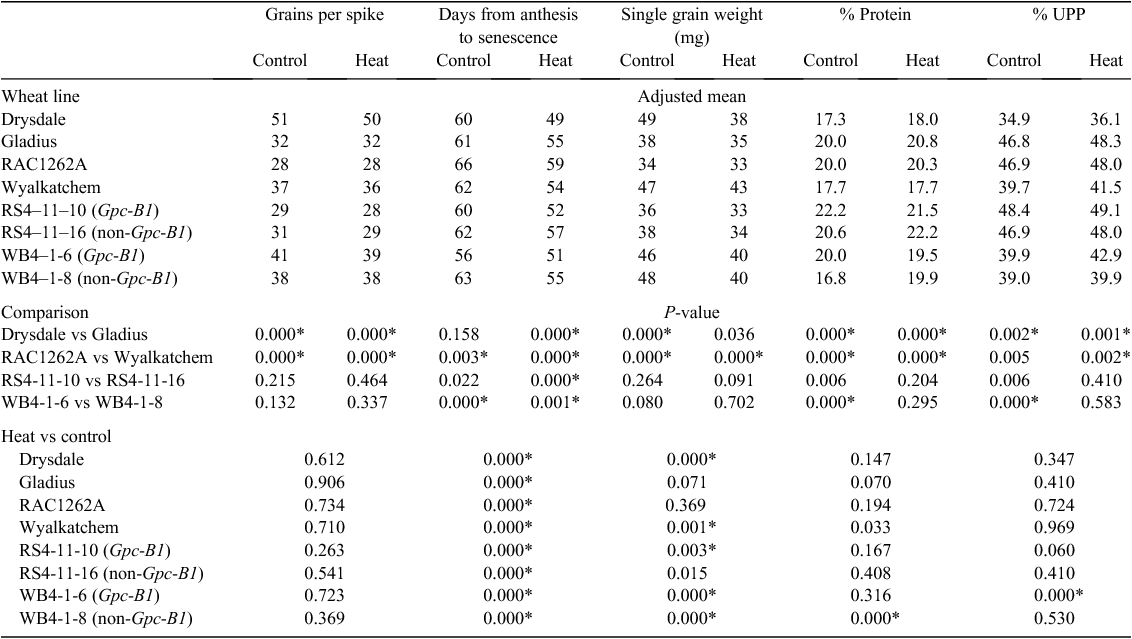
Post-anthesis heat and a Gpc-B1 introgression have similar but non-additive effects in bread wheat
Lancelot Maphosa A B C , Nicholas C. Collins A B , Julian Taylor B and Diane E. Mather A B DA Australian Centre for Plant Functional Genomics, Waite Research Institute, The University of Adelaide, PMB 1, Glen Osmond, SA 5064, Australia.
B School of Agriculture, Food and Wine, Waite Research Institute, The University of Adelaide, PMB 1, Glen Osmond, SA 5064, Australia.
C Present address: Department of Environment and Primary Industries, 110 Natimuk Road, Horsham, Vic. 3400, Australia.
D Corresponding author. Email: diane.mather@adelaide.edu.au
Functional Plant Biology 41(9) 1002-1008 https://doi.org/10.1071/FP14060
Submitted: 22 February 2014 Accepted: 8 May 2014 Published: 10 June 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
High temperatures during grain filling can reduce the yield of wheat and affect its grain protein concentration. The Gpc-B1 locus of wheat also affects grain protein concentration, but it is not known whether its effects interact with those of heat. The aim of this study was to investigate the effects of high temperature in lines with and without functional (high-protein) alleles at Gpc-B1. A highly replicated experiment was conducted in a glasshouse under control conditions (24/18°C, 14/10 h day/night), with half of the plants of each line or cultivar put into a heat chamber (37/27°C, 14/10 h day/night) at 15 days after anthesis for 3 days. Backcross derivatives with the Gpc-B1 introgression segment differed from their recurrent parents more than those without that segment. In some respects, the effects of the Gpc-B1 introgression were similar to those of the heat treatment: both could accelerate peduncle senescence, increase grain protein content and increase the percentage of unextractable polymeric protein. Unlike the heat treatment, Gpc-B1 did not reduce grain weight, indicating that factors that hasten senescence do not necessarily limit grain size. The presence of the Gpc-B1 segment did not exacerbate the effects of heat stress on any trait.
Additional keywords: protein, senescence, single nucleotide polymorphism, stress.
