Shoot–root carbon allocation, sugar signalling and their coupling with nitrogen uptake and assimilation
Lu Wang A B and Yong-Ling Ruan A CA School of Environmental and Life Sciences, University of Newcastle, Callaghan, NSW 2308, Australia.
B Present address: School of Plant Science, University of Tasmania, Hobart, Tas. 7001, Australia.
C Corresponding author. Email: yong-ling.ruan@newcastle.edu.au
Functional Plant Biology 43(2) 105-113 https://doi.org/10.1071/FP15249
Submitted: 19 August 2015 Accepted: 24 October 2015 Published: 20 November 2015
Abstract
Roots and shoots are distantly located but functionally interdependent. The growth and development of these two organ systems compete for energy and nutrient resource, and yet, they keep a dynamic balance with each other for growth and development. The success of such a relationship depends on efficient root-shoot communication. Aside from the well-known signalling processes mediated by hormones such as auxin and cytokinin, sugars have recently been shown to act as a rapid signal to co-ordinate root and shoot development in response to endogenous and exogenous clues, in parallel to their function as carbon and energy resources for biomass production. New findings from studies on vascular fluids have provided molecular insights into the role of sugars in long-distance communications between shoot and root. In this review, we discussed phloem- and xylem- translocation of sugars and the impacts of sugar allocation and signalling on balancing root–shoot development. Also, we have taken the shoot–root carbon–nitrogen allocation as an example to illustrate the communication between the two organs through multi-layer root–shoot–root signalling circuits, comprising sugar, nitrogen, cytokinin, auxin and vascular small peptide signals.
Additional keywords: carbon partitioning, phloem and xylem, root development, shoot growth, sugar signalling.
Introduction
Land plants possess two basic organ systems: the shoot (aboveground) and the root (underground). The former is responsible for capturing solar energy and atmospheric carbon dioxide, using photosynthesis to turn these into organic carbon (i.e. sugars), which is then used for development and biomass production of all plant organs. The latter, the roots, anchor the plant in and acquire water and inorganic nutrients from the soil. To optimise growth in an ever-changing environment, plants must balance the distribution between the two organ systems for energy, nutrient and water resources. The success of this co-ordination depends on effective root–shoot communications. For example, under soil water deficiency, quick stomatal closure is induced to minimise water loss by root-derived hormone, ABA, ahead of changes in leaf water status (Bates and Hall 1981; Stoll et al. 2000). Similarly, low leaf nitrogen status could generate a shoot-to-root signal to alter root physiology, leading to activation of nitrate uptake (Forde 2002). Numerous studies have established that a sophisticated hormonal signalling network regulates these root–shoot communications (e.g. Sauter et al. 2001; Dodd 2005; Schachtman and Goodger 2008; Puig et al. 2012). In contrast, much less attention has been paid to the impacts of carbon flow and sugar signalling on co-ordinating shoot and root development.
Sucrose (Suc) is the primary photoassimilate for long distance translocation through phloem in most higher plant species. Apart from its crucial role as carbon source for energy and building blocks, Suc has also been shown to act as a long-distance signal to co-ordinate plant development (Kircher and Schopfer 2012; Mason et al. 2014). The rapid transport of Suc in the phloem (e.g. ~150 cm h−1 vs that of auxin at just ~16 cm h−1 in Pisum sativum cv. Torsdag, see Mason et al. 2014) highlights its ability for instantaneous response. It remains unknown in many cases whether it is the Suc itself or its derivatives, such as glucose (Glc), fructose (Fru) or trahalose-6-phosphate (T6P) that function as signalling molecules to regulate development (Ruan 2014).
There are many sugars also detected in the xylem sap (Schill et al. 1996; Iwai et al. 2003; Secchi and Zwieniecki 2012), which opens up the possibility of an acropetal sugar transport from roots to shoots and leaves. Together, it becomes possible for a circuit sugar signalling pathway to operate at the whole-plant level through the vascular system. Furthermore, recent studies have revealed significant interactions between sugars and other signalling components including hormones, microRNAs, reactive oxygen species (ROS) and many other signalling molecules (Considine and Foyer 2014; Matsoukas 2014; Tsai and Gazzarrini 2014; Ljung et al. 2015; Yu et al. 2015). Clearly, sugars are major players in the global signalling network regulating plant development, crop yield and response to biotic and abiotic stress (Ruan 2014).
The aim of this review was to evaluate the current understanding of sugar allocation and signalling in root–shoot development, and to provide an insight into the global-level communication that integrates nutrition and growth signalling. First, we have discussed the possible routes for long-distance sugar transport and signalling. Then we have made an assessment of the roles of shoot-to-root sugar signalling in coupling root–shoot development. Finally, we have discussed root–shoot–root signalling circuits in co-ordinating the growth of these two distantly-located organs, using nitrogen-carbon balance as an example.
Long distance carbon flow and signalling circuit through phloem and xylem
Allocation of carbon resource from photosynthesising leaves (source) to non-photosynthetic organs (sink) relies on an efficient and highly controlled phloem transportation system. Among all the photosynthetically-fixed carbohydrates, only a few are able to be transported in the phloem over a long-distance (Lemoine et al. 2013). In this context, sucrose (Suc) is the primary form of assimilates translocated via phloem over a long-distance. The accumulation of Suc in the phloem of the source leaves attracts water osmotically, creating high turgor pressure, which drives mass flow of assimilates towards the sinks (Ruan et al. 1996). Recent reviews describing sugar transport pathways and transporters are also available elsewhere (Osorio et al. 2014; Chen et al. 2015; Fettke and Fernie 2015; Hedrich et al. 2015). Besides Suc, phloem hexose transport has also been reported for a limited number of species (van Bel and Hess 2008). However, these results were questioned by Liu et al. (2012). Oligosaccharides of raffinose family are indirectly involved in the building up of sugar concentrations in the phloem by polymer trapping (e.g. Rennie and Turgeon 2009). Plyols (mainly sorbitol and mannitol) are translocated through phloem, in some species such as apple (Noiraud et al. 2001). Overall, for most plant species, Suc is the main carbon resource moving from shoot to root or other sinks in the phloem, together with many other nutrients, transcripts, proteins and signalling molecules (Turgeon and Wolf 2009). For example, Suc was estimated to be 433 mM from phloem exudates of Ricinus communis L., accounting for 72% of total sap osmolality (Patrick 2013). Phloem sugar distribution is directly linked to the cellular pathways of assimilate transport and sugar metabolism in both ‘the giver’ and ‘the taker’ (Palmer et al. 2015), and can be affected by many environmental factors that alter shoot–root relationship (Liu and Vance 2010; Lemoine et al. 2013).
In contrast with the translocation of phloem sap from shoot to root, the root-to-shoot translocation is mediated by xylem sap flow driven by water loss through leaf transpiration. Some soluble sugars including Suc, Fru, and sugar polymers such as oligoarabinogalactan, oligoglycan and myo-inositol, have also been detected from the xylem sap of many species, such as maple (Schill et al. 1996), squash (Iwai et al. 2003), walnut (Améglio et al. 2004) and black poplar (Secchi and Zwieniecki 2012). However, it remains unclear how Suc or other sugars may have reached xylem vessels. One possibility is that Suc continuously leaks out from the phloem ((Minchin and McNaughton 1987; van Bel 2003) to the xylem/phloem parenchyma cells, which then diffuses to the transpiration stream. Indeed, apart from water and mineral elements, the xylem transpiration stream is full of hormones, transcripts, proteins, carbohydrates and other small organic chemicals (Pérez-Alfocea et al. 2011).
