Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: a review
Kunshan Gao A C and Douglas A. Campbell BA State Key Laboratory of Marine Environmental Science, Xiamen University, 361005 Xiamen, China.
B Department of Biology, Mount Allison University, Sackville, NB E4L 1G7, Canada.
C Corresponding author. Email: ksgao@xmu.edu.cn
Functional Plant Biology 41(5) 449-459 https://doi.org/10.1071/FP13247
Submitted: 21 August 2013 Accepted: 24 December 2013 Published: 13 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Diatoms dominate nearly half of current oceanic productivity, so their responses to ocean acidification are of general concern regarding future oceanic carbon sequestration. Community, mesocosm and laboratory studies show a range of diatom growth and photophysiological responses to increasing pCO2. Nearly 20 studies on effects of elevated pCO2 on diatoms have shown stimulations, no effects or inhibitions of growth rates. These differential responses could result from differences in experimental setups, cell densities, levels of light and temperature, but also from taxon-specific physiology. Generally, ocean acidification treatments of lowered pH with elevated CO2 stimulate diatom growth under low to moderate levels of light, but lead to growth inhibition when combined with excess light. Additionally, diatom cell sizes and their co-varying metabolic rates can influence responses to increasing pCO2 and decreasing pH, although cell size effects are confounded with taxonomic specificities in cell structures and metabolism. Here we summarise known diatom growth and photophysiological responses to increasing pCO2 and decreasing pH, and discuss some reasons for the diverse responses observed across studies.
Additional keywords: Bacillariophyceae, CO2, diatom, ocean acidification, photoinhibition, photosynthesis.
Introduction
Increasing atmospheric CO2 due to anthropogenic activities affects terrestrial photosynthesis, but is also causing pCO2 to rise and pH to drop in the surface oceans, which influence marine primary producers (Beardall et al. 2009; Riebesell and Tortell 2011; Gao et al. 2012a), in a yet more complicated way due to the concurrent changes in seawater chemistry and ocean mixing.
Diatoms are suggested to have evolved between 100 and 200 million years ago (Sims et al. 2006), when atmospheric CO2 is thought to have been ~2000 ppmv (Veron 2008), compared with current levels of ~400 ppmv and projected end-century levels of 750–1000 ppmv. The extant diatoms now contribute ~20% of the organic carbon generated globally each year by photosynthesis (Field et al. 1998). They exist both as phytoplankton and as benthic algae. At least some diatom species operate metabolic pathways unusual among studied phytoplankton, including a urea cycle (Allen et al. 2011) and a C4 carboxylation path (Reinfelder et al. 2004; Haimovich-Dayan et al. 2013). Diatoms run highly efficient CO2 concentrating mechanisms (CCMs) to achieve a high ratio of carboxylation to oxygenation (Raven et al. 2011; Reinfelder 2011). They tolerate high levels of UV radiation (Guan and Gao 2008; Wu et al. 2012a), enjoy a low susceptibility to photoinactivation of PSII compared with other phytoplankters (Six et al. 2007, 2009; Key et al. 2010; Wu et al. 2011) and successfully exploit variable light (Lavaud et al. 2004, 2007). They are, as a net result, by far the most successful group of eukaryotic aquatic primary producers, not only in terms of primary production but also in their number of species and their capacities to acclimate to environmental changes with diversified metabolisms. Diatom growth rates correlate closely with their size, decreasing almost linearly with the log of increasing cell volume, which ranges across eight orders of magnitude, with cell diameters ranging from ~2 µm to a few mm (Finkel et al. 2010). Diatoms have responded to past climate change through successions of differently sized cells, with a trend towards smaller cells under higher temperatures over the past 65 million years (Falkowski and Oliver 2007; Finkel et al. 2007).
The ongoing ocean acidification triggered by increasing atmospheric CO2 concentration alters seawater carbonate chemistry, the availabilities and toxicities of nutrients (Millero et al. 2009). Ocean warming will in concert tend to drive increased stratification, decreasing the thickness of the upper mixing layer and lowering transport of nutrients from interior or deeper layers to the surface ocean (Doney 2006; Steinacher et al. 2010). These changes will differentially affect differently sized diatom species (Flynn et al. 2012), and thereby alter sinking rates and organic carbon export (Finkel et al. 2010). Therefore, growth and physiological responses of diatoms to elevated CO2 concentrations have gained attention (Riebesell et al. 1993; Burkhardt and Riebesell 1997; Burkhardt et al. 1999). Stimulative, neutral and inhibitory effects of elevated CO2 concentrations on diatom growth have been reported in different species or even in the same species (Riebesell et al. 1993; Burkhardt et al. 1999; Chen and Gao 2003; Kim et al. 2006; Wu et al. 2010; Yang and Gao 2012; Li and Campbell 2013) (Table 1). In this review, we focus on the effects of elevated CO2 and lower pH on diatom photophysiology, by summarising studies of their growth and photophysiological responses to elevated CO2 under different experimental conditions, and discuss some potential mechanisms to resolve the diversity of responses observed across studies to date.

|
Ocean acidification
The oceans are presently absorbing ~25 million tons of CO2 from the atmosphere each day, an important role in counteracting global warming (Sabine et al. 2004). This dissolution of CO2 from the air is, however, acidifying the oceans (Doney 2006; Gattuso and Hansson 2011).
The exchange of CO2 between the sea and atmosphere depends on temperature, salinity, physical mixing of seawater, respiration and photosynthesis. Therefore, CO2 fluxes change horizontally due to physical, chemical and biological properties of waters. When CO2 dissolves in seawater, it combines with water to form carbonic acid (CO2 + H2O → H2CO3) which dissociates to bicarbonate (H2CO3 → H+ + HCO3–), discharging protons (H+) and so ultimately attaining a state of new equilibrium. However, as the H+ concentration increases with CO2 dissolution, the H+ releases can partially reverse the secondary dissociation reaction, leading to a decrease in carbonate ions (H+ + CO32– → HCO3–). Typical changes linked with ocean acidification are therefore increased concentrations of pCO2, H+ and HCO3–, decreases in the concentration of CO32– and decreases in the CaCO3 saturation state (Gattuso et al. 2010). Since the beginning of the industrial revolution, the pH of oceanic surface seawater has already dropped by ~0.1 unit due to atmospheric CO2 rise (Caldeira and Wickett 2003), equivalent to about a 30% increase in the H+ concentration. With a further increase of CO2 concentration in the atmosphere to 800–1000 ppmv under the IPCC A1F1 scenario (Houghton et al. 2001), by the end of this century, pH of the surface oceans will decrease by another 0.3–0.4 units (Feely et al. 2004; Sabine et al. 2004; Orr et al. 2005), thus, increasing [H+] by 100–150%. Consequently, organisms in the euphotic zone will be exposed to a higher CO2 and a lower pH, and their physiologies will respond to changes in seawater carbonate chemistry, as well as to secondary changes in ionic speciation and cell surface chemistry driven by decreasing pH (Millero et al. 2009; Flynn et al. 2012; Hervé et al. 2012; Sugie and Yoshimura 2013). These chemical changes can directly affect physiology of marine organisms (Pörtner and Farrell 2008), but can also indirectly influence organismal responses to other environmental factors including UV radiation (Sobrino et al. 2008; Gao et al. 2009; Chen and Gao 2011; Li et al. 2012a), light (Bartual and Galvez 2002; Sobrino et al. 2008; McCarthy et al. 2012; Li and Campbell 2013), temperature change (Pörtner and Farrell 2008; Zou et al. 2011) or nutrients (Burkhardt and Riebesell 1997; Burkhardt et al. 1999; Riebesell and Tortell 2011; Li et al. 2012b).