Introduction
In both hexaploid bread wheat (Triticum aestivum L.) and tetraploid durum wheat (Triticum turgidum L. ssp. durum (Desf.) Husn., a chromosome segment introgressed from wild emmer wheat (Triticum turgidum L. subsp. dicoccoides (Körn. Ex. Asch. and Graebn.)Thell.) is known to increase the concentration of protein, iron and zinc in the grain (Olmos et al. 2003; Uauy et al. 2006a, 2006b; Distelfeld et al. 2007). These effects have been attributed to a NAC transcriptional factor encoded by the NAM-B1 gene at the Gpc-B1 locus on chromosome arm 6BS (Uauy et al. 2006b). The functional NAM-B1 allele from wild emmer acts by accelerating senescence, shortening the grain filling period and enhancing remobilisation of nitrogen from other plant organs to the developing grain (Waters et al. 2009; Brevis and Dubcovsky 2010). Grain weight, test weight and grain yield are also reduced (Brevis and Dubcovsky 2010; Brevis et al. 2010). The reduction in grain yield is not as severe as the reduction in grain weight, indicating that there are compensating effects in other yield components. The reduction in grain weight is greater in tetraploid than in hexaploid wheats, possibly because of a dosage effect: tetraploids have two other functional NAM genes (on chromosomes 6A and 2B), whereas hexaploids have four (on chromosomes 6A, 2B, 2D and 6D). Traits that typically show a positive association with high protein concentration, including flour water absorption, mixing time and loaf volume have also been reported to be affected by Gpc-B1 (Brevis et al. 2010).
The effects of Gpc-B1 are similar in some respects to those that have been reported for heat stress during grain filling. Heat stress can shorten the duration of grain development (accelerate senescence) and affect grain yield and quality (Altenbach 2012). Starch synthesis is particularly sensitive to heat (Jenner 1994) and heat-induced loss of starch may largely account for the effects of heat on grain size and grain protein concentration. Heat stress has been reported to increase mixing time and loaf volume. This has been attributed to increased protein concentration (Li et al. 2013). However, Blumenthal et al. (1995) reported reduced mixing time due to heat. Differential responses in dough properties may be due to differences in the severity and timing of the heat stress. Increases in temperature up to 30°C can increase dough strength but further increases can decrease dough strength (Randall and Moss 1990). Heat-induced reductions in dough strength have been attributed to reductions in glutenin : gliadin ratios and the proportion of glutenin that is present as very large polymers (Blumenthal et al. 1995). Heat has been reported to decrease the percentage unextractable polymeric protein (% UPP, which is used as an indicator of dough strength) when applied at later grain filling stages (Irmak et al. 2008) but to increase % UPP when applied in early stages of grain filling (Balla et al. 2011).
Here, to directly compare the effects of heat stress with those of Gpc-B1 and to investigate how the effects of post-anthesis heat stress would affect the grain of wheat lines that carry the high-protein allele at Gpc-B1, pairs of backcross-derived wheat lines were grown with and without exposure to a high temperature treatment early in grain filling.
Materials and methods
Plant materials
The materials used here included the cultivars Gladius, Drysdale, Wyalkatchem, Burnside, Somerset and Glupro, a line designated RAC1262A (a selection from the breeding line RAC1262, which was later released as Gladius) and two pairs of backcross-derived lines (RS4-11-10 and RS4-11-16; WB4–1-6 and WB4-1-8). Gladius, a cultivar that is considered to be heat tolerant (Fleury et al. 2010) was derived from a complex cross involving derivatives of RAC875, Krichauff, Excalibur and Kukri. The pedigree of Drysdale is Hartog*3/Quarrion and that of Wyalkatchem is Machete///Gutha//Jacup*2/11thISEPTON-135. Burnside (pedigree Glenlea*2///Pasqua*2/Glupro///Glenlea*6/Kitt) and Somerset (pedigree AC Minto*5/Glupro//Pasqua) both carry the high-protein allele at Gpc-B1. Glupro (pedigree Columbus/FA15-2/Len) is the source of the high-protein allele in both Burnside and Somerset.
RS4-11-10 and RS4-11-16 were derived from a marker-assisted backcrossing program using RAC1262A as the recurrent parent and Somerset as the donor parent. RS4-11-10 and RS4-11-16 were both derived from selfed progeny of a BC4 progeny plant designated RS4-11. In the Gpc-B1 region of chromosome 6B, RS4-11-10 is homozygous for donor-parent alleles and RS4-11-16 is homozygous for recurrent-parent alleles. Similarly, WB4-1-6 and WB4-1-8 were derived from selfed progeny of a BC4 progeny plant (WB4-1) with Wyalkatchem as its recurrent parent and Burnside as its donor parent. In the Gpc-B1 region, WB4-1-6 carries donor-parent alleles and WB4–1-8 carries recurrent-parent alleles. For brevity, RS4-11-10 and WB4-1-6 will be referred to here as Gpc-B1+ lines, and RS-11-16 and WB4-1-8 will be referred to here as Gpc-B1– lines.
DNA extraction and SNP genotyping
DNA was extracted from ~2.0 g of leaf tissue from one 4-week-old plant of each of Glupro, Somerset, Burnside, Gladius, Drysdale, RAC1262A, Wyalkatchem, RS4-11-10, RS4-11-16, WB4-1-6 and WB4-1-8, using a modified mini prep ball bearing extraction method (Pallotta et al. 2000). DNA concentration was quantified using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Thermo Fisher Scientific, Wilmington, DE, USA). For each line, a 30 µL sample of about 100 ng µL–1 DNA was assayed on a 9K Infinium wheat SNP assay as described by Cavanagh et al. (2013).