Interestingly, analysis in Populus trichocarpa Torr. & A.Gray has revealed that Suc infiltration into xylem resulted in a simultaneous physiological and molecular response, including reduction of the starch pool in xylem parenchyma cells and induction of expression of genes encoding aquaporins, amylases and sugar transporters, similar to the response induced by embolism, a formation of air bubble in xylem blocking or impeding long distance transport of xylem sap (Secchi and Zwieniecki 2011). Evidence provided from this study indicates Suc may function in sensing embolism and triggering the refilling in xylem vessels. Further studies are required to understand the dynamics of this xylem-mediated carbon flow and to determine whether it plays a role in root-to-shoot communication. In addition to simple sugars (Suc, Glc and Fru), some oligosaccharides may also function as elicitors in stress responses (Fry et al. 1993; John et al. 1997; Shibuya and Minami 2001), and probably be involved in long-distance signalling through xylem transportation (MacDougall et al. 1992; Iwai et al. 2003).
Notably, recent research centered on the secreted oligopeptides shed lights on a root-to-shoot-to-root signalling feedback circuit in plants (reviewed by Notaguchi and Okamoto 2015). In this scenario, signal molecules generated in root system in response to the soil environments could move towards shoot via the xylem, translocated to the phloem through shared vascular parenchyma cells and perceived by potential receptors in the phloem. The perception of root-derived signals could elicit molecular and biochemical responses in shoot, which could relay the same signal or generate new signals for delivery through the phloem sap to the rest of the plants including the root. Although the mechanism by which sugar signalling participates in root-shoot-root signalling circuit remains to be elucidated, such a possibility of bi-directional signalling does appear to be plausible based on the observations on the presence of sugars in both xylem and phloem and the intimate anatomic and functional coupling between the two vascular systems.
Shoot-to-root Suc transport in co-ordinating plant development
As the main assimilate transported from shoot to root, Suc is required as the original and primary carbon resource for root development. Recent findings from Arabidopsis seedlings highlight the importance of Suc basipetal translocation in coupling shoot–root development as discussed below.
In early seedling development, light is known as a key factor affecting shoot and root development (Salisbury et al. 2007; Page et al. 2011). A more recent study revealed that photosynthetic Suc transported from shoot (cotyledon) to the root function as a direct signal to activate and promote Arabidopsis root elongation in a light-dependent manner (Kircher and Schopfer 2012). The findings from this report and other studies support the following model on how young seedlings efficiently use their carbon resource to co-ordinate root–shoot growth with light availability and quality. Firstly, at the early germination stage, seedling may be in the dark or in the shade with no photosynthesis. Most of the carbon resource pre-stored in the cotyledons fuels the hypocotyl elongation, whereas the root growth is stalled. At the meantime, a low ratio of red : far-red light under shade also induces hypocotyl elongation and promotes shoot over root growth (Page et al. 2011). As a result, cotyledons, together with shoot apex, are pushed towards the light. Thereafter, the de-etiolated seedling begins to photosynthesise to produce Suc acting as a shoot-to-root signal and nutrient and energy source to support root elongation to acquire belowground resources. The import of assimilate to roots is highly responsive to changes in Suc availability. For instance, studies in barley seedlings revealed a reduced carbon import to roots in response to either increased pool size of sugars within the roots (Farrar and Minchin 1991) or reduced supply from source leaves (Minchin et al. 1994).
Apart from Suc, other sugars such as Glc, were also reported to regulate root growth through: (i) driving target-of-rapamycin (TOR) signalling to instigates root meristem cell division (Xiong et al. 2013); (ii) interacting with auxin via heterotrimeric G protein complex to promote lateral root (LR) formation (Booker et al. 2010); and (iii) regulating brassinosteriod (BR) signalling and downstream auxin transportation to affect root directional responses and eventually root architecture (Singh et al. 2014). It may be that the phloem unloaded Suc may generate a Glc signal in the root, through the activities of Suc-cleavage enzymes, invertase and or sucrose synthase (Sus) to fulfil the reprogramming of gene expressions (Ruan 2014). Further research to determine the identity of sugar signalling pathway in the root is necessary to understand the nature of Suc- or Glc-mediated root growth.
Compared with the seedling stage, the regulation of Suc on root development at late growth stage is less studied. However, deficiency of Suc has been identified as a major cause of grain or seed abortion under drought, possibly owing to reduced Glc that activates programmed cell death (Ruan 2012; Liu et al. 2013). Whether this is the case in roots of mature plants remains to be tested.
In addition to Suc or Glc, shoot-derived auxin has also been identified to regulate primary root elongation in a light-dependent manner (Sassi et al. 2012). To this end, Suc moves much faster to axillary buds than local depletion of auxin (indole-3-acetic acid, IAA) to activate bud outgrowth in pea (Mason et al. 2014). This is likely due to the fact that Suc is translocated through mass flow in the phloem, whereas the cell-to-cell movement of auxin from shoot apex to axillary bud is probably mediated through the AUXs, PINs, auxin influxers and effluxers respectively (Wang and Ruan 2013). By deduction, it seems possible that the shoot-to-root Suc translocation may act as a light-induced signal, ahead of auxin, to promote root development. However, one cannot rule out the possibility that sugar and auxin may crosstalk with each other to modulate root development, as shown in other physiological and developmental contexts (Mishra et al. 2009; Wang and Ruan 2013; Ljung et al. 2015).
Clearly, the shoot-to-root Suc translocation is a critical factor in co-ordinating plant development, and must be tightly balanced. Brauner et al. (2014) provided an example that disruption of this balance limited the growths of both shoot and root. In the plastidial phosphoglucomutase (PGM) loss-of-function mutant (pgm), the inability of leaves to synthesise starch leads to the accumulation of Suc, Glc and Fru in the light, which are rapidly depleted during early night under a long-day light period, and resulted in carbon starvation during the remaining night (Apelt et al. 2015). However, the pgm mutant exhibited dramatically increased shoot-to-root sugar supply during the day and the first 4 h of the night, leading to exaggerated root respiration in pgm (Brauner et al. 2014) and inhibited protein synthesis and increased protein turnover at night (Ishihara et al. 2015). Consequently, the pgm mutant displayed restricted biomass formation in both the root and the shoot. We note that the sugar uptake by root largely exceeded its metabolic demand (Brauner et al. 2014). It remains unknown whether the enhanced Suc export by shoot functions as a mechanism to maintain leaf photosynthesis which was not altered in the pgm mutant, or Suc is ‘drawn’ to the root by an error signal derived from root. Further investigation of the dynamics of sugar metabolism in pgm root, especially the activities of Suc-cleavage enzymes and sugar transporters, and the subcellular compartmentation of the sugars (e.g. vacuole vs cytoplasm), could provide valuable information in understanding how root achieves this over-demanding for sugars.
Root-shoot-root signalling: coupling between C and N uptake and assimilation
Sugars also crosstalk with many other signalling and metabolic processes to regulate local and systemic signalling pathways (Bailey-Serres and Voesenek 2010; Eveland and Jackson 2012; Ruan 2014; Tsai and Gazzarrini 2014; Ljung et al. 2015). As an example, we evaluated below the current understanding on Suc signalling in controlling plant nitrogen (N) balancing. Recent studies have revealed intriguing interactions among sugars, nitrate and many other long-distance mobile signals including cytokinin, auxin, CEP/CLV peptides (Hartig and Beck 2006; Ruffel et al. 2011; Singh et al. 2014; Notaguchi and Okamoto 2015).
Nitrogen is the most abundant inorganic nutrient for plants. Most plant species use nitrate (NO3−) as a major N source, along with ammonium (NH4+) salts (Bloom 2015). Depending on species and soil NO3− abundance, root NO3− can be either assimilated in the root or translocated to the aerial parts of the plants through xylem sap for assimilation in the shoot (Andrews 1986). Typically, shoot dominates NO3− assimilation at high soil NO3− environment in most plants (Krapp 2015). Phloem loading of NO3− has been shown in both source leaves (Fan et al. 2009; Kiba et al. 2011; Hsu and Tsay 2013) and root companion cells (Wang and Tsay 2011), mediated by phloem-localised NO3− transporters. The former process contributes to NO3− remobilisation to younger leaves during N deficiency, whereas the latter facilitates NO3− loading into the root phloem for downward transport in the roots.