Growth responses
Dissolved inorganic carbon (DIC) in surface seawater, at present, is ~100–200 times that of CO2 in the atmosphere, but most seawater DIC is HCO3–, with CO2 typically accounting for less than 1% in pelagic waters (Gattuso et al. 2010). In addition, CO2 in seawater diffuses ~8000 times slower than in air, which can kinetically limit marine photosynthetic carbon fixation (Raven 1993; Riebesell et al. 1993; Morel et al. 1994). Growth of diatom species can, in turn, be limited by the availability of CO2 (Riebesell et al. 1993), and oceanic primary production might thus be enhanced by increasing atmospheric CO2 concentration (Hein and Sand-Jensen 1997; Schippers et al. 2004; Riebesell and Tortell 2011). However, the growth rate of diatom-dominated phytoplankton assemblages was not affected by an elevated pCO2 concentration of 800 μatm during 2–5 days shipboard incubation under ~30% of incident sunlight (Tortell et al. 2000). Growth of Skeletonema costatum was not stimulated by an enriched CO2 concentration (800 μatm) under laboratory conditions (Burkhardt and Riebesell 1997; Chen and Gao 2003, 2004a), but was enhanced in a mesocosm at an elevated CO2 concentration of 750 μatm (Kim et al. 2006). Growth of the diatoms Phaeodactylum tricornutum (Schippers et al. 2004; Wu et al. 2010), Navicula pelliculosa (Low-Décarie et al. 2011) and Attheya sp. (King et al. 2011) were also enhanced under elevated CO2 levels under laboratory conditions. However, in the diatom Chaetoceros muelleri, low-light treatments showed lower growth rates under elevated CO2 conditions, but no CO2 or pH effect was recorded under high light exposure (Ihnken et al. 2011). Under similar laboratory conditions, while growth of Thalassiosira pseudonana (CCMP 1335) was not stimulated at the elevated CO2 levels of 760 (Crawfurd et al. 2011) or 1000 μatm (Yang and Gao 2012), T. pseudonana (CCMP 1014 and 1335) grew faster under low (McCarthy et al. 2012) to moderate light (Li and Campbell 2013) with pCO2 of 750 μatm, but under higher light T. pseudonana CCMP 1335 suffered growth inhibition (Li and Campbell 2013). Growth rates of the diatoms S. costatum (CCMA110), P. tricornutum (CCMA 106) and T. pseudonana (CCMP 1335), when grown under different levels of sunlight and elevated CO2 of 1000 μatm, were stimulated under lower light levels (5–30% surface daytime mean solar PAR), but inhibited under higher light levels, with a drop in the PAR threshold for growth saturation (Fig. 1). These results show that elevated CO2 and light levels interact to affect diatom growth responses to ocean acidification, which may explain the different results obtained under different experimental setups, at least for the same species (Table 1). Some of these growth responses may relate to CO2 dependent changes in the cellular susceptibility to photoinactivation of PSII (Li and Campbell 2013) (see below). Since future shoaling of upper-mixed-layer (UML) depths is expected to expose phytoplankton to increased solar irradiance, marine primary producers within UML are expected to suffer from enhanced light stress. However, both low-light CO2 growth enhancement and high-light CO2 growth inhibition could occur even within a single daytime solar cycle or a vertical mixing path, making the net community outcomes difficult to predict. Boelen et al. (2011) did not find any interactive effects of elevated CO2 concentration and changing light levels nor fluctuating light on the growth and photosynthesis in the Antarctic diatom Chaetoceros brevis. Conversely, frequencies of light fluctuation that mimic different mixing regimes affect a coccolithophore’s response to ocean acidification (Jin et al. 2013a), implying an interactive effect of light fluctuation and ocean acidification, that could impose an additional layer of influence on the net species and community responses to increasing pCO2.
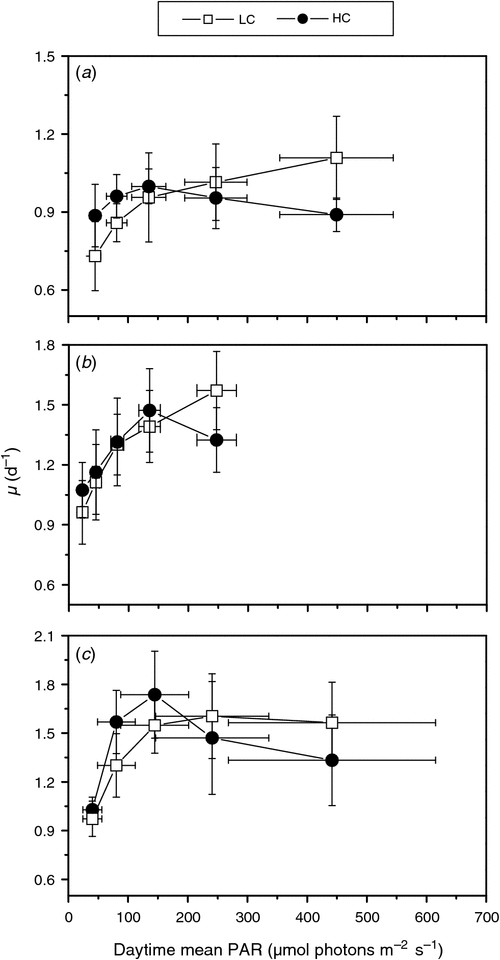
|
Inorganic carbon acquisition mediated by elevated CO2
Diatom Rubisco shows comparatively high CO2 affinity and CO2/O2 selectivity, and is served by CO2 concentrating mechanisms (CCMs) to supply CO2 to Rubisco, and thereby diminish photorespiration (Roberts et al. 2007a). CCMs differ among studied diatom species. Thalassionema nitzschioides (Trimborn et al. 2009), Thalassiosira weissflogii and P. tricornutum (Burkhardt et al. 2001) actively take up both CO2 and HCO3–, whereas Thalassiosira punctigera exclusively uses free CO2 (Elzenga et al. 2000). T. pseudonana, though lacking periplasmic (known also as extracellular) carbonic anhydrase (eCA), can take up HCO3– directly (Nimer et al. 1997; Elzenga et al. 2000; Nakajima et al. 2013), and indeed uses HCO3– as the dominant substrate for photosynthesis even under increased pCO2 (Hopkinson et al. 2013; Isensee et al. 2013). The eCA and intracellular carbonic anhydrase (iCA) facilitate Ci acquisition or utilisation by catalysing the inter-conversion of CO2 and HCO3–.
The activity of eCA can be downregulated under elevated CO2 concentrations relevant to climate change (Burkhardt et al. 2001; Chen and Gao 2003; Rost et al. 2003; Crawfurd et al. 2011). Therefore, active transport or use of HCO3– could be lowered under elevated CO2. Different growth conditions can therefore bring about different efficiencies of algal CCMs or changing preferences for CO2 or HCO3– (Korb et al. 1997; Nimer et al. 1997; Burkhardt et al. 2001). In microalgae and in cyanobacteria, very high CO2 concentrations of up to 50000 µatm (or ppmv in air) turn off CCMs (Kaplan et al. 1980; Tsuzuki and Miyachi 1989; Raven 1991; Matsuda et al. 2001). CCM induction is closely related to the intracellular Ci pool, ambient CO2 levels and oxygen availability (Woodger et al. 2005). CO2 levels (up to 1000 µatm) relevant to future CO2 levels projected for 2100 have been confirmed to partially downregulate CCMs in marine diatoms (Chen and Gao 2003; Trimborn et al. 2009; Wu et al. 2010, 2012a; Yang and Gao 2012) and to lower reliance upon an intracellular labile carbon pool (Isensee et al. 2013). Downregulation of CCMs can include decreased CO2 affinity resulting in an increased requirement for pCO2 to support photosynthesis, inhibition of carbonic anhydrase activity, depressed HCO3– transport, and downregulation of PEPCase and PEPCKase (Reinfelder et al. 2000; Giordano et al. 2005; Roberts et al. 2007a, 2007b; Raven 2010; Reinfelder 2011). Such CCM downregulation was found to be synchronised with diurnal photosynthetic performance in the diatom S. costatum (Chen and Gao 2004b).
CCMs in diatoms might connect to multiple metabolic pathways which differ among diatom species of differing physiology or sizes. In P. tricornutum, cAMP metabolism is involved to control CCM under elevated CO2 levels (Harada et al. 2006). T. weissflogii appears to run a C3–C4-intermediate photosynthesis (Roberts et al. 2007a), which may concentrate Ci through incorporation into an organic C4 carbon compound, before Rubisco-aided carboxylation (Reinfelder et al. 2000, 2004). However, in P. tricornutum, the C4 path way was recently suggested to contribute to pH homeostasis or excitation dissipation, but not to a CCM function (Haimovich-Dayan et al. 2013).
CCMs consume energy (Raven 1991; Bouma et al. 1994; Crawfurd et al. 2011; Hopkinson et al. 2011). Active uptake of HCO3– and CO2 is supported by cyclic and linear electron transport in cyanobacteria (Li and Canvin 1998). Pseudocyclic electron flow through the Mehler reaction can also contribute (Sültemeyer et al. 1993). The energisation mechanisms of diatom CCMs are as yet unclear. It addition to the initial uptake, it takes further energy to maintain high intracellular Ci levels by counteracting CO2 efflux (Sukenik et al. 1997; Tchernov et al. 1997) although in diatoms tested to date efflux rates appear small (Burkhardt et al. 2001; Trimborn et al. 2009). The major energy expenditure by the CCMs in diatoms is active Ci transport, and a doubling of ambient [CO2] could save ~20% of the CCM-related energy expenditure in several diatom species (Hopkinson et al. 2011). Under elevated CO2 concentrations, the growth enhancement under limiting light levels could be partially due to downregulation of CCMs, thereby lowering energy costs (Raven and Johnston 1991; Gao et al. 2012b). Alternatively, since CCMs can also be downregulated under low light, elevated pCO2 could have stimulated the low light growth of diatoms due to both increased availability of CO2 and savings on the energy cost of CCM operation.
Photosynthetic responses
Light energy captured and delivered via photochemical processes powers the active transport of CO2 and HCO3 in cyanobacteria and microalgae (Sültemeyer et al. 1993; Sukenik et al. 1997; Li and Canvin 1998), and then assimilatory carboxylation. Elevated CO2 concentration had no significant effect upon pigment contents nor upon the effective absorbance cross-section serving photosystem II photochemistry (σPSII) in S. costatum, P. tricornutum or T. pseudonana (Chen and Gao 2004a; Wu et al. 2010; Crawfurd et al. 2011; McCarthy et al. 2012; Li and Campbell 2013). Furthermore, elevated pCO2 had only limited effects on levels of the major protein complexes mediating photosynthesis across multiple species of centric diatoms, grown under low to saturating light (McCarthy et al. 2012; Li and Campbell 2013).