Glasshouse experiment
Plants were grown in a naturally-lit glasshouse in Urrbrae, Australia and the evaporative cooling system was set on a 24/18°C max/min 24 h sine wave cycle. The experimental design consisted of 30 complete blocks. Each block consisted of 16 pots (arranged in two rows of eight) and contained four sub-blocks, each of which consisted of four pots (arranged two-by-two). Each of four pairs of lines (RS4-11-10 and RS4-11-16; WB4-1-6 and WB4-1-8; RAC1262A and Wyalkatchem; Gladius and Drysdale) was assigned at random to a sub-block within each block. For each sub-block, two pots were used for each of the two lines in the pair and the pots were assigned at random to positions within the sub-block. One plant of each line was allocated at random to be subjected to a heat treatment, while the other one was used as a control.
Seeds were sown in pots containing a mixture of Waikerie sand and coco peat, 202 mg L–1 dolomite lime, 561 mg L–1 agricultural lime, 131 mg L–1 hydrated lime, 202 mg L–1 gypsum, 202 mg L–1 superphosphate, 505 mg L–1 iron phosphate, 33.7 mg L–1 iron chelate, 202 mg L–1 micronutrients (Scotts Micromax, MicroPlus Trace Element Fertilizer, Langley Fertilizers, Wangara WA, Australia), 505 mg L–1 calcium nitrate and 2022 mg L–1 slow-release fertiliser (Osmocote, Osmocote Exact Mini 16-3.4-9.1 (+1.2 Mg), Everris Australia, Bella Vista NSW, Australia). The pH of this mixture was between 6.0 and 6.5. Aquasol multi-nutrient fertiliser (nitrogen, phosphorus, potassium, sulfur, magnesium, manganese, copper, iron, zinc, boron and molybdenum) was dissolved in water (0.9 g L–1) and applied to the soil at 2 week intervals after anthesis.
For each plant, the date on which anthers were first extruded from florets on the first spike (anthesis date) was recorded. That spike was tagged, and other tillers were removed to reduce competition for light and assimilates. Each plant allocated for heat treatment was transferred at 15 days after its anthesis date to a growth chamber (BDW120, Conviron, Winnipeg, MB, Canada), where pots were placed in tubs containing around 3 cm of water to minimise drought stress. The temperature of the growth chamber was held at 37°C for 8 h each day, with 3 h transition periods used either side to linearly ramp the temperature up and down from and to a night-time temperature of 27°C. Lighting (mixture of metal halide and tungsten incandescent) was at a maximum of 630 µM m–2 s–1 at spike height for 10 h each day, with a 2 h transition period used either side to step the intensity at 460 micromole m–2 s–1. Average day/night RH in the chamber was measured at 60/80%. After 3 days in the growth chamber, the plants were returned to their original positions in the glasshouse and allowed to mature.
For each tagged tiller, the dates of anthesis and complete yellowing of the peduncle were recorded. Grain was harvested after all plants had ripened (complete yellowing of both peduncle and spike). The grains harvested from the spike of each tagged tiller were counted and weighed. Mean single grain weight was obtained by dividing grain weight by grain number.
Size-exclusion high-performance liquid chromatography (SE-HPLC)
Grain from each hand-threshed spike was crushed with a hammer. Flour that passed through a 280 µm sieve was retained. The flour was allowed to rest for at least 7 days in a refrigerator before protein extraction. Protein was extracted from flour using an established method (Appelbee 2007). It involved weighing 25 mg of flour into a 1.5 mL tube and adding 1 mL of 0.05 M phosphate extraction buffer (pH 6.9). Phosphate extraction buffer was made by preparing two solutions, solution A (3.55 g Na2HPO4; 2.5 g sodium dodecyl sulfate (SDS); 500 mL water) and solution B (3 g NaH2PO4; 2.5 g SDS; 500 mL water) then gradually adding solution B to solution A until the pH reached 6.9. The protein extraction procedure was a two-step process. In the first step, the flour was mixed with 1 mL of phosphate extraction buffer, and centrifuged for 10 min at 15 682g. The supernatant (containing SDS-soluble protein) was aspirated from the pellet to a new tube. In the second step, 1 mL of phosphate extraction buffer was added to the pellet, the tube was sonicated at room temperature using a sonifier (Branson model B-12 cell disrupter, Danbury, CT, USA) set at 10 W (output four) for 30 s, then spun again and the supernatant (containing SDS-insoluble protein) was collected. Both extracts were filtered through 0.45 µm PVDF filters into 1-mL glass HPLC vials. Proteases were inactivated by incubating the vials at 80°C for 2 min.