Carbon (C) skeletons are essential for the assimilation of inorganic N into amino acids, proteins and nucleic acids. Thus, N uptake is highly integrated with the availability of sugars (Ruffel et al. 2014). In contrast, N-deficiency negatively affects photosynthetic output, which can be recovered by N re-supply (Coruzzi and Bush 2001). On the other hand, the assimilation of NO3− is a highly energy-consuming process (Bloom 2015). Thus, increasing C supply promotes NO3– uptake and assimilation and vice versa (Cross et al. 2006; Weigelt et al. 2009; Schofield et al. 2009). The C : N ratio also affects NO3– root-to-shoot transportation. For example, under low soil NO3– level, root C : N ratio is high, which creates a condition to assimilate most of the NO3– into amino acid for synthesising proteins in the root, thereby stimulating root growth and the initiation of lateral roots (LR), but with little NO3– delivered to the shoot (Zheng 2009). As soil NO3– increases or root C reduces, unassimilated NO3– could be transported to the shoot through xylem, stimulating shoot growth (Takei et al. 2001, 2002). Similarly, high leaf N promotes shoot development and its sink strength. Under this scenario, the photosynthetic C tends to be retained in the shoot with less C exported to roots. In contrast, low leaf N limits shoot growth and more C is likely to be translocated to the root (Bloom et al. 1993). The exact NO3– level and root C : N ratio below or above which plant growth may be affected vary from species to species since different species have different normal C : N ratio. For example, the root C : N ratio of canola, mustard and wheat is ~44, which is higher than chickpea and pea with their root C : N ratio being 33 and 18 respectively (Gan et al. 2011). The low C : N ratio of the latter two legume species may reflect their ability of N2-fixation. Interestingly, survey from three oilseed, three pulse crops and spring wheat revealed that root C : N ratio ranged from 17 to 75, higher than that in straw (shoot) of 14–55 and seed of 6–17 (Gan et al. 2011).
At the molecular level, the balance between C and N affects the expression of more than half of Arabidopsis genes (Palenchar et al. 2004; Gutiérrez et al. 2007), indicating a sophisticated C : N response network. Research in Arabidopsis roots has identified three candidates which probably sense and respond to C : N status. A putative glutamate receptor AtGLR1.1 was first reported as a sensor of intracellular Suc and NO3– status in Arabidopsis. This proposition was based on the following observations: (i) AtGLR1.1-deficient (antiArGLR1.1) seed failed to germination in the presence of Suc, but not Glc, mannitol or sorbitol, while the germination could be restored upon co-incubation with NO3–, but not NH4+; (ii) the antiArGLR1.1 seedlings exhibited a conditional phenotype that was sensitive to the C : N ratio (Kang and Turano 2003). Moreover, a putative methyltransferase AtOSU1 was identified as a critical modulator in balancing C and N nutrient response in Arabidopsis (Gao et al. 2008). The Atosu1 mutant was more sensitive than the wild type to both low C/high N and high C/low N conditions, of which the AtOSU1 mutation strongly upregulated the expression of Asn synthetase isoform 1 (AtASN1) and MYB75 transcription factor in response to low C/high N and high C/low N respectively (Gao et al. 2008).
The nitrate transporter AtNRT2.1 was also proposed to act either as a high C/low N sensor or signal transducer to co-ordinate the development of the root system with nutritional cues (Little et al. 2005). Several molecular components have been identified to regulate AtNRT2.1 expression. A dual affinity NO3− transporter AtNTR1.1 was shown to act as a NO3− transceptor (transporter/sensor, Gojon A et al. 2011), required for both local induction (short term) and repression (long term) of a high-affinity nitrate transporter gene AtNRT2.1 (Muños et al. 2004; Krouk et al. 2006; Ho et al. 2009). The phosphorylation of AtNRT1.1 by CBL-interacting protein kinase AtCIPK23 switches AtNRT1.1 to the high-affinity function leading to a weak induction of AtNRT2.1 (Ho et al. 2009; Parker and Newstead 2014; Sun et al. 2014). Another CBL-interacting protein kinase AtCIPK8 (Hu et al. 2009) and a putative transcription factor NIN-Like Protein 7 (AtNLP7, Castaings et al. 2009; Marchive et al. 2013) were also found to be involved in the induction of AtNRT2.1 expression by NO3−. In addition, Arabidopsis high nitrogen-insensitive 9 (AtHNI9)/ INTERACT WITH SPT6 (AtIWS1), an evolutionarily-conserved component of the RNA polymerase II complex, acts in roots to repress AtNRT2.1 transcription in response to high N supply (Girin et al. 2010; Widiez et al. 2011). Furthermore, the transcription factors AtLBD37/38/39 seem to mimic the effect of organic N compounds at high N status, and act as a long-distance N signalling to downregulate nitrate transport AtNTR2.1 (Rubin et al. 2009). These findings indicate a mechanism of managing whole-plant level NO3− (for recent reviews see Krapp et al. 2014; Ruffel et al. 2014; Bloom 2015; Krapp 2015).
de Jong and co-workers (2014) demonstrated in Arabidopsis that Glc is coupled to nitrate uptake and assimilation through hexose kinase1(HXK1)-mediated oxidative pentose phosphate pathway (OPPP). The interaction regulates the expression and activity of NO3– transporter NRT2.1 from transcriptional to post-translational levels. Reda (2015) reported Suc signalling from shoot enhances the nitrate reductase (NR) activity in Arabidopsis root by inducing NIA genes (encoding NR) expression and NR activation state, and probably through an HXK1-independent pathway.
Regulatory proteins and hormonal pathways involved in balancing shoot–root C : N status
Along with the root-shoot-root N–C signal circuit as outlined above, other signalling components are also involved in modulating C and N metabolism and allocation. To this end, SnRK1 (SNF1-related protein kinase-1) is a plant protein kinase activated by low levels of Glc, but inhibited by glucose 6-phosphate, trehalose-6-phosphate and Suc (Toroser et al. 2000; Halford et al. 2003; Zhang et al. 2009; Ruan 2014). In addition to participating carbon allocation by modulation gene expression of Sus, ADP glucose pyrophosphorylase (AGPase) and α-amylase (Laurie et al. 2003; McKibbin et al. 2006), SnRK1-mediated signalling was also revealed to regulate nitrogen assimilation and amino acid biosynthesis through phosphorylation and inactivation of NR, and regulation of asparagine synthase gene expression (Baena-González et al. 2007). These findings indicate the potential role of SnRK1 in C–N balancing.
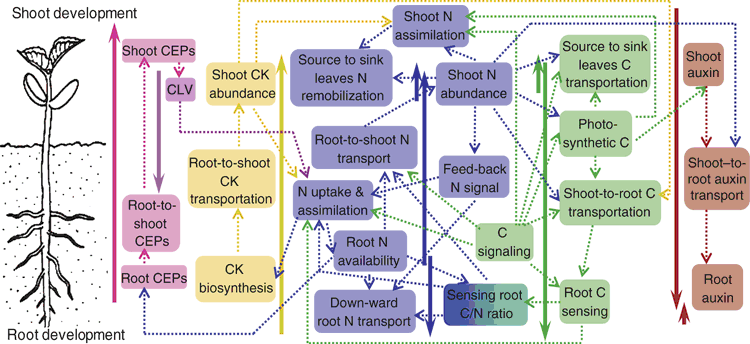
Plant 14-3-3 proteins could also act as regulators of carbon and nitrogen metabolism through direct interaction with many essential enzymes such as the plasma membrane H+-ATPase, sucrose phosphate synthase, ADP-glucose pyrophosphorylase, NR, and glutamine synthase (Chung et al. 1999) and their overall control over the TCA cycle (Diaz et al. 2011). Overexpression of 14-3-3 protein in Arabidopsis results in hypersensitity to C–N stress conditions, which could further be aggravated by the absence of its upstream regulator ubiquitin ligases ATL31 (Sato et al. 2011). The observations suggest that ATL31 regulates C–N response by degrading the 14-3-3 proteins. Finally, Dof1 (DNA binding with one finger), a gene expression activator for organic acid metabolism, has been shown to improve N assimilation under low-nitrogen condition in Arabidopsis. Overexpression of Dof1 was accompanied with reduced Glc level and increased gene expressions responsible for carbon-skeleton production, required for amino acid biosynthesis (Yanagisawa et al. 2004). Similarly, introduction of a maize orthologue, ZmDof1, into rice also enhanced N and C assimilations under N-deficiency (Kurai et al. 2011). Together, a root–shoot-root signal circuit in sensing C–N status is equipped with a cohort of regulatory proteins to achieve C–N balancing at the whole-plant level (Fig. 1).