The photochemical quantum yield of S. costatum, P. tricornutum and T. pseudonana decreases faster with increasing levels of PAR under elevated, than under ambient CO2 levels (Gao et al. 2012b). Non-photochemical quenching (NPQ), however, increases faster in the high-CO2 grown cells with increasing light levels compared with the ambient CO2 grown cells (Gao et al. 2012b). Modulating NPQ helps diatoms, like other photoautoauphs, to withstand high or fluctuating levels of PAR (Niyogi et al. 2005; Lavaud et al. 2007; Zhu and Green 2010; Wu et al. 2012b). T. pseudonana employs NPQ to cope with light stress, even under elevated CO2 levels, more effectively than does a strain of P. tricornutum (Yang and Gao 2012), so that photoinhibition of electron transport was observed in P. tricornutum, but not T. pseudonana, when grown under elevated CO2 of 1000 µatm (Wu et al. 2010). These differential responses between two model diatoms show taxon-specific mechanisms in coping with the combined impacts of ocean acidification and light stress.
At a functional level, the diatoms P. tricornutum and T. pseudonana grown under elevated CO2 of 1000 µatm, at subsaturating photosynthetically active radiation, showed an increase in photosynthetic carbon fixation rate per cell of more than 20% (Wu et al. 2010; Yang and Gao 2012). Their growth rate was, however, only enhanced by ~5% in P. tricornutum and was unaffected in T. pseudonana. Enhanced respiratory and photorespiratory carbon losses under elevated CO2 are likely responsible for this discrepancy (Wu et al. 2010; Gao et al. 2012b; Yang and Gao 2012). In the toxic diatom Pseudo-nitzschia multiseries, maximum carbon fixation rates per cell also increased with elevated CO2 levels, although the apparent light use efficiency was not affected (Sun et al. 2011). In Cylindrotheca closterium f. minutissima, when grown at 1000 µatm CO2 under sunlight, rates of electron transport and O2 evolution dropped compared with the cells grown at the ambient CO2 concentration (Wu et al. 2012a).
PSII photoinactivation and UV responses
Diatoms, like all photoautotrophs, suffer light- and UV-dependent photoinactivation of their PSII centres (Kok 1956). To maintain their photosynthesis in the face of light-dependent photoinactivation, diatoms must use a metabolically expensive PSII repair cycle (Aro et al. 1993) to proteolytically remove photoinactivated protein subunits (Nixon et al. 2010; Nagao et al. 2012; Campbell et al. 2013) and replace them with newly synthesised subunits (Edelman and Mattoo 2008). In comparison with other phytoplankton groups, diatoms enjoy a relatively low susceptibility to photoinactivation of their PSII (Key et al. 2010; Wu et al. 2011, 2012b). In T. pseudonana CCMP 1335, however, the primary susceptibility to photoinactivation of PSII changes under elevated pCO2 (Sobrino et al. 2008; McCarthy et al. 2012; Li and Campbell 2013). As a net result, cells under high pCO2 and high light incur an increased metabolic expense to accelerate PSII protein cycling, to counter increased photoinactivation (G Li, DA Campbell, unpubl. data). This increased metabolic cost to maintain PSII function is a possible explanation for the pattern of growth stimulation under elevated pCO2 under low to moderate light, but growth inhibition under excess light (Gao et al. 2012b; McCarthy et al. 2012; Li and Campbell 2013). We are as yet unsure as to the mechanism(s) for the changes in susceptibility to primary photoinactivation under elevated pCO2. Decreased silification under elevated pCO2 (Mejía et al. 2013) might alter cellular optics. Or, a drop in excitation dissipation capacity, as reported in cyanobacteria (Tchernov et al. 1997) and now suggested in diatoms (Haimovich-Dayan et al. 2013) could result if the CCM is partly downregulated. Evidence in this direction is that the content of the reactive-oxygen toxicity indicator malondialdehyde increases in T. pseudonana CCMP 1335 growing under elevated pCO2 (Li and Campbell 2013), consistent with a downregulation of paths with photoprotective roles under elevated pCO2.
Solar UV radiation (UVR, 280–400 nm) affects phytoplankton physiology and primary productivity (Häder 2011; and literatures cited therein). In T. pseudonana, acclimation to UVR, partially relieved the increased susceptibility to photoinhibition under elevated pCO2 (Sobrino et al. 2008), consistent with a hormetic protective induction of reactive oxygen species (ROS) detoxification by UV acclimation. The effect of UV-B irradiance (280–320 nm) on P. tricornutum was counteracted under ocean acidification conditions (Li et al. 2012a). Cylindrotheca closterium f. minutissima did not show any significant growth response to solar UVR after acclimation to solar radiation, though a combination of UVR and elevated CO2 concentration led to significant drop in maximal electron transport (Wu et al. 2012a).
Respiratory responses
Altered seawater carbonate chemistry due to ocean acidification could perturb energy requirements for the diatom cells, leading to changes in respiration. Mitochondrial respiration indeed increases under ocean acidification conditions of 1000 µatm pCO2 (pH 7.8) by ~34% in P. tricornutum (Wu et al. 2010) and by 35% in T. pseudonana (Yang and Gao 2012). Increased acidity of seawater associated with increased pCO2 could disturb cell surface (Flynn et al. 2012) or even intracellular pH stability, so that phytoplankton cells may need to allocate additional energy to transport ions against the acid–base perturbation. Cell surface effects of increasing pCO2 and decreasing pH will vary with cell size and with the co-varying cellular metabolic rate (Flynn et al. 2012). Thus, increasing pCO2 is likely to increase the influences of cell size on phytoplankton responses to environmental forcings (Finkel et al. 2010; Flynn et al. 2012).
Photorespiration and electron flows to oxygen can be important in photoprotection and short-term responses to excess light in diatoms (Wingler et al. 2000; Waring et al. 2010). Both P. tricornutum and T. pseudonana showed enhanced photorespiration by up to 23–27% under elevated CO2 or ocean acidification conditions (Gao et al. 2012b). Cells of T. pseudonana grown under the elevated CO2 level of 1000 µatm showed higher carbon fixation rates but a lower net O2 evolution rate compared with cells grown under the ambient CO2, although the cells exhibited equivalent electron transfer rates from PSII (Yang and Gao 2012), providing evidence for enhanced photorespiratory or pseudocyclic re-consumption of O2 released from PSII, under ocean acidification. Enhanced excretion of organic compounds due to photorespiration can connect to production of transparent exopolymers in phytoplankton communities that include diatoms (Engel 2002). These metabolic pathways could result in discrepant effects of ocean acidification on different species or under different light levels.
Effects on diatom communities across diverse habitats
Although photosynthesis of diatoms is likely to be stimulated by increased availability of CO2, lower pH might increase their respiration (Wu et al. 2010; Yang and Gao 2012) and their costs for photoprotection, therefore, the net effect of ocean acidification on diatoms will depend on multiple environmental forcings and possibly species-specific metabolic pathways.
Community level responses to rising pCO2 and temperature vary across oceanic regions. In the north-east Atlantic and North Sea a 50 year (1960–2009) time series survey revealed a decline of dinoflagellate abundance, whereas diatoms showed relatively constant richness (Hinder et al. 2012). The transition over the half century was attributed to ocean warming and windy conditions. In contrast, elevated temperatures lowered the short-term abundance of diatoms in a North Atlantic Bloom incubation study (Feng et al. 2009), although CO2 changes had no apparent effect. A shipboard incubation study that examined rising temperature and CO2 in two natural Bering Sea assemblages also found large community shifts away from diatoms towards nanoflagellates in the ‘greenhouse’ treatment (Hare et al. 2007), though diatom-dominated phytoplankton growth increased in the Ross Sea under elevated CO2 levels (Tortell et al. 2008).
Across a CO2 and pH gradient off the volcanic island of Vulcano (Mediterranean, NE Sicily), periphyton communities altered significantly as CO2 concentrations increased, with significant increases in chlorophyll a concentrations and in diatom abundance (Johnson et al. 2013).
Feng et al. (2010) found no interactive effects of light and CO2 on community photosynthesis during an experiment using a Ross Sea diatom/Phaeocystis assemblage, but the diatom community structure shifted away from small pennate diatoms towards much larger centric diatoms. In the very different conditions of the South China Sea a shipboard incubation combining elevated CO2 concentration and near surface solar irradiances, showed decreased photosynthesis while diatom abundance declined (Gao et al. (2012b).
In hypoxic seawaters, algae may experience large changes in the ratio of pO2 to pCO2 or respiration index (RI = log10 (pO2/pCO2)), which is predicted to decline in future oceans (Brewer and Peltzer 2009). Together with lower pH, hypoxic areas represent a future situation of combined ocean acidification and deoxygenation. There has been little documented on the interactive effects of these two climate-change factors on diatoms nor upon other marine primary producers. However, changes in RI could affect net photosynthesis (Fig. 2) (Gao et al. 2012b; Xu and Gao 2012). For diatoms grown under either elevated or ambient levels of CO2, net photosynthetic O2 evolution decreases with increased RI (Fig. 2a, b).