The protein extracts were run on a Waters (Milford, MA, USA) HPLC system using a Protein-Pak 300TM column (C18, 300 Å pore size, 3.5 µm particle size, 150 × 4.6 mm) along with a Waters 717 plus auto sampler, a Waters 600 system controller and a Waters 486 detector. The samples were run in 50% (v/v) acetonitrile containing 0.1% trifluoroacetic acid (TFA), for 50 min (soluble fraction) or 40 min (insoluble fraction) (Batey et al. 1991) at a flow rate of 0.5 mL min–1. The software Millennium32 (ver. 3.2, Waters, Milford, MA, USA) was used for data acquisition and processing. Protein was detected by UV absorbance at 214 nm (Stone and Nicolas 1996) and the areas under specific peaks were used to estimate amounts of soluble and insoluble protein. The first major peak eluting at ~14.5 min in the soluble fraction was defined as total soluble polymeric protein and the first major peak in the insoluble fraction eluting at ~15.5 min was defined as total insoluble polymeric protein. Two aliquots of each sample were each run separately through the HPLC and the values averaged. Total polymeric protein and percentage of SDS-unextractable polymeric protein (% UPP) were calculated as follows:
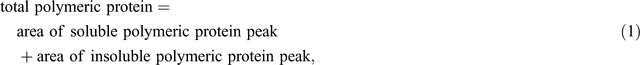
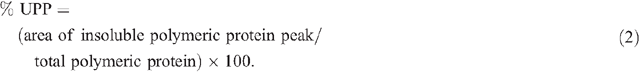
Total protein measurement
For each line, total grain protein concentration was estimated using grain harvested from each of three blocks of the experiment. Total nitrogen content was measured using the Dumas total combustion method (Buckee 1994) using an Elementar Rapid N III Nitrogen Analyser, ver. J (Elementar, Hanau, Germany). Total protein concentration expressed on an ‘as is’ basis was obtained by multiplying nitrogen concentration by 5.7 (conversion factor for wheat).
Statistical analysis
For each of the measured traits, a spatial analysis was conducted using a linear mixed model (Gilmour et al. 1997) that took into account the design factors of the experiment (blocks and sub-blocks) and the layout of pots within the glasshouse. The model also contained a fixed treatment-by-line interaction term which provided adjusted means for the measured traits of each factorial combination of the treatment levels. All models were analysed using ASReml-R (Butler et al. 2009) in the R statistical computing environment (R Development Core Team 2012). With each trait model, post-analysis multiple comparisons were performed to test the significance of the effects of the heat treatment on each of the lines and to directly compare the lines within pairs (Gladius and Drysdale, RAC1262A and Wyalkatchem, RS4-11-10 and RS4-11-16, and WB4-1-6 and WB4-1-8) under heat treatment and control conditions. Correction for the family-wise error rate (FWER) for the number of comparisons performed was done using a Bonferroni-corrected significance level calculated as 0.05 divided by number of comparisons (eight for time to anthesis and 16 for the other traits).
Results
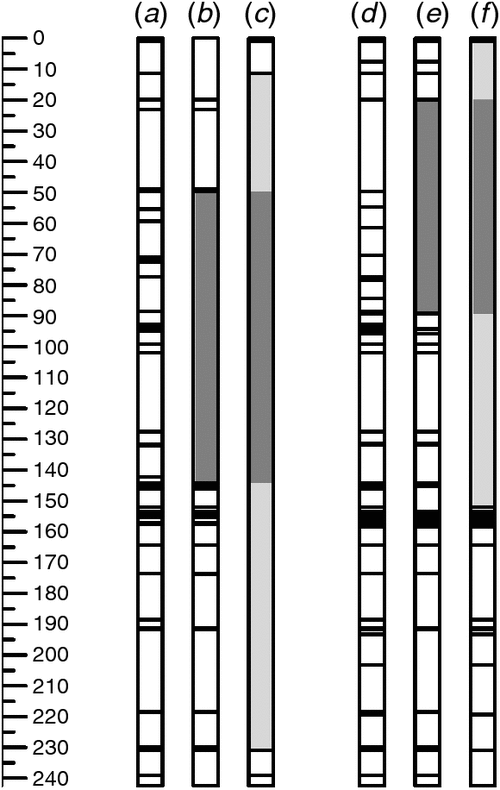
As expected, data for SNP markers that had previously been mapped on chromosome 6B (Cavanagh et al. 2013), confirmed the presence of donor-parent (Somerset or Burnside) introgressions in both Gpc-B1+ lines (RS-11-10 and WB4-1-6) (Fig. 1). In addition to containing the Gpc-B1-containing segment derived from wild emmer wheat, these introgressions could include flanking regions derived from the original donor Glupro and donor wheat cultivars Somerset and Burnside. Considering that there would have been at least five opportunities for recombination in the development of the BC4-F1-derived lines, the introgression segments on chromosome 6B are surprisingly long (up to 220 cM in RS4-11-10 and up to 151 cM in WB4-1-6), possibly indicating suppression of recombination around the Gpc-B1 locus. Elsewhere in the genome, only a few polymorphisms were detected between the backcross derivatives and their recurrent parents (Fig. S1, available as Supplementary Material to this paper), with RS4-11-10, RS4-11-16, WB4-1-6 and WB4-1-8 differing from their recurrent parents for only two, seven, four and five markers respectively. Thus, although differences between the Gpc-B1+ lines and their Gpc-B1− counterparts cannot be attributed exclusively to the presence or absence of the functional NAM-B1 allele at the Gpc-B1 locus, it is reasonable to attribute them to the presence or absence of the Gpc-B1-containing introgression. With continued backcrossing there would be further opportunities for recombination, and it might be possible to reduce the introgression segment size, especially if marker-based selection is used to favour recombinant progeny. However, given that the donor parents used here are modern cultivars, there is no reason to expect negative effects from these long introgression segments.