In parallel to regulatory proteins involved, plants also recruit hormone signals for root–shoot communications with respect of C–N status. Root cytokinkins (CKs), for example, are transported acropetally in the xylem sap to increase the expression of N-uptake/assimilation genes in both shoot and root, and to promote lateral root (LR) development (Takei et al. 2001; Kiba et al. 2011). Reciprocally, the supply of NO3– induces CK biosynthesis (Kiba et al. 2011). The synergistic NO3–-CK relay provides a positive feedback system contributing to N uptake and assimilation (Ruffel et al. 2011) and allows the root to actively ‘forage’ soil NO3– by increasing lateral root formation in N-limited conditions (Ruffel et al. 2011). We note that CK deficiency in the Arabidopsis cytokinin oxidase (CKX) overexpressing plants leads to high root to shoot ratio, largely owing to stunted shoot growth with enhanced root system, a phenomenon probably reflecting a shift of carbon allocation from shoot to root (Werner et al. 2003, 2008). The findings suggest a linkage between N–CK and C–N loop under N-limitation condition: CK-deficiency signal may trigger more C allocated from shoot to root whereby promoting root growth. The N–CK positive feedback loop then kicks in to strengthen root N uptake and assimilation, and to induce CK biosynthesis. The increased CK, once translocated to the shoot, could shift root-over-shoot growth back to a balanced shoot–root development (Fig. 1).
Similar to CK, auxin also exerts sophisticate interactions with both N and sugar signalling pathways (Ljung et al. 2015). Auxin was found to be a positive regulator for NO3– accumulation in leaves. Treatment with exogenous auxin or introduction of the auxin overproducing mutation in Arabidopsis resulted in a strong increase in the transcription of AtNRT1.1, a dual-affinity NO3– transporter/sensor, in root tips (Guo et al. 2002). N-deficiency trends to trigger auxin translocated from shoot to root (Caba et al. 2000; Krouk et al. 2010), which could promote root growth and LR formation (Giehl and von Wirén 2014). Further, AtNRT1.1 not only transports NO3– but also facilitates auxin translocation from shoot to root when Arabidopsis seedlings were grown in medium containing less than 0.2 mM NO3– (Krouk et al. 2010). Together with strong upregulation of IAA biosynthesis by soluble sugars (LeClere et al. 2010; Lilley et al. 2012; Sairanen et al. 2012), the basipetal allocation of auxin probably function as a shoot-to-root feedback signal to stimulate root development under low N condition in accompany with C flow from shoots (Fig. 1). Further addition to the shoot-root bio-directional C–N signalling loop comes from the recently identified xylem signalling peptides C-TERMINALLY ENCODED PEPTIDE (CEP) and their receptor protein CLAVATAV (CLV) in phloem. Tabata et al. (2014) identified two CEP1 receptors in Arabidopsis, XIP1/CEPR1 and CEPR2. Application of CEP1 peptides into roots resulted in upregulation of NRT2.1, in a shoot CEPRs-dependent manner. The findings suggest a root–shoot-root CEP/CLV signalling pathway in promoting N uptake. Together, a multi-layer global signalling circuit appears to have been employed by plants to balance C–N status and co-ordinate root–shoot development (Fig. 1).
Concluding Remarks
Significant progress has been made over the last several decades on roles of C allocation and sugar signalling in shoot-root communication and development. Plants have clearly evolved sophisticated mechanisms to manage the allocation of C, N and other nutrients between shoot and root through sugar signalling and other interacting pathways. The advances in genomic and molecular tools including large scale data analyses have provided great opportunities to study the systemic sugar signalling network along with the roles of sugars as building blocks and energy resource. Among various challenges, tracing the spatial-temporal changes of sugars at the cellular and subcellular levels remains essential for better understating shoot-root communication. New technologies such as biosensors and magnetic resonance imaging (MRI) have opened up new windows to tackle this challenge. The former are genetically encoded fluorescent protein-based sensors for ion and metabolites including Suc and Glc, allowing in vivo visualisation of the spatial and temporal dynamics of sugars and other small molecules at the cellular and subcellular levels (e.g. Jones et al. 2013). The MRI permits non-destructive monitoring of sap flow through phloem and xylem at the whole-plant level (e.g. Windt et al. 2009). Application of these techniques together with other molecular and analytical tools will undoubtedly deepen and broaden our understanding on roles of sugars in co-ordinating shoot–root nutrient allocation and development.
Acknowledgement
We gratefully acknowledge funding support from Australia Research Council (ARC DP110104931 and DP120104148).
References
Améglio T, Decourteix M, Alves G, Valentin V, Sakr S, Julien JL, Petel G, Guilliot A, Lacointe A (2004) Temperature effects on xylem sap osmolarity in walnut trees: evidence for a vitalistic model of winter embolism repair. Tree Physiology 24, 785–793.| Temperature effects on xylem sap osmolarity in walnut trees: evidence for a vitalistic model of winter embolism repair.Crossref | GoogleScholarGoogle Scholar | 15123450PubMed |
Andrews M (1986) The partitioning of nitrate assimilation between root and shoot of higher plants. Plant, Cell & Environment 9, 511–519.
Apelt F, Breuer D, Nikoloski Z, Stitt M, Kragler F (2015) Phytotyping (4D): a light-field imaging system for non-invasive and accurate monitoring of spatio-temporal plant growth. The Plant Journal 82, 693–706.
| Phytotyping (4D): a light-field imaging system for non-invasive and accurate monitoring of spatio-temporal plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXotVWntb0%3D&md5=7a0d0135870b203cb449644cc64e8255CAS | 25801304PubMed |
Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942.
| A central integrator of transcription networks in plant stress and energy signalling.Crossref | GoogleScholarGoogle Scholar | 17671505PubMed |
Bailey-Serres J, Voesenek LA (2010) Life in the balance: a signaling network controlling survival of flooding. Current Opinion in Plant Biology 13, 489–494.
| Life in the balance: a signaling network controlling survival of flooding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKls7%2FM&md5=8a71c0e56cbe4945f48044b3fe10f0a3CAS | 20813578PubMed |
Bates LM, Hall AE (1981) Stomatal closure with soil water depletion not associated with changes in bulk leaf water status. Oecologia 50, 62–65.
| Stomatal closure with soil water depletion not associated with changes in bulk leaf water status.Crossref | GoogleScholarGoogle Scholar |
Bloom AJ (2015) The increasing importance of distinguishing among plant nitrogen sources. Current Opinion in Plant Biology 25, 10–16.
| The increasing importance of distinguishing among plant nitrogen sources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmsFaqs7o%3D&md5=d03b4f7e9ff96136b2685f972ff1a38dCAS | 25899331PubMed |
Bloom AJ, Jackson IE, Smart DR (1993) Root growth as a function of ammonium and nitrate in the root zone. Plant, Cell & Environment 16, 199–206.
| Root growth as a function of ammonium and nitrate in the root zone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkvFCms70%3D&md5=28f818667face306c107af93ee4e9517CAS |
Booker KS, Schwarz J, Garrett MB, Jones AM (2010) Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by heterotrimetic G protein complex. PLoS One 5, e12833
| Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by heterotrimetic G protein complex.Crossref | GoogleScholarGoogle Scholar | 20862254PubMed |
Brauner K, Hörmiller I, Nägele T, Heyer A (2014) Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana. The Plant Journal 79, 82–91.
| Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVahtb3E&md5=2934ee436f4219b2efba97e8da8f09dbCAS | 24836712PubMed |
Caba JM, Centeno ML, Fernandez B, Gresshoff PM, Ligero F (2000) Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta 211, 98–104.
| Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjvVyhsLk%3D&md5=d65ddb299cbb172a4556311c580381bfCAS | 10923709PubMed |
Castaings L, Camargo A, Pocholle D, Gaudon V, Texier Y, Boutet-Mercey S, Taconnal L, Renou J-P, Daniel-Vedele F, Fernandez E, Meyer C, Krapp A (2009) The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. The Plant Journal 57, 426–435.
| The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Kitb4%3D&md5=a0e6fec493a512d708d56f96a499f947CAS | 18826430PubMed |
Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB (2015) Transport of sugars. Annual Review of Biochemistry 84, 865–894.
| Transport of sugars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVyrt7%2FO&md5=593191b6a8855fc3163d6102f6414cc4CAS | 25747398PubMed |
Chung HJ, Sehnke PC, Ferl RJ (1999) The 14-3-3 proteins: cellular regulators of plant metabolism. Trends in Plant Science 4, 367–371.
| The 14-3-3 proteins: cellular regulators of plant metabolism.Crossref | GoogleScholarGoogle Scholar | 10462770PubMed |
Considine MJ, Foyer CH (2014) Redox regulation of plant development. Antioxidants & Redox Signalling 21, 1305–1326.
| Redox regulation of plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsV2ltrzK&md5=af747e6d525552802d529773576182deCAS |
Coruzzi G, Bush DR (2001) Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiology 125, 61–64.
| Nitrogen and carbon nutrient and metabolite signaling in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjslymtL8%3D&md5=6d4edf2d278ce07c752871952d568525CAS | 11154297PubMed |
Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiology 142, 1574–1588.
| Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCns7vL&md5=ac5f15d0463a9f9c2e1213fdfa907965CAS | 17085515PubMed |
de Jong F, Thodey K, Lejay LV, Bevan MW (2014) Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPORTER2.1 expression. Plant Physiology 164, 308–320.
| Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPORTER2.1 expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks1Cqt7c%3D&md5=5e85b1bbe7af49ab5d1e31121c94196cCAS | 24272701PubMed |
Diaz C, Kusano M, Sulpice R, Araki M, Redestig H, Saito K, Stitt M, Shin R (2011) Determining novel functions of Arabidopsis 14-3-3 proteins in central metabolic processes. BMC Systems Biology 5, 192–201.
| Determining novel functions of Arabidopsis 14-3-3 proteins in central metabolic processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisV2nsrc%3D&md5=2be610ee417c1a02af67a1ae14c5991aCAS | 22104211PubMed |
Dodd IC (2005) Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil 274, 251–270.
| Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWiurfM&md5=ca37afd3be77db737f85b9f1b8bacdb6CAS |
Eveland AL, Jackson DP (2012) Sugars, signaling, and plant development. Journal of Experimental Botany 63, 3367–3377.
| Sugars, signaling, and plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVSitbw%3D&md5=70ec813f05d1ad8cf0a07d36ddd880f0CAS | 22140246PubMed |
Fan SC, Lin CS, Hsu PK, Lin SH, Tsay YF (2009) The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. The Plant Cell 21, 2750–2761.
| The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVejtbfE&md5=4312f97c6cf9347f2301ee27180ef4faCAS | 19734434PubMed |
Farrar JF, Minchin PEH (1991) Carbon partitioning in split root systems of barley: relation to metabolism. Journal of Experimental Botany 42, 1261–1269.
| Carbon partitioning in split root systems of barley: relation to metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhvVCnsQ%3D%3D&md5=1d59b4a2e724ba5ab8d95f9fa98085e1CAS |
Fettke J, Fernie AR (2015) Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism. Trends in Plant Science
| Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism.Crossref | GoogleScholarGoogle Scholar | 26008154PubMed |
Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology 53, 203–224.
| Local and long-range signaling pathways regulating plant responses to nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVWhtbk%3D&md5=edbc37634c3636321bfe2769581f9489CAS | 12221973PubMed |
Fry SC, Aldington S, Hetherington PR, Aitken J (1993) Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiology 103, 1–5.
| Oligosaccharides as signals and substrates in the plant cell wall.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksFar&md5=11e7b51d7b60160bebdb6b69485b716bCAS | 8208845PubMed |
Gan YT, Liang BC, Liu LP, Wang X, McDonald CL (2011) C : N ratios and carbon distribution profile across rooting zones in oilseed and pulse crops. Crop and Pasture Science 62, 496–503.
| C : N ratios and carbon distribution profile across rooting zones in oilseed and pulse crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosFCgsLs%3D&md5=2356799ef3dde8bc286febab514b0ebfCAS |
Gao P, Xin Z, Zheng ZL (2008) The OSU1/QUA2/TSD2-encoded putative methyltransferase is a critical modulator of carbon and nitrogen nutrient balance response in Arabidopsis. PLoS One 3, e1387
| The OSU1/QUA2/TSD2-encoded putative methyltransferase is a critical modulator of carbon and nitrogen nutrient balance response in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 18167546PubMed |
Giehl RF, von Wirén N (2014) Root nutrient foraging. Plant Physiology 166, 509–517.
| Root nutrient foraging.Crossref | GoogleScholarGoogle Scholar | 25082891PubMed |
Girin T, El-Kafafi el-S, Widiez T, Erban A, Hubberten HM, Kopka J, Hoefgen R, Gojon A, Lepetit M (2010) Identification of Arabidopsis mutants impaired in the systemic regulation of root nitrate uptake by the nitrogen status of the plant. Plant Physiology 153, 1250–1260.
| Identification of Arabidopsis mutants impaired in the systemic regulation of root nitrate uptake by the nitrogen status of the plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsFejtr0%3D&md5=d81d57e90df3a912e72deed5c1660669CAS | 20448103PubMed |
Gojon A, Krouk G, Perrine-Walker F, Laugier E (2011) Nitrate transceptor(s) in plants. Journal of Experimental Botany 62, 2299–2308.
| Nitrate transceptor(s) in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlt1CltLY%3D&md5=60df1f8a5cd294798263d4ea7d4d1f9fCAS | 21239382PubMed |
Guo FQ, Wang R, Crawford NM (2002) The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots. Journal of Experimental Botany 53, 835–844.
| The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFSntLk%3D&md5=5314a8ab9888d51f35f8791142c891e8CAS | 11912226PubMed |
Gutiérrez RA, Lejay LV, Dean A, Chiaromonte F, Shasha DE, Coruzzi GM (2007) Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biology 8, R7
| Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 17217541PubMed |
Halford NG, Hey S, Jhurreea D, Laurie S, McKibbin RS, Paul M, Zhang Y (2003) Metabolic signalling and carbon partitioning: role of Snf1-related (SnRK1) protein kinase. Journal of Experimental Botany 54, 467–475.
| Metabolic signalling and carbon partitioning: role of Snf1-related (SnRK1) protein kinase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFKgtb4%3D&md5=cdf971d85f34742892fa6b38f7a2f541CAS | 12508057PubMed |
Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biology 8, 389–396.
| Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosFahtbY%3D&md5=506b04eb77ec73ebcebdde1f83103910CAS | 16807832PubMed |
Hedrich R, Sauer N, Neuhaus HE (2015) Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins. Current Opinion in Plant Biology 25, 63–70.
| Sugar transport across the plant vacuolar membrane: nature and regulation of carrier proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXotlWktr8%3D&md5=232c505a2f6ac7e416169d8cc7adfceeCAS | 26000864PubMed |
Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194.
