubMed |
Sugden MC, Holness MJ (2011) The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: going round in circles? Islets 3, 302–319.
| The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: going round in circles?Crossref | GoogleScholarGoogle Scholar | 21934355PubMed |
Ünyayar S, Ünal E, Ünyayar A (2001) Relationship between production of 3-indoleacetic acid and peroxidase-laccase activities depending on the culture periods in Funalia trogii (Trametes trogii). Folia Microbiologica 46, 123–126.
| Relationship between production of 3-indoleacetic acid and peroxidase-laccase activities depending on the culture periods in Funalia trogii (Trametes trogii).Crossref | GoogleScholarGoogle Scholar | 11501398PubMed |
van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B (1995) Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 378, 62–65.
| Cell fate in the Arabidopsis root meristem determined by directional signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXptVyqsrw%3D&md5=515561a3c04838e01a1f1b56ddf394dfCAS | 7477287PubMed |
Wakabayashi K, Soga K, Kamisaka S, Hoson T (2003) Modification of cell wall architecture of wheat coleoptiles grown under hypergravity conditions. Biological Science in Space 17, 228–229.
Wang T, Zhang E, Chen X, Li L, Liang X (2010) Identification of seed proteins associated with resistance to pre-harvested aflatoxin contamination in peanut (Arachis hypogaea L.). BMC Plant Biology 10, 267
| Identification of seed proteins associated with resistance to pre-harvested aflatoxin contamination in peanut (Arachis hypogaea L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFOgsbnF&md5=48077052a2d1dc68cf12ead3d5981521CAS | 21118527PubMed |
Wang H, Zhou L, Fu Y, Cheung MY, Wong FL, Phang TH, Sun Z, Lam HM (2012) Expression of an apoplast-localized BURP-domain protein from soybean (GmRD22) enhances tolerance towards abiotic stress. Plant Cell Environ 35, 1932–1947.
| Expression of an apoplast-localized BURP-domain protein from soybean (GmRD22) enhances tolerance towards abiotic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVKrtLjE&md5=4a1662311d9a6e736ef1e16aea9b1f58CAS | 22548236PubMed |
Wang T, Chen XP, Li HF, Liu HY, Hong YB, Yan QL, Chi XY, Yan Z, Yu SL, Li L, Liang XQ (2013) Transcriptome identification of the resistance-associated genes (RAGs) to Aspergillus flavus infection in pre-harvested peanut (Arachis hypogaea). Functional Plant Biology 40, 292–303.
| Transcriptome identification of the resistance-associated genes (RAGs) to Aspergillus flavus infection in pre-harvested peanut (Arachis hypogaea).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktVOgtbc%3D&md5=6cd717a841d13a09bb67671290ada276CAS |
Weise SE, Kiss JZ (1999) Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. International Journal of Plant Sciences 160, 521–527.
| Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnmtVynsQ%3D%3D&md5=f6db82340162ceadeaac2432a26c0947CAS | 11542271PubMed |
Wimalasekera R, Tebartz F, Scherer GF (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Science 181, 593–603.
| Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFaru7bI&md5=8baf5d92b9fdd47b4e1f4a8cbffa19feCAS | 21893256PubMed |
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology 126, 485–493.
| Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1Gnu74%3D&md5=da70f877dea794044a1c8d4e2b6259c8CAS | 11402179PubMed |
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Molecular & General Genetics 238, 17–25.
Yendo AC, de Costa F, Gosmann G, Fett-Neto AG (2010) Production of plant bioactive triterpenoid saponins: elicitation strategies and target genes to improve yields. Molecular Biotechnology 46, 94–104.
| Production of plant bioactive triterpenoid saponins: elicitation strategies and target genes to improve yields.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXps1Crtr4%3D&md5=6bab4b71a6c80f57c008d2f68fa18069CAS | 20204713PubMed |
Zeeman SC, Smith SM, Smith AM (2007) The diurnal metabolism of leaf starch. Biochem J 401, 13–28.
| The diurnal metabolism of leaf starch.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlShsLnL&md5=978816d645ee5a1213280333872f19ffCAS | 17150041PubMed |
Zelitch I (1975) Pathways of carbon fixation in green plants. Annual Review of Biochemistry 44, 123–145.
| Pathways of carbon fixation in green plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXlt1ylsLs%3D&md5=e5f41a175d3d8fe36f5067fa6196696bCAS | 1094907PubMed |
Zhang Y, Lai C, Duan J, Guan N, Ullah K, Deng Y (2012) Simulated microgravity affects semicarbazide-sensitive amine oxidase expression in recombinant Escherichia coli by HPLC-ESI-QQQ analysis. Applied Microbiology and Biotechnology 94, 809–816.
| Simulated microgravity affects semicarbazide-sensitive amine oxidase expression in recombinant Escherichia coli by HPLC-ESI-QQQ analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvVOmtL8%3D&md5=c8f8f121df5c040f000fc0929d7065acCAS | 22170102PubMed |
Ziv MZE (1975) Geotropic responses and pod development in gynophore explants of peanut (Arachis hypogaea L.) cultured in vitro. Annals of Botany 39, 5