|
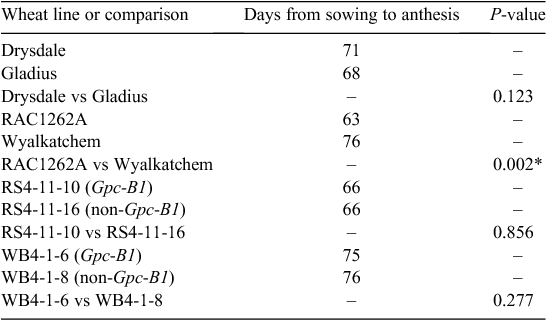
The two recurrent parents differed substantially in flowering time, with Wyalkatchem reaching anthesis 13 days after RAC1262A (Table 1). Compared with RAC1262A, Wyalkatchem consistently (i.e. under both control conditions and after the heat treatment) had a shorter grain filling duration (anthesis to complete peduncle senescence), more grains per spike, larger grain and lower grain protein concentration. Each of the other lines reached anthesis at a very similar time as the line with which it had been paired (Gladius with Drysdale; RS4-11-10 with RS4-11-16; WB4-1-6 with WB4-1-8) (Table 1). For these pairs of lines, there were numerous cases in which the two lines differed for a particular trait under one set of conditions but not the other (Table 2). There were no cases in which the direction of a significant difference differed between the two sets of conditions.
|
In the Wyalkatchem background, the Gpc-B1 segment significantly increased grain protein content (20.0% for WB4-1-6 compared with 16.8% for WB4-1-8). In the higher-protein RAC1262A background, the difference (22.2% for RS4-11-10 compared with 20.6% in RS4-11-16) was not quite significant with the stringent (Bonferroni-corrected) Type I error control employed here. This failure to detect significant differences in protein content for one pair of lines is probably due to the relatively low number of replicates used for this trait. With more replicates, the statistical test would likely have had sufficient power to detect an increase in grain protein content due to Gpc-B1 in both backgrounds.
As expected, the heat treatment, which was applied after grain had set, had no effect on the number of grains per spike. In each of the eight lines evaluated, the period between anthesis and complete peduncle senescence was significantly shortened by exposure to heat (Table 2). The heat treatment reduced grain weight in Drysdale, Wyalkatchem, RS4-11-10, WB4-1-6 and WB4-1-8, increased grain protein concentration in WB4-1-8 and increased % UPP in WB4-1-6.
Discussion
Although the cultivars Gladius and Drysdale did not differ in time to anthesis, they differed from each other in grain number, senescence, single grain weight and grain protein content. In Drysdale, heat treatment accelerated senescence more than in Gladius (by 11 days compared with 7 days) and significantly reduced grain weight. In Gladius, which is considered to be heat tolerant (Fleury et al. 2010), exposure to heat accelerated senescence by 7 days but did not significantly reduce grain weight. Similarly, RAC1262A (Gladius or a close sister line of Gladius) did not show significant reduction in grain weight under heat stress. In Drysdale, the acceleration of senescence was greater (11 days) and grain weight was significantly reduced, possibly due to early senescence shortening the grain filling duration and interfering with the supply of substrates to the developing grain. Heat stress also shortens the duration of grain filling and in sensitive cultivars it decreases the rate of grain growth (Tashiro and Wardlaw 1989; Stone and Nicolas 1995a, 1995b; Viswanathan and Khanna-Chopra 2001; Zahedi and Jenner 2003; Altenbach 2012).
Given that the whole plant was exposed to the heat treatment, the heat-induced reduction in grain weight may have involved interference with important physiological processes in vegetative tissues and/or in the developing grain. These could include nutrient uptake, carbon translocation and starch synthesis. Soluble starch synthase, which catalyses starch biosynthesis, is particularly sensitive to high temperatures, leading to reduction of starch deposition in the developing grain under heat (Jenner 1994). Since a large proportion of the wheat grain is starch, suppression of starch synthesis would be expected to reduce grain weight. Reduction of starch synthesis might be expected to be accompanied by increased grain protein concentration due to a concentration effect. Here, significant heat-induced reduction in grain weight was accompanied by significant increase in grain protein content only in the Gpc-B1− line WB4-1-8. We noted that although the heat treatment resulted in a significant increase in grain protein content of that line, and the Gpc-B1 introgression segment significantly increased grain protein content in the same background (WB4-1-6 vs WB4-1-8), there was no further increase due to the combination of Gpc-B1 and heat. Grain protein concentration may have already reached an upper limit.
In contrast to the effects of heat, the Gpc-B1 introgression segment accelerated senescence without significantly decreasing grain weight, indicating that factors that hasten senescence do not necessarily limit grain size. Although acceleration of senescence by Gpc-B1 was expected (Uauy et al. 2006b; Brevis and Dubcovsky 2010), we noted that the extent of this effect differed between genetic backgrounds (Wyalkatchem vs RAC1262A) and between the control and heat treatments.