| CHL1 functions as a nitrate sensor in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVyrsbvF&md5=e4ddfe975a458f023447e8f16e59f14bCAS | 19766570PubMed |
Hsu PK, Tsay YF (2013) Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem-borne nitrate to enhance plant growth. Plant Physiology 163, 844–856.
| Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem-borne nitrate to enhance plant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1OjsL3K&md5=76b72b236a859e12267d90adefbb457dCAS | 24006285PubMed |
Hu HC, Wang YY, Tsay YF (2009) AtCIPK8, a CBL-interacting protein kinase regulates the low-affinity phase of the primary nitrate response. The Plant Journal 57, 264–278.
| AtCIPK8, a CBL-interacting protein kinase regulates the low-affinity phase of the primary nitrate response.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1SgtLg%3D&md5=e40caf4d43c181824fe32f42164e6944CAS | 18798873PubMed |
Ishihara H, Obata T, Sulpice R, Fernie AR, Stitt M (2015) Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein. Plant Physiology 168, 74–93.
| Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFSiu7w%3D&md5=0bafdca161b5f683e8c04d0f998b78d8CAS | 25810096PubMed |
Iwai H, Usui M, Hoshino H, Kamada H, Matsunage T, Kakegawa K, Ishii T, Satoh S (2003) Analysis of sugars in squash xylem sap. Plant & Cell Physiology 44, 582–587.
| Analysis of sugars in squash xylem sap.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFSjsL0%3D&md5=0d931832cec3e433a338c6369f985130CAS |
John M, Rohrig H, Schmidt J, Walden R, Schell J (1997) Cell signaling by oligosaccharides. Trends in Plant Science 2, 111–115.
| Cell signaling by oligosaccharides.Crossref | GoogleScholarGoogle Scholar |
Jones AM, Grossmann G, Danielson JÅ, Sosso D, Chen LQ, Ho CH, Frommer WB (2013) In vivo biochemistry: applications for small molecule biosensors in plant biology. Current Opinion in Plant Biology 16, 389–395.
| In vivo biochemistry: applications for small molecule biosensors in plant biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmt1Onu7Y%3D&md5=a4da12b5d7f94f597ce9ee291ed1b9f5CAS | 23587939PubMed |
Kang J, Turano FJ (2003) The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 100, 6872–6877.
| The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktlygt74%3D&md5=4a401220642459f124e72aefad2769e3CAS | 12738881PubMed |
Kiba T, Kudo T, Kojima M, Sakakibara H (2011) Hormonal control of nitrogen acquisition: roles of auxin, abscissic acid, and cytokinin. Journal of Experimental Botany 62, 1399–1409.
| Hormonal control of nitrogen acquisition: roles of auxin, abscissic acid, and cytokinin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gjtrs%3D&md5=e3789d626b8bcb11b02bedeb08bd6884CAS | 21196475PubMed |
Kircher S, Schopfer P (2012) Photosynthetic sucrose acts as a cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 109, 11217–11221.
| Photosynthetic sucrose acts as a cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1WrurjM&md5=9f9f98c148ab254f18897d668157d010CAS | 22733756PubMed |
Krapp A (2015) Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Current Opinion in Plant Biology 25, 115–122.
| Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpt1Krt7o%3D&md5=d783822c631adb1a97fe5b853564ab78CAS | 26037390PubMed |
Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Méry S, Meyer C, Daniel-Vedele F (2014) Nitrate transport and signalling in Arabidopsis. Journal of Experimental Botany 65, 789–798.
| Nitrate transport and signalling in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXisFOjt78%3D&md5=c14f004982268b5d20662aedeb4dff40CAS | 24532451PubMed |
Krouk G, Tillard P, Gojon A (2006) Regulation of the high-affinity NO3 – uptake system by NRT1.1-mediated NO3 – demand signaling in Arabidopsis. Plant Physiology 142, 1075–1086.
| Regulation of the high-affinity NO3 – uptake system by NRT1.1-mediated NO3 – demand signaling in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1ejurfK&md5=71ad9e4df2019d1514145867f9748913CAS | 16998085PubMed |
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nacry P, Gojon A (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937.
| Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXos1Kqt70%3D&md5=29fbfe7087b7889ef68419a00371427cCAS | 20627075PubMed |
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnology Journal 9, 826–837.
| Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlemtrjM&md5=88a1f57f185f484c9208cf347c059437CAS | 21624033PubMed |
Laurie S, McKibbin RS, Halford NG (2003) Antisense SNF1-related 554 Carbon and nitrogen balancing in plants (SnRK1) protein kinase gene represses transient activity of an α-amylase (α-Amy2) gene promoter in cultured wheat embryos. Journal of Experimental Botany 54, 739–747.
| Antisense SNF1-related 554 Carbon and nitrogen balancing in plants (SnRK1) protein kinase gene represses transient activity of an α-amylase (α-Amy2) gene promoter in cultured wheat embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXit1aru78%3D&md5=49ea8b27f9b0824a95d81b92d067f53cCAS | 12554717PubMed |
LeClere S, Schmelz EA, Chourey PS (2010) Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiology 153, 306–318.
| Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1Ggs7k%3D&md5=9a2c9540a1b101a1255d5fd9ecdf7047CAS | 20237017PubMed |
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Frontiers in Plant Science 4, 272
| Source-to-sink transport of sugar and regulation by environmental factors.Crossref | GoogleScholarGoogle Scholar | 23898339PubMed |
Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL (2012) An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiology 160, 2261–2270.
| An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKmtLzI&md5=1d8b1ca7a2c041828920c4dd5aca6026CAS | 23073695PubMed |
Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proceedings of the National Academy of Sciences of the United States of America 102, 13693–13698.
| The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVygtrzE&md5=1c200bb1cf03a91aaeaa48389549754fCAS | 16157886PubMed |
Liu J, Vance CP (2010) Crucial roles of sucrose and microRNA399 in systemic signaling of P deficiency: a tale of two team players? Plant Signaling & Behavior 5, 1556–1560.
| Crucial roles of sucrose and microRNA399 in systemic signaling of P deficiency: a tale of two team players?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gqtr7M&md5=67ab07b942552aecffd075141c03aa30CAS |
Liu DD, Chao WM, Turgeon R (2012) Transport of sucrose, not hexose, in the phloem. Journal of Experimental Botany 63, 4315–4320.
| Transport of sucrose, not hexose, in the phloem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSlsLvI&md5=cedb97684c7c51694922c8dc67da4747CAS | 22553289PubMed |
Liu Y, Offler CE, Ruan Y-L (2013) Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Frontiers in Plant Science 4, article 282
| Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals.Crossref | GoogleScholarGoogle Scholar |
Ljung K, Nemhauser JL, Perata P (2015) New mechanistic links between sugar and hormone signalling networks. Current Opinion in Plant Biology 25, 130–137.
| New mechanistic links between sugar and hormone signalling networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpt1KrurY%3D&md5=0d7ff72848d6383c2ae38960e2cc8811CAS | 26037392PubMed |
MacDougall AJ, Rigby NM, Need PW, Selvendran RR (1992) Movement and metabolism of oligogalacturonide elicitors in tomato shoots. Planta 188, 566–574.
| Movement and metabolism of oligogalacturonide elicitors in tomato shoots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXls1CqsQ%3D%3D&md5=676c5d26cb7cd06b43e849a35319db4dCAS | 24178390PubMed |
Marchive C, Roudier F, Castaings L, Brehaut V, Blondet E, Colot V, Meyer C, Krapp A (2013) Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nature Communications 4, 1713
| Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants.Crossref | GoogleScholarGoogle Scholar | 23591880PubMed |
Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America 111, 6092–6097.
| Sugar demand, not auxin, is the initial regulator of apical dominance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtlWksbY%3D&md5=0598de89d12f0028c1c79b9ab735ff66CAS | 24711430PubMed |
Matsoukas IG (2014) Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Frontiers in Plant Science 5, 147
McKibbin RS, Muttucumaru N, Paul MJ, Powers SJ, Burrell MM, Coates S, Purcell PC, Tiessen A, Geigenberger P, Halford NG (2006) Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnology Journal 4, 409–418.
| Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XoslKnsLg%3D&md5=1a9a0f8c42b5bc7b8520097da7b4d9d6CAS | 17177806PubMed |
Minchin PEH, McNaughton GS (1987) Xylem transport of recently fixed carbon with lupin. Australian Journal of Plant Physiology 14, 325–329.