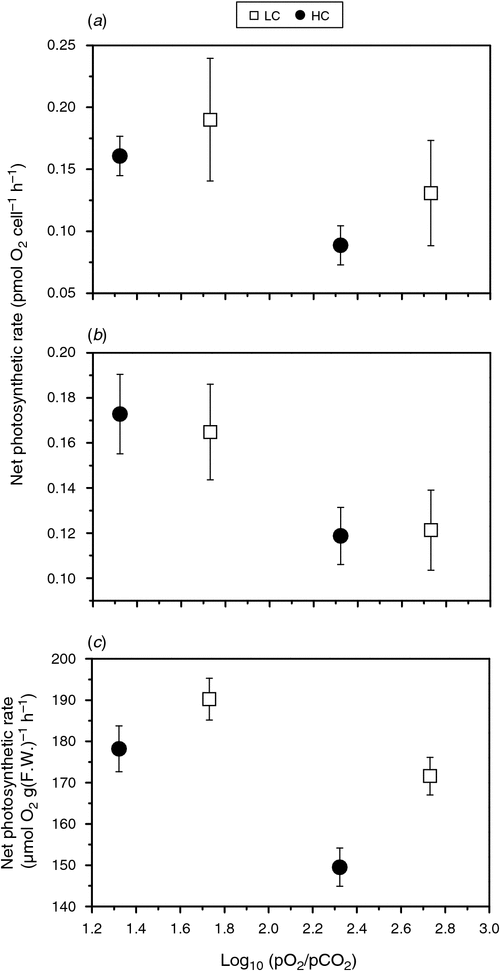
|
Future efforts
Based on the work summarised here, priorities for future study on diatoms include the following.
-
Response of frustule mineralisation to elevated CO2 concentrations. Biogenic silicate content of some diatoms decreases under ocean acidification conditions (Hervé et al. 2012; Tatters et al. 2012; Mejía et al. 2013). However, we do not know the mechanisms involved, nor the interactive effects of multiple factors, such as warming, UV radiation, nutrient limitation, deoxygenation and cell surface pH (Flynn et al. 2012; Milligan and Morel 2002) upon silicate mineralisation.
-
Higher CO2 concentrations appear to favour growth enhancement of larger rather than smaller diatoms (Feng et al. 2010) (Y Wu, AJ Irwin, D Suggett, D Campbell, ZV Finkel, unpubl. data). Mechanistic studies are needed to examine responses of differently sized diatoms to ocean acidification, to discriminate among direct size effects (Barton et al. 2013) and effects of taxonomic distinctions in cell structures (Mitchell et al. 2013) or metabolisms.
-
Coastal and pelagic water diatoms may react differently to ocean acidification due to their pre-adaptations to different regimes of mixing, nutrient and diel pH changes. In coastal waters, photosynthetic carbon fixation, and night-time respiration per volume of seawater is much higher, leading to high pH during the day and low pH during the night. Little is known about diatom responses to diel pH changes under elevated CO2 concentrations as well as to diel changes in the respiration index of the water.
-
To guide the scope of studies responses of diatoms to ocean acidification should be examined under expected combinations of environmental changes. Although UV radiation appears not to influence the growth of some diatoms under elevated CO2 level (Wu et al. 2012a), UV-B (280–315 nm) seems to counteract some effects of high CO2 and low pH (Li et al. 2012a). Further studies are needed to explore physiological responses of diatoms to reasonable exposures to UV, temperature rise (Shatwell et al. 2012), fluctuation of irradiance to model changing mixing conditions, nutrient limitation and deoxygenation under elevated CO2 and acidification conditions. Hundreds of genes in P. tricornutum are upregulated after acclimation to ocean acidification conditions (Y Li, F Su, Y Wu, KJ Wang, K Gao, unpubl. data), but little has been documented on long-term acclimation of open ocean diatoms, and studies on interactions between ocean acidification and the progression from exponential to stationary phase are just beginning (Orellana et al. 2013).
-
Evolutionary responses to increasing CO2 concentration in diatoms should be examined for hundreds to thousands of generations. The coastal strain Thalassiosira pseudonana CCMP 1335 showed little evidence of evolutionary adaptation over months of growth at elevated CO2 (Crawfurd et al. 2011). Evolutionary responses of the freshwater green alga Chlamydomonas sp. at high CO2 demonstrated that some adapted cell lines lost CCM capabilities (Collins and Bell 2004; Collins et al. 2006). Lohbeck et al. (2012) found that 500 generations of selection at high CO2 led to recovery of a coccolithophore’s growth rates and calcification, although 680 generations of selection at high CO2 did not show such a trend in Gephyrocapsa oceanica (Jin et al. 2013b).
-
Monitoring community abundance of diatoms together with other key taxa over longer time scales is important to gain in situ information on their responses to environmental changes (Mutshinda et al. 2013). These field data obtained from different waters, when combined with mechanistic from controlled experiments would provide valuable insight into future climate change impacts upon phytoplankton communities.
References
Allen AE, Dupont CL, Oborník M, Horák A, Nunes-Nesi A, McCrow JP, Zheng H, Johnson DA, Hu H, Fernie AR, Bowler C (2011) Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 473, 203–207.| Evolution and metabolic significance of the urea cycle in photosynthetic diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlvFGmur0%3D&md5=3ece83ae079e300a9cb9d6db4c11106cCAS | 21562560PubMed |
Aro E, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1143, 113–134.
| Photoinhibition of photosystem II. Inactivation, protein damage and turnover.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXms1Kgtb0%3D&md5=da736f4d10c5888b10eda7b2f6780120CAS |
Barton AD, Finkel ZV, Ward BA, Johns DG, Follows MJ (2013) On the roles of cell size and trophic strategy in North Atlantic diatom and dinoflagellate communities. Limnology and Oceanography 58, 254–266.
| On the roles of cell size and trophic strategy in North Atlantic diatom and dinoflagellate communities.Crossref | GoogleScholarGoogle Scholar |
Bartual A, Galvez JA (2002) Growth and biochemical composition of the diatom Phaeodactylum tricornutum at different pH and inorganic carbon levels under saturating and subsaturating light regimes. Botanica Marina 45, 491–501.
| Growth and biochemical composition of the diatom Phaeodactylum tricornutum at different pH and inorganic carbon levels under saturating and subsaturating light regimes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXls1Gjtrg%3D&md5=4b596268f5327474f759c6f14b162619CAS |
Beardall J, Sobrino C, Stojkovic S (2009) Interactions between the impacts of ultraviolet radiation, elevated CO2, and nutrient limitation on marine primary producers. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology 8, 1257–1265.
| Interactions between the impacts of ultraviolet radiation, elevated CO2, and nutrient limitation on marine primary producers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGgs7fL&md5=5c4a3bfa92e60497a02a4e74997c9608CAS |
Boelen P, Van de Poll WH, Van der Strate HJ, Neven IA, Beardall J, Buma AGJ (2011) Neither elevated nor reduced CO2 affects the photophysiological performance of the marine Antarctic diatom Chaetoceros brevis. Journal of Experimental Marine Biology and Ecology 406, 38–45.
| Neither elevated nor reduced CO2 affects the photophysiological performance of the marine Antarctic diatom Chaetoceros brevis.Crossref | GoogleScholarGoogle Scholar |
Bouma TJ, Visser R, Janssen JHJA, Kock MJ, Leeuwen PH, Lambers H (1994) Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect. Physiologia Plantarum 92, 585–594.
| Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXivVSls7o%3D&md5=dfe3eaa7f25c02db2f635b0b8e8a3571CAS |
Brewer PG, Peltzer ET (2009) Limits to marine life. Science 324, 347–348.
| Limits to marine life.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFant7g%3D&md5=760743a098b9f978dca212efbfbf7f4cCAS | 19372421PubMed |
Burkhardt S, Riebesell U (1997) CO2 availability affects elemental composition (C : N : P) of the marine diatom Skeletonema costatum. Marine Ecology Progress Series 155, 67–76.
| CO2 availability affects elemental composition (C : N : P) of the marine diatom Skeletonema costatum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmt12hsb4%3D&md5=ec9f468a6dc115bc33c102c73c4d71feCAS |
Burkhardt S, Zondervan I, Riebesell U (1999) Effect of CO2 concentration on the C : N : P ratio in marine phytoplankton: a species comparison. Limnology and Oceanography 44, 683–690.
| Effect of CO2 concentration on the C : N : P ratio in marine phytoplankton: a species comparison.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1yjs7g%3D&md5=2a1b8347a0666ff89f1717f0cc18f6c9CAS |
Burkhardt S, Amoroso G, Riebesell U (2001) CO2 and HCO3 – uptake in marine diatoms acclimated to different CO2 concentrations. Limnology and Oceanography 46, 1378–1391.