Estimation of % UPP made it possible to investigate the effects of Gpc-B1 and/or heat on a functional property of grain protein. Measurement of % UPP, which requires only a small sample of flour, provides an indication of the extent of glutenin polymerisation and is a predictor of dough strength. These characteristics are affected by allelic variation at the Glu-1 loci, which encode high-molecular-weight glutenins (HMW-GS). Gladius, RAC1262A, RS4-11-10 and RS411-16 all carry the Glu-A1a, Glu-B1b and Glu-D1d alleles, which encode the HMW-GS Ax1, Bx7, By8, Dx5 and Dy10. Wyalkatchem, WB4-1-6 and WB4-1-8 all carry the Glu-A1a, Glu-B1b and Glu-D1a alleles, which encode the HMW-GS Ax1, Bx7, By8, Dx2 and Dy12. Drysdale carries Glu-A1a, Glu-B1i and Glu-D1d alleles, which encode the HWM-GS Ax1, Bx17, By18, Dx5 and Dy10. According to effect estimates presented by Eagles et al. (2002), the allelic combination carried by Gladius should lead to stronger dough (measured as the maximum dough resistance to extension, Rmax) than the combinations carried by Wyalkatchem and Drysdale. Consistent with these expectations, the lines carrying the Gladius allelic combination had consistently higher % UPP than Drysdale or those carrying the Wyalkatchem combinations, under control conditions and after post-anthesis heat treatment. Such differences in dough strength are partly due to the extent of glutenin polymerisation.
Accumulation of SDS-insoluble polymers has been shown to begin at ~31 days after anthesis and to be accompanied by reductions in monomers and SDS-soluble polymers (Gupta et al. 1996; Carceller and Aussenac 1999). Consistent with this, exposure to heat late in grain filling (in field experiments or applied at 25 days after anthesis) has been reported to reduce % UPP and dough mixing time and strength (Irmak et al. 2008; Cavanagh et al. 2010). In contrast, earlier heat treatments (applied at 10 or 12 days after anthesis) have been reported to increase % UPP (Balla et al. 2011) and increase SDS sedimentation height : volume ratio (another indicator of glutenin polymerisation and dough strength) (Beecher et al. 2012). In the experiment conducted here, plants were exposed to heat early in grain filling (15 days after anthesis) and for only 3 days. In the Gpc-B1− lines, this heat treatment had no significant effects on % UPP. In the Gpc-B1+ line WB4-1-6; however, the heat treatment increased % UPP from 39.9 to 42.9%. Despite high grain protein concentration achieved in that line under control conditions (20.0%), % UPP was relatively low (39.9% compared with 48.4% for RS4-11-10, probably due to the Wyalkatchem background). Perhaps in the high-protein condition conferred by the Gpc-B1 introgression (20.0% compared with 16.8% for WB4-1-8) the heat treatment triggered a change in glutenin polymerisation. In the other Gpc-B1+ line, RS4-11-10, the corresponding % UPP values were 48.4% (control) and 49.1% (heat); the smaller difference between these was in the same direction, but not statistically significant (P = 0.056). Compared with WB4-1-6, RS4-11-10 may have had less capacity for heat to increase % UPP, given that the control level of % UPP was already high.
In the current study, the use of controlled environment facilities made it possible to expose wheat plants to high temperatures at a specific developmental stage and without the soil moisture deficit that often accompanies heat events in the field. Under the experimental conditions used here, it was not possible to realistically measure bread making quality or grain yield, but data were obtained for indicator variables including grain protein concentration, % UPP, grain number and grain weight. In the experimental conditions used here, the Gpc-B1 introgression increased not only grain protein concentration but also protein polymerisation. It accelerated senescence yet did not reduce grain number or grain weight. Exposure to heat between 15 and 17 days after anthesis similarly hastened senescence, but reduced grain size. This exposure to heat early in grain filling did not affect % UPP in most lines, and it increased grain protein concentration in only one of the eight lines investigated. Although these effects have not been tested in the field, it is worth noting that the presence of the Gpc-B1 introgression did not exacerbate the effects of heat stress on any quality or yield-related parameters, in either of the Australian genetic backgrounds used here.
Acknowledgements
This work was supported by the Grains Research and Development Corporation, the Australian Research Council, the South Australian Government and by scholarships awarded to the first author by the University of Adelaide and the Australian Centre for Plant Functional Genomics. We thank Howard Eagles for providing seed of the Gpc-B1 lines and acknowledge Hugh Wallwork and the late Genet Mekuria for their contributions to the development of those lines. We thank Iman Lohraseb and Hamid Shirdelmoghanloo for technical assistance, Matthew Hayden for SNP genotyping and Marie Appelbee for advice on HPLC analysis for estimating % unextractable polymeric protein.