Minchin PEH, Thorpe MR, Farrar JF (1994) Short-term control of root: shoot partitioning. Journal of Experimental Botany 45, 615–622.
| Short-term control of root: shoot partitioning.Crossref | GoogleScholarGoogle Scholar |
Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One 4, e4502
| Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development.Crossref | GoogleScholarGoogle Scholar | 19223973PubMed |
Muños S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A (2004) Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. The Plant Cell 16, 2433–2447.
| Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1.Crossref | GoogleScholarGoogle Scholar | 15319483PubMed |
Noiraud N, Maurousset L, Lemoine RM (2001) Transport of polyols in higher plants. Plant Physiology and Biochemistry 39, 717–728.
| Transport of polyols in higher plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvVKqsrc%3D&md5=bf42c35e35e066c4cb61f1955aac886bCAS |
Notaguchi M, Okamoto S (2015) Dynamics of long-distance signaling via plant vascular tissues. Frontiers in Plant Science 6, 161
| Dynamics of long-distance signaling via plant vascular tissues.Crossref | GoogleScholarGoogle Scholar | 25852714PubMed |
Osorio S, Ruan YL, Fernie AR (2014) An update on source-to-sink carbon partitioning in tomato. Frontiers in Plant Science 5, 516
| An update on source-to-sink carbon partitioning in tomato.Crossref | GoogleScholarGoogle Scholar | 25339963PubMed |
Page ER, Liu W, Cerrudo D, Lee EA, Swanton CJ (2011) Shade avoidance influences stress tolerance in maize. Weed Science 59, 326–334.
| Shade avoidance influences stress tolerance in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWqtro%3D&md5=f24ba2168371f52b435949df7a45cb99CAS |
Palenchar PM, Kouranov A, Lejay LV, Coruzzi GM (2004) Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants. Genome Biology 5, R91
| Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants.Crossref | GoogleScholarGoogle Scholar | 15535867PubMed |
Palmer WM, Ru L, Jin Y, Patrick JW, Ruan Y-L (2015) Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements. Molecular Plant 8, 315–328.
| Tomato ovary-to-fruit transition is characterized by a spatial shift of mRNAs for cell wall invertase and its inhibitor with the encoded proteins localized to sieve elements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmtValtrk%3D&md5=77998e34c70afab4fbc242cf382c644bCAS | 25680776PubMed |
Parker JL, Newstead S (2014) Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507, 68–72.
| Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjs1Gntrs%3D&md5=8ead1f3e035d391b61da6b93dcf0748cCAS | 24572366PubMed |
Patrick JW (2013) Fundamentals of phloem transport physiology. In ‘Phloem: molecular cell biology, systemic communication, biotic interactions’. (Eds GA Thompson, AJE van Bel) pp. 30–59. (Wiley-Blackwell Publishing: London)
Pérez-Alfocea F, Ghanem ME, Gómez-Cadenas A, Dodd IC (2011) Omics of root-to-shoot signaling under salt stress and water deficit. OMICS: A Journal of Integrative Biology 15, 893–901.
| Omics of root-to-shoot signaling under salt stress and water deficit.Crossref | GoogleScholarGoogle Scholar | 22136663PubMed |
Puig J, Pauluzzi G, Guiderdoni E, Gantet P (2012) Regulation of shoot and root development through mutual signalling. Molecular Plant 5, 974–983.
| Regulation of shoot and root development through mutual signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlKlu7fJ&md5=643ae6887120f5c7d32e9e6bbde022b6CAS | 22628542PubMed |
Reda M (2015) Response of nitrate reductase activity and NIA genes expression in roots of Arabidopsis hxk1 mutant treated with selected carbon and nitrogen metabolites. Plant Science 230, 51–58.
| Response of nitrate reductase activity and NIA genes expression in roots of Arabidopsis hxk1 mutant treated with selected carbon and nitrogen metabolites.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVGksrbK&md5=b906433b7e552da10c666d539041754eCAS | 25480007PubMed |
Rennie EA, Turgeon R (2009) A comprehensive picture of phloem loading strategies. Proceedings of the National Academy of Sciences of the United States of America 106, 14162–14167.
| A comprehensive picture of phloem loading strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFWksLvL&md5=23d85eea329306fbb2e0cd76d5481a8aCAS | 19666555PubMed |
Ruan Y-L (2012) Signaling roles of sucrose metabolism in plant development. Molecular Plant 5, 763–765.
| Signaling roles of sucrose metabolism in plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSlsb7E&md5=f14fc752b45d6dff4f8565a3e1d7fd9fCAS | 22532605PubMed |
Ruan Y-L (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology 65, 33–67.
| Sucrose metabolism: gateway to diverse carbon use and sugar signaling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFWhtrrI&md5=26a26cfcc7e6e682ba6a62f77f7637e4CAS | 24579990PubMed |
Ruan Y-L, Patrick JW, Brady CJ (1996) The composition of apoplastic fluid recovered from intact developing tomato fruit. Australian Journal of Plant Physiology 23, 9–13.
| The composition of apoplastic fluid recovered from intact developing tomato fruit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhslanur4%3D&md5=9c94a8082be98e0658fd9c83a692aec1CAS |
Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. The Plant Cell 21, 3567–3584.
| Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovVWqsQ%3D%3D&md5=fde842939b34881eb69f0d0f8de07d01CAS | 19933203PubMed |
Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proceedings of the National Academy of Sciences of the United States of America 108, 18524–18529.
| Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFymu7bO&md5=953b28d40d7baa7ffc910ee58a29630fCAS | 22025711PubMed |
Ruffel S, Gojon A, Lejay L (2014) Signal interactions in the regulation of root nitrate uptake. Journal of Experimental Botany 65, 5509–5517.
| Signal interactions in the regulation of root nitrate uptake.Crossref | GoogleScholarGoogle Scholar | 25165146PubMed |
Sairanen I, Novák O, Pencík A, Ikeda Y, Jones B, Sandberg G, Ljung K (2012) Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. The Plant Cell 24, 4907–4916.
| Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXisVKkt78%3D&md5=085cc47c4b0d43d8b328b3d4537f391bCAS | 23209113PubMed |
Salisbury FJ, Hall A, Grierson CS, Halliday KJ (2007) Phytochrome co-ordinates Arabidopsis shoot and root development. The Plant Journal 50, 429–438.
| Phytochrome co-ordinates Arabidopsis shoot and root development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsVWms74%3D&md5=9100bb2081e43cf96e221e505e775bfeCAS | 17419844PubMed |
Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, Dai M, Li J, Gong X, Jaillais Y, Yu X, Traas J, Ruberti I, Wang H, Scheres B, Vernoux T, Xu J (2012) COP1 mediates the co-ordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 139, 3402–3412.
| COP1 mediates the co-ordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFGru7%2FJ&md5=dd8389dfdc880c58430bdab9a0188bfaCAS | 22912415PubMed |
Sato T, Maekawa S, Yasuda S, Domeki Y, Sueyoshi K, Fujiwara M, Fukao Y, Goto DB, Yamaguchi J (2011) Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis. The Plant Journal 68, 137–146.
| Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSis73K&md5=6c8880ec21c6e16ff91af0f39aed6aa8CAS | 21668537PubMed |
Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. Journal of Experimental Botany 52, 1991–1997.
| The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXns1Wkt78%3D&md5=c00e685666f6b039035c7763a899459dCAS | 11559734PubMed |
Schachtman DP, Goodger JQD (2008) Chemical root to shoot signaling under drought. Trends in Plant Science 13, 281–287.