| CO2 and HCO3 – uptake in marine diatoms acclimated to different CO2 concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnsVSktbs%3D&md5=c39d51d42420ebb0d429b98a148f3b79CAS |
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365
| Oceanography: anthropogenic carbon and ocean pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnsV2ktrs%3D&md5=8b6921755b2c2d70746ab46735261235CAS | 14508477PubMed |
Campbell DA, Hossain Z, Cockshutt AM, Zhaxybayeva O, Wu H, Li G (2013) Photosystem II protein clearance and FtsH function in the diatom Thalassiosira pseudonana. Photosynthesis Research 115, 43–54.
| Photosystem II protein clearance and FtsH function in the diatom Thalassiosira pseudonana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsFSnsb4%3D&md5=8749506f6b9b209ec908aab4b0547516CAS | 23504483PubMed |
Chen X, Gao K (2003) Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracelluar carbonic anhydrase in the marine diatom Skeletonema costatum. Chinese Science Bulletin 48, 2616–2620.
| Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracelluar carbonic anhydrase in the marine diatom Skeletonema costatum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmslyktA%3D%3D&md5=baedc8b9c044bc1706015d09c08984e3CAS |
Chen X, Gao K (2004a) Photosynthetic utilisation of inorganic carbon and its regulation in the marine diatom Skeletonema costatum. Functional Plant Biology 31, 1027–1033.
| Photosynthetic utilisation of inorganic carbon and its regulation in the marine diatom Skeletonema costatum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXosFSqu7Y%3D&md5=06e8361fd1b4b5ec71ac24d281f562e2CAS |
Chen X, Gao K (2004b) Characterization of diurnal photosynthetic rhythms in the marine diatom Skeletonema costatum grown in synchronous culture under ambient and elevated CO2. Functional Plant Biology 31, 399–404.
| Characterization of diurnal photosynthetic rhythms in the marine diatom Skeletonema costatum grown in synchronous culture under ambient and elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs12mu7w%3D&md5=531bdea2df00ed198bcaebb6cb0e5769CAS |
Chen S, Gao K (2011) Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae). Hydrobiologia 675, 105–117.
| Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWgsrfL&md5=e41c18bd468e03dfff57981d836c968cCAS |
Collins S, Bell G (2004) Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga. Nature 431, 566–569.
| Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvFCnurk%3D&md5=6df5db54de405e0192ee9539b379f679CAS | 15457260PubMed |
Collins S, Sültemeyer D, Bell G (2006) Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2. Plant, Cell & Environment 29, 1812–1819.
| Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWktLnF&md5=fce451fb7f440c0e2241c2c9e661a8c1CAS |
Crawfurd KJ, Raven JA, Wheeler GL, Baxter EJ, Joint I (2011) The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS ONE 6, e26695
| The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVyqu7zK&md5=6079760c0b68bd5f96408f76845099c8CAS | 22053201PubMed |
Doney SC (2006) Oceanography: plankton in a warmer world. Nature 444, 695–696.
| Oceanography: plankton in a warmer world.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Ontb%2FN&md5=e1333420e8940632531b01ecfd8dbcfeCAS | 17151650PubMed |
Edelman M, Mattoo AK (2008) D1-protein dynamics in photosystem II: the lingering enigma. Photosynthesis Research 98, 609–620.
| D1-protein dynamics in photosystem II: the lingering enigma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOgt7fN&md5=059466e02edc90e30b02f681b670545fCAS | 18709440PubMed |
Elzenga JTM, Prins HBA, Stefels J (2000) The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Pyrmnesiophyceae): a comparison with other marine algae using isotopic disequilibrium technique. Limnology and Oceanography 45, 372–380.
| The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Pyrmnesiophyceae): a comparison with other marine algae using isotopic disequilibrium technique.Crossref | GoogleScholarGoogle Scholar |
Engel A (2002) Direct relationship between CO2 uptake and transparent exopolymer particles production in natural phytoplankton. Journal of Plankton Research 24, 49–53.
| Direct relationship between CO2 uptake and transparent exopolymer particles production in natural phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitFOntbw%3D&md5=25602f5bf56116580591ffe521e1effbCAS |
Falkowski PG, Oliver MJ (2007) Mix and match: how climate selects phytoplankton. Nature Reviews. Microbiology 5, 813–819.
| Mix and match: how climate selects phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVeis7nE&md5=0dca66c2e7209db7c8d97714f36912f9CAS | 17853908PubMed |
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366.
| Impact of anthropogenic CO2 on the CaCO3 system in the oceans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXls1egsbY%3D&md5=ecb5338a1b9c6edc091884ed853e5488CAS | 15256664PubMed |
Feng Y, Hare CE, Leblanc K, Rose JM, Zhang Y, DiTullio GR, Lee P, Wilhelm S, Rowe JM, Sun J, Nemcek N, Gueguen C, Passow U, Benner I, Brown C, Hutchins DA (2009) Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Marine Ecology Progress Series 388, 13–25.
| Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1SrtrjO&md5=677b14e256c574f1ab8b840a474c9457CAS |
Feng Y, Hare CE, Rose JM, Handy SM, DiTullio GR, Lee PA, Smith WO, Peloquin J, Tozzi S, Sun J, Zhang Y, Dunbar RB, Long MC, Sohst B, Lohan M, Hutchins DA (2010) Interactive effects of iron, irradiance and CO2 on Ross Sea phytoplankton. Deep-sea Research. Part I, Oceanographic Research Papers 57, 368–383.
| Interactive effects of iron, irradiance and CO2 on Ross Sea phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFSntrw%3D&md5=fc8a35400c09b11a2c5e2e44cb7f6638CAS |
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240.
| Primary production of the biosphere: integrating terrestrial and oceanic components.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXksFKitb0%3D&md5=f7ef5b418ebeed232244a9fb0bc32d6cCAS | 9657713PubMed |
Finkel ZV, Sebbo J, Feist-Burkhardt S, Irwin AJ, Katz ME, Schofield OME, Young JR, Falkowski PG (2007) A universal driver of macroevolutionary change in the size of marine phytoplankton over the Cenozoic. Proceedings of the National Academy of Sciences of the United States of America 104, 20416–20420.
| A universal driver of macroevolutionary change in the size of marine phytoplankton over the Cenozoic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjslemsw%3D%3D&md5=b90f189dec2161b9e71bb1cfbeb6c6e0CAS | 18077334PubMed |
Finkel ZV, Beardall J, Flynn KJ, Quigg A, Rees TAV, Raven JA (2010) Phytoplankton in a changing world: cell size and elemental stoichiometry. Journal of Plankton Research 32, 119–137.
| Phytoplankton in a changing world: cell size and elemental stoichiometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFarsbrK&md5=7e9bd6a5aa4e5658f03c004d3d8c21a2CAS |
Flynn KJ, Blackford JC, Baird ME, Raven JA, Clark DR, Beardall J, Brownlee C, Fabian H, Wheeler GL (2012) Changes in pH at the exterior surface of plankton with ocean acidification. Nature Climate Change 2, 510–513.
| Changes in pH at the exterior surface of plankton with ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVGiu7w%3D&md5=0a2c5d02dc9ce1d15935b97c03caf0a6CAS |
Gao K, Ruan Z, Villafañe VE, Gattuso J-P, Helbling EW (2009) Ocean acidification exacerbates the effect of UV radiation on the calcifying phytoplankter Emiliania huxleyi. Limnology and Oceanography 54, 1855–1862.
| Ocean acidification exacerbates the effect of UV radiation on the calcifying phytoplankter Emiliania huxleyi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFKku7rO&md5=9da9f773843100216b1358d3d9d80c65CAS |
Gao K, Helbling EW, Häder DP, Hutchins DA (2012a) Ocean acidification and marine primary producers under the sun: a review of interactions between CO2, warming, and solar radiation. Marine Ecology Progress Series 470, 167–189.
| Ocean acidification and marine primary producers under the sun: a review of interactions between CO2, warming, and solar radiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXisVSis70%3D&md5=f72972f3a173c713c3a46f4c4adc79daCAS |
Gao K, Xu J, Gao G, Li Y, Hutchins DA, Huang B, Wang L, Zheng Y, Jin P, Cai X, Häder D, Li W, Xu K, Liu N, Riebesell U (2012b) Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nature Climate Change 2, 519–523.