References
Altenbach SB (2012) New insights into the effects of high temperature, drought and post-anthesis fertilizer on wheat grain development. Journal of Cereal Science 56, 39–50.| New insights into the effects of high temperature, drought and post-anthesis fertilizer on wheat grain development.Crossref | GoogleScholarGoogle Scholar |
Appelbee MJ (2007) Quality potential of gluten proteins in hexaploid wheat and related species. PhD Thesis, School of Agriculture, Food and Wine, The University of Adelaide, SA, Australia.
Balla K, Rakszegi M, Li Z, Békés F, Bencze S, Veisz O (2011) Quality of winter wheat in relation to heat and drought shock after anthesis. Czech Journal of Food Sciences 29, 117–128.
Batey IL, Gupta RB, MacRitchie F (1991) Use of size-exclusion high-performance liquid chromatography in the study of wheat flour proteins: an improved chromatographic procedure. Cereal Chemistry 68, 207–209.
Beecher FW, Mason E, Mondal S, Awika J, Dirk Hays D, Ibrahim A (2012) Identification of quantitative trait loci (QTLs) associated with maintenance of wheat (Triticum aestivum Desf.) quality characteristics under heat stress conditions. Euphytica 188, 361–368.
| Identification of quantitative trait loci (QTLs) associated with maintenance of wheat (Triticum aestivum Desf.) quality characteristics under heat stress conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12rsLrK&md5=006bb398d6ceabbcce44739ba5b5b3cdCAS |
Blumenthal CS, Bekes F, Gras PW, Barlow EWR, Wrigley CW (1995) Identification of wheat genotypes tolerant to the effects of heat stress on grain quality. Cereal Chemistry 72, 539–544.
Brevis JC, Dubcovsky J (2010) Effects of the chromosome region including the Gpc-B1 locus on wheat grain and protein yield. Crop Science 50, 93–104.
| Effects of the chromosome region including the Gpc-B1 locus on wheat grain and protein yield.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFSkt74%3D&md5=446a492acaf0a5c484b0c61063ae2a45CAS |
Brevis JC, Morris CF, Manthey F, Dubcovsky J (2010) Effect of the grain protein content locus Gpc-B1 on bread and pasta quality. Journal of Cereal Science 51, 357–365.
| Effect of the grain protein content locus Gpc-B1 on bread and pasta quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVeksrw%3D&md5=950643f2bde3355d65155dc83b89f829CAS |
Buckee GK (1994) Determination of total nitrogen in barley, malt and beer by Kjeldahl procedures and the Dumas combustion method-collaborative trial. Journal of the Institute of Brewing. Institute of Brewing 100, 57–64.
Butler DG, Cullis BR, Gilmour AR, Gogel BJ (2009) ‘ASReml-R Reference Manual.’ (ver. 3) (Queensland Department of Primary Industries: Brisbane)
Carceller JL, Aussenac T (1999) Accumulation and changes in molecular size distribution of polymeric proteins in developing grains of hexaploid wheats: role of the desiccation phase. Australian Journal of Plant Physiology 26, 301–310.
| Accumulation and changes in molecular size distribution of polymeric proteins in developing grains of hexaploid wheats: role of the desiccation phase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvFGlsLc%3D&md5=0661a77b2f9b0a2ca8bb42d524a954c9CAS |
Cavanagh C, Taylor J, Larroque O, Coombes N, Verbyla A, Nath Z, Kutty I, Rampling L, Butow B, Ral J-P, Tomoskozi S, Balazs G, Békés F, Mann G, Quail K, Southan M, Morell M, Newberry M (2010) Sponge and dough bread making: genetic and phenotypic relationships with wheat quality traits. Theoretical and Applied Genetics 121, 815–828.
| Sponge and dough bread making: genetic and phenotypic relationships with wheat quality traits.Crossref | GoogleScholarGoogle Scholar | 20495901PubMed |
Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proceedings of the National Academy of Sciences of the United States of America 110, 8057–8062.
| Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtV2ksL%2FJ&md5=2ac518560f5795dc4c80dd01c1e5c5bfCAS | 23630259PubMed |
Distelfeld A, Cakmak I, Peleg Z, Ozturk L, Yazici AM, Budak H, Saranga Y, Fahima T (2007) Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiologia Plantarum 129, 635–643.
| Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVyku7o%3D&md5=d5df32d7b39b669035200d9fc33fb000CAS |
Eagles HA, Hollamby GJ, Gororo NN, Eastwood RF (2002) Estimation and utilisation of glutenin gene effects from the analysis of unbalanced data from wheat breeding programs. Australian Journal of Agricultural Research 53, 367–377.
| Estimation and utilisation of glutenin gene effects from the analysis of unbalanced data from wheat breeding programs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtlWju7c%3D&md5=e0a91fa7028c45fdd53c8624ae44e8b8CAS |
Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany 61, 3211–3222.
| Genetic and genomic tools to improve drought tolerance in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVymsLc%3D&md5=5c66e9f2b8ff0fa2bfa7a74aedbabd61CAS | 20525798PubMed |
Gilmour AR, Cullis BR, Verbyla AP (1997) Accounting for natural and extraneous variation in the analysis of field experiments. Journal of Agricultural Biological & Environmental Statistics 2, 269–293.