| Chemical root to shoot signaling under drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1artbg%3D&md5=55a831cd311a0f98650a93f677f7a0a6CAS | 18467158PubMed |
Schill V, Hartung W, Orthen B, Weisenseel MH (1996) The xylem sap of maple (Acer platanoides) trees-sap obtained by a novel method shows changes with season and height. Journal of Experimental Botany 47, 123–133.
| The xylem sap of maple (Acer platanoides) trees-sap obtained by a novel method shows changes with season and height.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhtlajtb0%3D&md5=5d72aa1bb092aa85b29eafa45330c04aCAS |
Schofield RA, Bi YM, Kant S, Rothstein SJ (2009) Overexpression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant, Cell & Environment 32, 271–285.
| Overexpression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsVKlsL0%3D&md5=66b4a8485d6778034ca0a7505af5bbc5CAS |
Secchi F, Zwieniecki MA (2011) Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant, Cell & Environment 34, 514–524.
| Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvFOgtr4%3D&md5=63a1fd0ed5979ed1342c4f2008fe2316CAS |
Secchi F, Zwieniecki MA (2012) Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling. Plant Physiology 160, 955–964.
| Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFaksbbF&md5=77386eda5cc2571dc6b8e1ec68ded577CAS | 22837359PubMed |
Shibuya N, Minami E (2001) Oligosaccharide signaling for defense responses in plant. Physiological and Molecular Plant Pathology 59, 223–233.
| Oligosaccharide signaling for defense responses in plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovV2mt78%3D&md5=1e1eb3cce18815c13d6f49799600bc2bCAS |
Singh M, Gupta A, Laxmi A (2014) Glucose control of root growth direction in Arabidopsis thaliana. Journal of Experimental Botany 65, 2981–2993.
| Glucose control of root growth direction in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 24719453PubMed |
Stoll M, Loveys B, Dry P (2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. Journal of Experimental Botany 51, 1627–1634.
| Hormonal changes induced by partial rootzone drying of irrigated grapevine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnt12ju7o%3D&md5=35d3f316c9f237252ec2d6a30827dac4CAS | 11006312PubMed |
Sun J, Bankston JR, Payandeh J, Hinds TR, Zagotta WN, Zheng N (2014) Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 507, 73–77.
| Crystal structure of the plant dual-affinity nitrate transporter NRT1.1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjs1Gnurc%3D&md5=625ba5540f93a3af1e3b2da4874395a3CAS | 24572362PubMed |
Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346.
| Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslChtb3O&md5=f3fa13f73cfc5ab46f6be8366121a023CAS | 25324386PubMed |
Takei K, Sakakibara H, Taniguchi M, Sugiyama T (2001) Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator. Plant & Cell Physiology 42, 85–93.
| Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXns1CntA%3D%3D&md5=7b01a5fdd93484b2510d11d7af9e5963CAS |
Takei K, Takahashi T, Sugiyama T, Yamaya T, Sakakibara H (2002) Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. Journal of Experimental Botany 53, 971–977.
| Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFSnur4%3D&md5=649f1d829dccc19925e7cba2b6973670CAS | 11912239PubMed |
Toroser D, Plaut Z, Huber SC (2000) Regulation of a plant SNF1- related protein kinase by glucose-6-phosphate. Plant Physiology 123, 403–412.
| Regulation of a plant SNF1- related protein kinase by glucose-6-phosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsFemsLs%3D&md5=c3dbf868708003b78f76188c81530824CAS | 10806257PubMed |
Tsai AY, Gazzarrini S (2014) Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: the emerging picture. Frontiers in Plant Science 5, 119
| Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: the emerging picture.Crossref | GoogleScholarGoogle Scholar | 24744765PubMed |
Turgeon R, Wolf S (2009) Phloem transport: cellular pathways and molecular trafficking. Annual Review of Plant Biology 60, 207–221.
| Phloem transport: cellular pathways and molecular trafficking.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFGls7s%3D&md5=4f94106f794c94852791fdcef9c13bb0CAS | 19025382PubMed |
van Bel AJ (2003) Transport phloem: low profile, high impact. Plant Physiology 131, 1509–1510.
van Bel AJE, Hess PH (2008) Hexoses as phloem transport sugars: the end of a dogma? Journal of Experimental Botany 59, 261–272.
| Hexoses as phloem transport sugars: the end of a dogma?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVamtLw%3D&md5=4864f1c7724d1c0dbc6f09f75bee3286CAS |
Wang L, Ruan YL (2013) Regulation of cell division and expansion by sugar and auxin signaling. Frontiers in Plant Science 4, 163
| Regulation of cell division and expansion by sugar and auxin signaling.Crossref | GoogleScholarGoogle Scholar | 23755057PubMed |
Wang YY, Tsay YF (2011) Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. The Plant Cell 23, 1945–1957.
| Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWqsbw%3D&md5=b4c61f5d6de358be0bbd759afae6ababCAS | 21571952PubMed |
Weigelt K, Kuster H, Rutten T, Fait A, Fernie AR, Miersch O, Wasternack C, Emery RJ, Desel C, Hosein F, Müller M, Saalbach I, Weber H (2009) ADP-glucose pyrophosphorylase-deficient pea embryos reveal specific transcriptional and metabolic changes of carbon-nitrogen metabolism and stress responses. Plant Physiology 149, 395–411.
| ADP-glucose pyrophosphorylase-deficient pea embryos reveal specific transcriptional and metabolic changes of carbon-nitrogen metabolism and stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1Wqt7k%3D&md5=73c6a11a41659480eb4c05c1bacaee46CAS | 18987213PubMed |
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550.
| Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpt1Ortbw%3D&md5=682cb695ccc3beb178c741e89254f282CAS | 14555694PubMed |
Werner T, Holst K, Pors Y, Guivarc’h A, Mustroph A, Chriqui D, Grimm B, Schmülling T (2008) Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. Journal of Experimental Botany 59, 2659–2672.
| Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXot1Witr8%3D&md5=933089dca36c05031de86b0d34196783CAS | 18515826PubMed |
Widiez T, El Kafafi el S, Girin T, Berr A, Ruffel S, Krouk G, Vayssières A, Shen W-H, Coruzzi GM, Gojon A, Lepetit M (2011) High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3 – uptake is associated with changes in histone methylation. Proceedings of the National Academy of Sciences of the United States of America 108, 13329–13334.
| High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3 – uptake is associated with changes in histone methylation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVGqsLfL&md5=503f0d40d9d0fc7bb57ec84b01d10b10CAS | 21788519PubMed |
Windt CW, Gerkema E, Van As H (2009) Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study. Plant Physiology 151, 830–842.
| Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSjsr%2FP&md5=05cfe5dd525bf47d1de0ee53618d4980CAS | 19710234PubMed |
Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186.
| Glucose–TOR signalling reprograms the transcriptome and activates meristems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkvFOksbw%3D&md5=60cbb16d70f68b58a66dce4e2b63f6deCAS | 23542588PubMed |
Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T (2004) Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions. Proceedings of the National Academy of Sciences of the United States of America 101, 7833–7838.
| Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXktlOksrk%3D&md5=d6ffc0e3bb305b6452e3fc0747a7dce6CAS | 15136740PubMed |
Yu S, Lian H, Wang JW (2015) Plant development transitions: the role of microRNAs and sugars. Current Opinion in Plant Biology 27, 1–7.
| Plant development transitions: the role of microRNAs and sugars.Crossref | GoogleScholarGoogle Scholar | 26042537PubMed |
Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiology 149, 1860–1871.
| Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXks1Wgu7o%3D&md5=f523131495d839b187e687c6683f9860CAS | 19193861PubMed |
Zheng ZL (2009) Carbon and nitrogen nutrient balance signaling in plants. Plant Signaling & Behavior 4, 584–591.
| Carbon and nitrogen nutrient balance signaling in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGmsbjF&md5=f47574d911bc431eb5ec2380707eff68CAS |