Gattuso J-P, Hansson L (2011) ‘Ocean acidification.’ (Oxford University Press: Oxford)
Gattuso J-P, Gao K, Lee K, Rost B, Schulz KG (2010) Approaches and tools to manipulate the carbonate chemistry. In ‘Guide to best practices for ocean acidification research and data reporting’. (Eds U Riebesell, VJ Fabry, L Hansson, JP Gattuso) pp. 41–52. (Publications Office of the European Union: Luxembourg)
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131.
| CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtVaru7w%3D&md5=8c4b5aae2facd09285c7681a28b1376eCAS | 15862091PubMed |
Guan W, Gao K (2008) Light histories influence the impacts of solar ultraviolet radiation on photosynthesis and growth in a marine diatom, Skeletonema costatum. Journal of Photochemistry and Photobiology. B, Biology 91, 151–156.
| Light histories influence the impacts of solar ultraviolet radiation on photosynthesis and growth in a marine diatom, Skeletonema costatum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVSgsbc%3D&md5=5c25dc92d8c529bad374859c7c9fcf1dCAS | 18462948PubMed |
Häder D-P (2011) Does enhanced solar UV-B radiation affect marine primary producers in their natural habitats? Photochemistry and Photobiology 87, 263–266.
| Does enhanced solar UV-B radiation affect marine primary producers in their natural habitats?Crossref | GoogleScholarGoogle Scholar | 21208211PubMed |
Haimovich-Dayan M, Garfinkel N, Ewe D, Marcus Y, Gruber A, Wagner H, Kroth PG, Kaplan A (2013) The role of C4 metabolism in the marine diatom Phaeodactylum tricornutum. New Phytologist 197, 177–185.
| The role of C4 metabolism in the marine diatom Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslelsL%2FK&md5=0670558ce317cee968db1d162afe503aCAS | 23078356PubMed |
Harada H, Nakajima K, Sakaue K, Matsuda Y (2006) CO2 sensing at ocean surface mediated by cAMP in a marine diatom. Plant Physiology 142, 1318–1328.
| CO2 sensing at ocean surface mediated by cAMP in a marine diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1eju7%2FJ&md5=f55cee28e1603d13e43828cf86d54788CAS | 17012409PubMed |
Hare CE, Leblanc K, DiTullio GR, Kudela RM, Zhang Y, Lee PA, Riseman S, Hutchins DA (2007) Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea. Marine Ecology Progress Series 352, 9–16.
| Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlKhsrw%3D&md5=bef21e7a1003d54972a235372424c305CAS |
Hein M, Sand-Jensen K (1997) CO2 increases oceanic primary production. Nature 388, 526–527.
| CO2 increases oceanic primary production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlt1arsbk%3D&md5=2a3a3e555ad6b2499c0c4f0216232ec2CAS |
Hervé V, Derr J, Douady S, Quinet M, Moisan L, Lopez PJ (2012) Multiparametric analyses reveal the pH-dependence of silicon biomineralization in diatoms. PLoS ONE 7, e46722
| Multiparametric analyses reveal the pH-dependence of silicon biomineralization in diatoms.Crossref | GoogleScholarGoogle Scholar | 23144697PubMed |
Hinder SL, Hays GC, Edwards M, Roberts EC, Walne AW, Gravenor MB (2012) Changes in marine dinoflagellate and diatom abundance under climate change. Nature Climate Change 2, 271–275.
| Changes in marine dinoflagellate and diatom abundance under climate change.Crossref | GoogleScholarGoogle Scholar |
Hopkinson BM, Dupont CL, Allen AE, Morel FMM (2011) Efficiency of the CO2-concentrating mechanism of diatoms. Proceedings of the National Academy of Sciences of the United States of America 108, 3830–3837.
| Efficiency of the CO2-concentrating mechanism of diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1Wqsb8%3D&md5=168a7eee2603ae4518190e12b17e2312CAS | 21321195PubMed |
Hopkinson BM, Meile C, Shen C (2013) Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role. Plant Physiology 162, 1142–1152.
| Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Oqsbw%3D&md5=6e0b87a440d2530cd19e9402b2a5c09bCAS | 23656892PubMed |
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (2001) ‘IPCC climate change: the scientific basis.’ (Cambridge University Press: Cambridge, UK)
Ihnken S, Roberts S, Beardall J (2011) Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability. Phycologia 50, 182–193.
| Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFKit7k%3D&md5=bd6d777749a015291cdd5f4568e1911bCAS |
Isensee K, Erez J, Stoll H (2013) Detection of a variable internal C i pool in Thalassiosira weissflogii (Heterokontophyta) and Emiliania huxleyi (Haptophyta) in response to changes in the seawater carbon system. Physiologia Plantarum
| Detection of a variable internal C i pool in Thalassiosira weissflogii (Heterokontophyta) and Emiliania huxleyi (Haptophyta) in response to changes in the seawater carbon system.Crossref | GoogleScholarGoogle Scholar | 23992373PubMed | n press
Jin P, Gao K, Villafañe VE, Campbell DA, Helbling W (2013a) Ocean acidification alters the photosynthetic responses of a coccolithophorid to fluctuating UV and visible radiation. Plant Physiology 162, 2084–2094.
| Ocean acidification alters the photosynthetic responses of a coccolithophorid to fluctuating UV and visible radiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlWlt7vO&md5=b61e81995a203ce9bbdd54ba7d2475e2CAS | 23749851PubMed |
Jin P, Gao K, Beardall J (2013b) Evolutionary responses of a coccolithophorid Gephyrocapsa oceanica to ocean acidification. Evolution 67, 1869–1878.
| Evolutionary responses of a coccolithophorid Gephyrocapsa oceanica to ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1WiurrM&md5=e9d3fae9c70dc0f300e2c6687e7a0c43CAS | 23815645PubMed |
Johnson VR, Brownlee C, Rickaby REM, Graziano M, Milazzo M, Hall-Spencer JM (2013) Responses of marine benthic microalgae to elevated CO2. Marine Biology 160, 1813–1824.
| Responses of marine benthic microalgae to elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1Ggs7jL&md5=d4d3d72f5ee3ba38d20484515641200cCAS |
Kaplan A, Badger MR, Berry JA (1980) Photosynthesis and the intracellular inorganic carbon pool in the bluegreen alga Anabaena variabilis: response to external CO2 concentration. Planta 149, 219–226.
| Photosynthesis and the intracellular inorganic carbon pool in the bluegreen alga Anabaena variabilis: response to external CO2 concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXlsVKmt74%3D&md5=593e157573d5401d351d9f606fd57f56CAS | 24306290PubMed |
Key T, Mccarthy A, Campbell D, Six C, Roy S, Finkel Z (2010) Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environmental Microbiology 12, 95–104.
| Cell size trade-offs govern light exploitation strategies in marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFaisLg%3D&md5=39a7599d74eb09dc59ba2606675e5487CAS | 19735282PubMed |
Kim J-M, Lee K, Shin K, Kang J-H, Lee H-W, Kim M, Jang P-G, Jang M-C (2006) The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment. Limnology and Oceanography 51, 1629–1636.
| The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1antLo%3D&md5=d5eb98bf26ac035d09a1bcaedeb2f76cCAS |
King AL, Sañudo-Wilhelmy SA, Leblanc K, Hutchins DA, Fu F (2011) CO2 and vitamin B12 interactions determine bioactive trace metal requirements of a subarctic Pacific diatom. ISME Journal 5, 1388–1396.
| CO2 and vitamin B12 interactions determine bioactive trace metal requirements of a subarctic Pacific diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptVynurg%3D&md5=69facaf3064bbb5b9796db64d9e9592dCAS | 21248860PubMed |
Kok B (1956) On the inhibition of photosynthesis by intense light. Biochimica et Biophysica Acta 21, 234–244.
| On the inhibition of photosynthesis by intense light.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG28XptVeitw%3D%3D&md5=2d59c552c6d5a41cee05cd89d6dc28ffCAS | 13363902PubMed |
Korb RE, Saville PJ, Johnston AM, Raven JA (1997) Sources of inorganic carbon for photosynthesis by three species of marine diatom. Journal of Phycology 33, 433–440.
| Sources of inorganic carbon for photosynthesis by three species of marine diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkslOisro%3D&md5=4ddf9216800c413baf898fadabfc5f70CAS |
Lavaud J, Rousseau B, Etienne A-L (2004) General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae). Journal of Phycology 40, 130–137.
| General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae).Crossref | GoogleScholarGoogle Scholar |
Lavaud J, Strzepek RF, Kroth PG (2007) Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnology and Oceanography 52, 1188–1194.
| Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXms1ymtbs%3D&md5=098016f4dcd8e5b6628242847251a295CAS |
Li G, Campbell DA (2013) Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS ONE 8, e55562
| Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1yltLY%3D&md5=32cd7f2fb9c1e0aaa08649ce96d91472CAS | 23383226PubMed |
Li Q, Canvin DT (1998) Energy sources for HCO3 − and CO2 transport in air-grown cells of Synechococcus UTEX 625. Plant Physiology 116, 1125–1132.
| Energy sources for HCO3 − and CO2 transport in air-grown cells of Synechococcus UTEX 625.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitVOjsLk%3D&md5=92998e755624e1ea38baa0fc35c52825CAS | 9501145PubMed |
Li YH, Gao K, Villafañe VE, Helbling EW (2012a) Ocean acidification mediates photosynthetic response to UV radiation and temperature increase in the diatom Phaeodactylum tricornutum. Biogeosciences 9, 3931–3942.
| Ocean acidification mediates photosynthetic response to UV radiation and temperature increase in the diatom Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXit1Gku7w%3D&md5=d0529721796d2127b3b50e397675405eCAS |
Li W, Gao K, Beardall J (2012b) Interactive effects of ocean acidification and nitrogen-limitation on the diatom Phaeodactylum tricornutum. PLoS ONE 7, e51590
| Interactive effects of ocean acidification and nitrogen-limitation on the diatom Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvV2qtr%2FJ&md5=7661f4f8abe6811cd3bfeb0c259d3660CAS | 23236517PubMed |
Lohbeck KT, Riebesell U, Reusch TBH (2012) Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience 5, 346–351.