| Accounting for natural and extraneous variation in the analysis of field experiments.Crossref | GoogleScholarGoogle Scholar |
Gupta RB, Masci S, Lafiandra D, Bariana HS, MacRitchie F (1996) Accumulation of protein subunits and their polymers in developing grains of hexaploid wheats. Journal of Experimental Botany 47, 1377–1385.
| Accumulation of protein subunits and their polymers in developing grains of hexaploid wheats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmslWlsro%3D&md5=1410259f110a3f7a8a4d3355e882a358CAS |
Irmak S, Naeem HA, Lookhart GL, MacRitchie F (2008) Effect of heat stress on wheat proteins during kernel development in wheat near-isogenic lines differing at Glu-D1. Journal of Cereal Science 48, 513–516.
| Effect of heat stress on wheat proteins during kernel development in wheat near-isogenic lines differing at Glu-D1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVarsLnM&md5=1c68e81ac0a227501d612235549ec5c9CAS |
Jenner CF (1994) Starch synthesis in the kernel of wheat under high temperature conditions. Australian Journal of Plant Physiology 21, 791–806.
| Starch synthesis in the kernel of wheat under high temperature conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkslequrk%3D&md5=f5f6080f04f261a55928fd045d2b3c93CAS |
Li Y, Wu Y, Hernandez-Espinosa N, Peña RJ (2013) The influence of drought and heat stress on the expression of end use quality parameters of common wheat. Journal of Cereal Science 57, 73–78.
| The influence of drought and heat stress on the expression of end use quality parameters of common wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1ajsb%2FE&md5=331e29198c7f8a584b0d0730dcbca3d6CAS |
Olmos S, Distelfeld A, Chicaiza O, Schlatter AR, Fahima T, Echenique V, Dubcovsky J (2003) Precise mapping of a locus affecting grain protein content in durum wheat. Theoretical and Applied Genetics 107, 1243–1251.
| Precise mapping of a locus affecting grain protein content in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXos1Oktbw%3D&md5=66be8fd528d90737b4cad188d16818e9CAS | 12923624PubMed |
Pallotta MA, Graham RD, Langridge P, Sparrow DHB, Barker SJ (2000) RFLP mapping of manganese efficiency in barley. Theoretical and Applied Genetics 101, 1100–1108.
| RFLP mapping of manganese efficiency in barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXovVaks7o%3D&md5=6c18b7839fb50bce6844e8ecc6a36196CAS |
R Development Core Team (2012) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna)
Randall P, Moss H (1990) Some effects of temperature regime during grain filling on wheat quality. Australian Journal of Agricultural Research 41, 603–617.
| Some effects of temperature regime during grain filling on wheat quality.Crossref | GoogleScholarGoogle Scholar |
Stone P, Nicolas M (1995a) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Functional Plant Biology 22, 927–934.
Stone P, Nicolas M (1995b) Comparison of sudden heat stress with gradual exposure to high temperature during grain filling in two wheat varieties differing in heat tolerance. I. Grain growth. Functional Plant Biology 22, 935–944.
Stone PJ, Nicolas ME (1996) Varietal differences in mature protein composition of wheat resulted from different rates of polymer accumulation during grain filling. Australian Journal of Plant Physiology 23, 727–737.
| Varietal differences in mature protein composition of wheat resulted from different rates of polymer accumulation during grain filling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXms1Ggsg%3D%3D&md5=b032d3e461edd36e7464c66cc1a0b540CAS |
Tashiro T, Wardlaw IF (1989) A comparison of the effect of high temperature on grain development in wheat and rice. Annals of Botany 64, 59–65.
Uauy C, Brevis JC, Dubcovsky J (2006a) The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. Journal of Experimental Botany 57, 2785–2794.
| The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xotl2mtbk%3D&md5=5a845fc25dd0f078b2db60fdfaae61c9CAS | 16831844PubMed |
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006b) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314, 1298–1301.
| A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1agt7jL&md5=ccd00356986d0bb068bd97b3243308b6CAS | 17124321PubMed |
Viswanathan C, Khanna-Chopra R (2001) Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat (Triticum aestivum L.) varieties differing in grain weight stability. Journal Agronomy & Crop Science 186, 1–7.
| Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat (Triticum aestivum L.) varieties differing in grain weight stability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjt1Smsb8%3D&md5=951f9674daf57f2b9d76124d2524672fCAS |
Waters BM, Uauy C, Dubcovsky J, Grusak MA (2009) Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. Journal of Experimental Botany 60, 4263–4274.
| Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlGitL%2FN&md5=5444ebe0beed8b59de5d4e83aad371e4CAS | 19858116PubMed |
Zahedi M, Jenner CF (2003) Analysis of effects in wheat of high temperature on grain filling attributes estimated from mathematical models of grain filling. Journal of Agricultural Science 141, 203–212.
| Analysis of effects in wheat of high temperature on grain filling attributes estimated from mathematical models of grain filling.Crossref | GoogleScholarGoogle Scholar |