| Adaptive evolution of a key phytoplankton species to ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltFOgsbg%3D&md5=307d2692703707d96636b8423cbae1f9CAS |
Low-Décarie E, Fussmann GF, Bell G (2011) The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Global Change Biology 17, 2525–2535.
| The effect of elevated CO2 on growth and competition in experimental phytoplankton communities.Crossref | GoogleScholarGoogle Scholar |
Matsuda Y, Hara T, Colman B (2001) Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum. Plant, Cell & Environment 24, 611–620.
| Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltVOgtb0%3D&md5=edb611b415ab3b43bbd85d0b9bbbe34fCAS |
McCarthy A, Rogers SP, Duffy SJ, Campbell DA (2012) Elevated carbon dioxide differentially alters the photophysiology of Thalassiosira pseudonana (Bacillariophyceae) and Emiliania huxleyi (Haptophyta). Journal of Phycology 48, 635–646.
| Elevated carbon dioxide differentially alters the photophysiology of Thalassiosira pseudonana (Bacillariophyceae) and Emiliania huxleyi (Haptophyta).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSrtbjF&md5=ec15866bc98886a980e73588311a155fCAS |
Mejía LM, Isensee K, Méndez-Vicente A, Pisonero J, Shimizu N, González C, Monteleone B, Stoll H (2013) B content and Si/C ratios from cultured diatoms (Thalassiosira pseudonana and Thalassiosira weissflogii): relationship to seawater pH and diatom carbon acquisition. Geochimica et Cosmochimica Acta 123, 322–337.
| B content and Si/C ratios from cultured diatoms (Thalassiosira pseudonana and Thalassiosira weissflogii): relationship to seawater pH and diatom carbon acquisition.Crossref | GoogleScholarGoogle Scholar |
Millero FJ, Woosley R, Ditrolio B, Waters J (2009) Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22, 72–85.
| Effect of ocean acidification on the speciation of metals in seawater.Crossref | GoogleScholarGoogle Scholar |
Milligan AJ, Morel FMM (2002) A proton buffering role for silica in diatoms. Science 297, 1848–1850.
| A proton buffering role for silica in diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvFGgsrs%3D&md5=3e8bab26444affb93329d939990883c6CAS | 12228711PubMed |
Mitchell JG, Seuront L, Doubell MJ, Losic D, Voelcker NH, Seymour J, Lal R (2013) The role of diatom nanostructures in biasing diffusion to improve uptake in a patchy nutrient environment. PLoS ONE 8, e59548
| The role of diatom nanostructures in biasing diffusion to improve uptake in a patchy nutrient environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnslGjtLw%3D&md5=da7e127a739799283fbf8dc4170a0b3bCAS | 23667421PubMed |
Morel FMM, Reinfelder JR, Roberts SB, Chamberlain CP, Lee JG, Yee D (1994) Zinc and carbon co-limitation of marine phytoplankton. Nature 369, 740–742.
| Zinc and carbon co-limitation of marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlt12lur8%3D&md5=c11e035a280497e70acc0b2c2767d853CAS |
Mutshinda CM, Troccoli-Ghinaglia L, Finkel ZV, Müller-Karger FE, Irwin AJ (2013) Environmental control of the dominant phytoplankton in the Cariaco basin: a hierarchical Bayesian approach. Marine Biology Research 9, 246–260.
| Environmental control of the dominant phytoplankton in the Cariaco basin: a hierarchical Bayesian approach.Crossref | GoogleScholarGoogle Scholar |
Nagao R, Tomo T, Noguchi E, Suzuki T, Okumura A, Narikawa R, Enami I, Ikeuchi M (2012) Proteases are associated with a minor fucoxanthin chlorophyll a/c-binding protein from the diatom, Chaetoceros gracilis. Biochimica et Biophysica Acta 1817, 2110–2117.
| Proteases are associated with a minor fucoxanthin chlorophyll a/c-binding protein from the diatom, Chaetoceros gracilis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsV2qtbjI&md5=3326125180c2c1c8d42e56023d216e5cCAS | 22967834PubMed |
Nakajima K, Tanaka A, Matsuda Y (2013) SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proceedings of the National Academy of Sciences of the United States of America 110, 1767–1772.
| SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXislChsLo%3D&md5=d194959a057130e222739bf00f58afcfCAS | 23297242PubMed |
Nimer NA, Iglesias-Rodriguez MD, Merrett MJ (1997) Bicarbonate utilization by marine phytoplankton species. Journal of Phycology 33, 625–631.
| Bicarbonate utilization by marine phytoplankton species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvV2qtrg%3D&md5=f3184b4702e4639772ffe7196df0c8efCAS |
Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J (2010) Recent advances in understanding the assembly and repair of photosystem II. Annals of Botany 106, 1–16.
| Recent advances in understanding the assembly and repair of photosystem II.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVWjsb4%3D&md5=46047e7eb26177dabfe1b4eb47a2d3e5CAS | 20338950PubMed |
Niyogi KK, Li X-P, Rosenberg V, Jung H-S (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? Journal of Experimental Botany 56, 375–382.
| Is PsbS the site of non-photochemical quenching in photosynthesis?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXovVymuw%3D%3D&md5=651acca7516f18f197337eb3c383d39aCAS | 15611143PubMed |
Orellana M, Ashworth J, Lee A, Armbrust EV (2013) ‘A systems approach to diatom responses to ocean acidification.’ (The Molecular Life of Diatoms EMBO Workshop: Paris)
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner G, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig M, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686.
| Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsL%2FE&md5=3764c303dd2dac3f7a26ed0fb69f00e5CAS | 16193043PubMed |
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322, 690–692.
| Physiology and climate change.Crossref | GoogleScholarGoogle Scholar | 18974339PubMed |
Raven JA (1991) Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature. Plant, Cell & Environment 14, 779–794.
| Physiology of inorganic C acquisition and implications for resource use efficiency by marine phytoplankton: relation to increased CO2 and temperature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyisbc%3D&md5=dc15a859ae05c58b7070d11b8d0731f0CAS |
Raven JA (1993) Limits on growth rates. Nature 361, 209–210.
| Limits on growth rates.Crossref | GoogleScholarGoogle Scholar |
Raven JA (2010) Inorganic carbon acquisition by eukaryotic algae: four current questions. 106, 123–134.
Raven JA, Johnston AM (1991) Mechanisms of inorganic-carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnology and Oceanography 36, 1701–1714.
| Mechanisms of inorganic-carbon acquisition in marine phytoplankton and their implications for the use of other resources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xkt1entr0%3D&md5=2c2cf2ed1f7bc6cf1beb26833700d368CAS |
Raven JA, Giordano M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynthesis Research 109, 281–296.
| Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFOqu7vO&md5=53fe332da4c683da6da089878c189e27CAS | 21327536PubMed |
Reinfelder JR (2011) Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annual Review of Marine Science 3, 291–315.
| Carbon concentrating mechanisms in eukaryotic marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 21329207PubMed |
Reinfelder JR, Kraepiel AM, Morel FM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407, 996–999.
| Unicellular C4 photosynthesis in a marine diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnvVaru7k%3D&md5=c3c42fe0f57d92c13e64f123bbe48436CAS | 11069177PubMed |
Reinfelder JR, Milligan AJ, Morel FMM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiology 135, 2106–2111.
| The role of the C4 pathway in carbon accumulation and fixation in a marine diatom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnt1Ggs70%3D&md5=a557c1a95d9c74af85ec7d63153ebf52CAS | 15286292PubMed |
Riebesell U, Tortell PD (2011) Effects of ocean acidification on pelagic organisms and ecosystems. In ‘Ocean acidification’. (Oxford University Press: Oxford)
Riebesell U, Wolf-Gladrow DA, Smetacek V (1993) Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249–251.
| Carbon dioxide limitation of marine phytoplankton growth rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhs1Cqurc%3D&md5=7cb4a95f1c213acfcc142134f83a2300CAS |
Roberts K, Granum E, Leegood RC, Raven JA (2007a) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiology 145, 230–235.
| C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVKqtr%2FN&md5=79d832f9c6aaf7d48f0cc4aaa44cb9f1CAS | 17644625PubMed |
Roberts K, Granum E, Leegood RC, Raven JA (2007b) Carbon acquisition by diatoms. Photosynthesis Research 93, 79–88.
| Carbon acquisition by diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptlKgt7o%3D&md5=d5db097a80799f8806cad4a3452cdb70CAS | 17497225PubMed |
Rost B, Riebesell U, Burkhardt S, Sültemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnology and Oceanography 48, 55–67.
| Carbon acquisition of bloom-forming marine phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Tilbrook B, Millero FJ, Peng T, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305, 367–371.
| The oceanic sink for anthropogenic CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXls1egsbc%3D&md5=6212d8dddbd30d2a8b300fd70f98da95CAS | 15256665PubMed |
Schippers P, Lürling M, Scheffer M (2004) Increase of atmospheric CO2 promotes phytoplankton productivity. Ecology Letters 7, 446–451.
| Increase of atmospheric CO2 promotes phytoplankton productivity.Crossref | GoogleScholarGoogle Scholar |
Shatwell T, Nicklisch A, Köhler J (2012) Temperature and photoperiod effects on phytoplankton growing under simulated mixed layer light fluctuations. Limnology and Oceanography 57, 541–553.
| Temperature and photoperiod effects on phytoplankton growing under simulated mixed layer light fluctuations.Crossref | GoogleScholarGoogle Scholar |
Sims PA, Mann DG, Medlin LK (2006) Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45, 361–402.
| Evolution of the diatoms: insights from fossil, biological and molecular data.Crossref | GoogleScholarGoogle Scholar |
Six C, Finkel ZV, Irwin AJ, Campbell DA (2007) Light variability illuminates niche-partitioning among marine picocyanobacteria. PLoS ONE 2, e1341
| Light variability illuminates niche-partitioning among marine picocyanobacteria.Crossref | GoogleScholarGoogle Scholar | 18092006PubMed |
Six C, Sherrard R, Lionard M, Roy S, Campbell D (2009) Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiology 151, 379–390.
| Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOjsbrM&md5=9643dbb13aa43346a070387cbf46d88eCAS | 19587099PubMed |
Sobrino C, Ward ML, Neale PJ (2008) Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: effects on growth, photosynthesis, and spectral sensitivity of photoinhibition. Limnology and Oceanography 53, 494–505.
| Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: effects on growth, photosynthesis, and spectral sensitivity of photoinhibition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFKjtbk%3D&md5=1a3c275f859338f1f8b69f44a4257eddCAS |
Steinacher M, Joos F, Frölicher TL, Bopp L, Cadule P, Cocco V, Doney SC, Gehlen M, Lindsay K, Moore JK, Schneider B, Segschneider J (2010) Projected 21st century decrease in marine productivity: a multi-model analysis. Biogeosciences 7, 979–1005.
| Projected 21st century decrease in marine productivity: a multi-model analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1Cgu7c%3D&md5=0f3d32f632036cc6223f4283fe6645eeCAS |
Sugie K, Yoshimura T (2013) Effects of pCO2 and iron on the elemental composition and cell geometry of the marine diatom Pseudo-nitzschia pseudodelicatissima (Bacillariophyceae). Journal of Phycology 49, 475–488.
| Effects of pCO2 and iron on the elemental composition and cell geometry of the marine diatom Pseudo-nitzschia pseudodelicatissima (Bacillariophyceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVaisrvL&md5=0625fba50ab780b31178352f70cbd862CAS |
Sukenik A, Tchernov D, Kaplan A, Huertas E, Lubian LM, Livne A (1997) Uptake, efflux, and photosynthetic utilization of inorganic carbon by the marine eustigmatophyte Nannochloropsis Sp.1. Journal of Phycology 33, 969–974.
| Uptake, efflux, and photosynthetic utilization of inorganic carbon by the marine eustigmatophyte Nannochloropsis Sp.1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtVyrsg%3D%3D&md5=fa2d085fb016a7a64c33d6fc0b963c52CAS |
Sültemeyer D, Schmidt C, Fock HP (1993) Carbonic anhydrases in higher plants and aquatic microorganisms. Physiologia Plantarum 88, 179–190.
| Carbonic anhydrases in higher plants and aquatic microorganisms.Crossref | GoogleScholarGoogle Scholar |
Sun J, Hutchins DA, Feng Y, Seubert EL, Caron DA, Fu F-X (2011) Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnology and Oceanography 56, 829–840.
| Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotVKjt7s%3D&md5=7a6bb07b14683f1824425c02b93d52b4CAS |
Tatters AO, Fu F-X, Hutchins DA (2012) High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta. PLoS ONE 7, e32116
| High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVarurs%3D&md5=e476b0387da052fbdd4d56400cde650bCAS | 22363805PubMed |
Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A (1997) Sustained net CO2 evolution during photosynthesis by marine microorganisms. Current biology 723–728.
| Sustained net CO2 evolution during photosynthesis by marine microorganisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntVKltb8%3D&md5=7572469990b33914ea80e4efd2cb3274CAS | 9368754PubMed |
Torstensson A, Chierici M, Wulff A (2012) The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biology 35, 205–214.
| The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa.Crossref | GoogleScholarGoogle Scholar |
Tortell PD, Rau GH, Morel FMM (2000) Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnology and Oceanography 45, 1485–1500.
| Inorganic carbon acquisition in coastal Pacific phytoplankton communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosFymtrc%3D&md5=ae173ab6771bc48919182efeae1ca9b8CAS |
Tortell PD, Payne CD, Li YY, Trimborn S, Rost B, Smith WO, Riesselman C, Dunbar RB, Sedwick P, DiTullio GR (2008) CO2 sensitivity of Southern Ocean phytoplankton. Geophysical Research Letters 35, L04605
| CO2 sensitivity of Southern Ocean phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Trimborn S, Wolf-Gladrow D, Richter K-U, Rost B (2009) The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. Journal of Experimental Marine Biology and Ecology 376, 26–36.
| The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosVaisL0%3D&md5=1bd8d13156b78b925eaab938dc658db4CAS |
Tsuzuki M, Miyachi S (1989) The function of carbonic anhydrase in aquatic photosynthesis. Aquatic Botany 34, 85–104.
| The function of carbonic anhydrase in aquatic photosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXltlSltLg%3D&md5=a7b1e76cc563fa38967437b52ad67702CAS |
Veron JEN (2008) Mass extinctions and ocean acidification: biological constraints on geological dilemmas. Coral Reefs 27, 459–472.
| Mass extinctions and ocean acidification: biological constraints on geological dilemmas.Crossref | GoogleScholarGoogle Scholar |
Waring J, Klenell M, Bechtold U, Underwood GJC, Baker NR (2010) Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. Journal of Phycology 46, 1206–1217.
| Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species.Crossref | GoogleScholarGoogle Scholar |
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 355, 1517–1529.
| Photorespiration: metabolic pathways and their role in stress protection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXovFKltrc%3D&md5=26a1bd7acaf46ea3a3935bef52d8cd6eCAS | 11128005PubMed |
Woodger FJ, Badger MR, Price GD (2005) Sensing of inorganic carbon limitation in Synechococcus PCC7942 is correlated with the size of the internal inorganic carbon pool and involves oxygen. Plant Physiology 139, 1959–1969.
| Sensing of inorganic carbon limitation in Synechococcus PCC7942 is correlated with the size of the internal inorganic carbon pool and involves oxygen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlGmsb%2FE&md5=d1c508008e2a430417388f531e9e0b0aCAS | 16306144PubMed |
Wu Y, Gao K, Riebesell U (2010) CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923.
| CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFCns7jM&md5=d2834a069c9ba917b1fba1ab67a339a7CAS |
Wu H, Cockshutt AM, McCarthy A, Campbell DA (2011) Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms. Plant Physiology 156, 2184–2195.
| Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOrurrP&md5=c64356fe903904d8d7fc84a2ef317141CAS | 21617029PubMed |
Wu X, Gao G, Giordano M, Gao K (2012a) Growth and photosynthesis of a diatom grown under elevated CO2; in the presence of solar UV radiation. Fundamental and Applied Limnology. Archiv fuer Hydrobiologie 180, 279–290.
Wu H, Roy S, Alami M, Green BR, Campbell DA (2012b) Photosystem II photoinactivation, repair, and protection in marine centric diatoms. Plant Physiology 160, 464–476.
| Photosystem II photoinactivation, repair, and protection in marine centric diatoms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlOmtL3L&md5=669376e585bd6657d978f057bd233a54CAS | 22829321PubMed |
Xu JT, Gao K (2012) Future CO2-induced ocean acidification mediates physiological performance of a green tide alga. Plant Physiology 160, 1762–1769.
| Future CO2-induced ocean acidification mediates physiological performance of a green tide alga.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKmt7rK&md5=dd8da24c40c9b48d4dc7af8d8dd425c7CAS |
Yang G, Gao K (2012) Physiological responses of the marine diatom Thalassiosira pseudonana to increased pCO2 and seawater acidity. Marine Environmental Research 79, 142–151.
| Physiological responses of the marine diatom Thalassiosira pseudonana to increased pCO2 and seawater acidity.Crossref | GoogleScholarGoogle Scholar | 22770534PubMed |
Zhu S-H, Green BR (2010) Photoprotection in the diatom Thalassiosira pseudonana: role of LI818-like proteins in response to high light stress. Biochimica et Biophysica Acta 1797, 1449–1457.
| Photoprotection in the diatom Thalassiosira pseudonana: role of LI818-like proteins in response to high light stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvFSjtL0%3D&md5=c5c4852460239ec66ca78bd5af571322CAS | 20388491PubMed |
Zou D, Gao K, Luo H (2011) Short- and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia fusiformis (sargassaceae, Phaeophyta) grown at low and high N supplies. Journal of Phycology 47, 87–97.
| Short- and long-term effects of elevated CO2 on photosynthesis and respiration in the marine macroalga Hizikia fusiformis (sargassaceae, Phaeophyta) grown at low and high N supplies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktF2lurg%3D&md5=ede87ab9b9f0802ccbe3c52e593b7f46CAS |


