Tissue tolerance: an essential but elusive trait for salt-tolerant crops
Rana Munns A B C G , Richard A. James B , Matthew Gilliham D , Timothy J. Flowers A E and Timothy D. Colmer A FA School of Plant Biology, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B CSIRO Agriculture, GPO Box 1600, Canberra, ACT 2601, Australia.
C ARC Centre of Excellence in Plant Energy Biology, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
D ARC Centre of Excellence in Plant Energy Biology, School of Agriculture, Food and Wine, Waite Research Institute, University of Adelaide, Australia.
E School of Life Sciences, University of Sussex, Falmer, Brighton, BN1 9QG, UK.
F Institute of Agriculture, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
G Corresponding author. Email: rana.munns@uwa.edu.au
Functional Plant Biology 43(12) 1103-1113 https://doi.org/10.1071/FP16187
Submitted: 21 May 2016 Accepted: 20 August 2016 Published: 12 October 2016
Abstract
For a plant to persist in saline soil, osmotic adjustment of all plant cells is essential. The more salt-tolerant species accumulate Na+ and Cl– to concentrations in leaves and roots that are similar to the external solution, thus allowing energy-efficient osmotic adjustment. Adverse effects of Na+ and Cl– on metabolism must be avoided, resulting in a situation known as ‘tissue tolerance’. The strategy of sequestering Na+ and Cl– in vacuoles and keeping concentrations low in the cytoplasm is an important contributor to tissue tolerance. Although there are clear differences between species in the ability to accommodate these ions in their leaves, it remains unknown whether there is genetic variation in this ability within a species. This viewpoint considers the concept of tissue tolerance, and how to measure it. Four conclusions are drawn: (1) osmotic adjustment is inseparable from the trait of tissue tolerance; (2) energy-efficient osmotic adjustment should involve ions and only minimal organic solutes; (3) screening methods should focus on measuring tolerance, not injury; and (4) high-throughput protocols that avoid the need for control plants and multiple Na+ or Cl– measurements should be developed. We present guidelines to identify useful genetic variation in tissue tolerance that can be harnessed for plant breeding of salt tolerance.
Additional keywords: barley, chickpea, chloride, osmoregulation, rice, sodium, wheat.
Introduction
Osmotic adjustment is an essential plant response to a saline soil. The osmotic pressure in every cell must increase in order to maintain turgor, and in every organelle in order to maintain volume. This is achieved by increased accumulation of Na+, Cl– or organic solutes. Halophytic plants can grow in soils with over 500 mM NaCl (in excess of seawater), and tolerate over 500 mM Na+ and Cl– in their tissues (Flowers and Colmer 2008). This must be, at least in part, due to their ability to sequester much of the salt in the cell vacuoles, as there is a limit to which Na+ and Cl– can accumulate in the cytoplasm without an adverse effect on metabolic processes. It is not known what this limit is, because the cytoplasm is made up of many compartments that are difficult to isolate and measure, but for chloroplasts the upper limit is possibly 100–200 mM Na+ (Flowers et al. 2015). The concentration in the cytosol is likely to be considerably less, possibly 10–30 mM (Munns and Tester 2008; Kronzucker and Britto 2011).
Salt-tolerant crop species like barley are able to grow productively in soils with up to 200 mM NaCl in the soil solution (Ayers et al. 1952) and tolerate similar concentrations of Na+ and Cl– in their leaves (e.g. Fricke et al. 1994; Boyer et al. 2008). The ions therefore provide osmotic adjustment with little need for organic solutes except in the cytoplasm.
In contrast, in some of the more salt-sensitive species, Na+ is at much lower concentration than Cl– in the leaves and Cl– contributes more to osmotic adjustment. Examples are grapevine (Walker et al. 2004), bread wheat (Gorham et al. 1990; Husain et al. 2004) and soybean (Ledesma et al. 2016). In chickpea, a particularly salt-sensitive species, Na+ rather than Cl– is the major cause of damage to leaves (Khan et al. 2016). Such findings are consistent with the conclusion that Na+ is potentially a more toxic ion than Cl–. A large portion of osmotic adjustment in some salt-sensitive species occurs with organic solutes that are expensive to synthesise and divert assimilate from growth processes such as cell wall and protein synthesis (Munns and Gilliham 2015). The ‘cost’ of this osmotic adjustment can be seen in the yield reductions in saline soil despite lowered leaf Na+ concentration. Although introduction of Nax2 (Na+-excluding gene, TmHKT1;5-A) into durum wheat increased yield by 25% in saline soil, the yield was still less than in non-saline soil (Munns et al. 2012).
The capacity of tissues to function while containing a high internal Na+ and Cl– concentration is known as ‘tissue tolerance’. A key mechanism is intracellular compartmentation of Na+ and Cl– so that most of the ions are contained in vacuoles and the concentrations in the cytoplasm remains relatively low (Flowers et al. 1977). This mechanism can also be called ‘cellular tolerance’ and defined as the ability of a cell to compartmentalise Na+ and Cl– in vacuoles at concentrations that would be toxic in the cytoplasm. However, as we explain below, other factors are also involved in tissue tolerance.
This viewpoint paper explores the concept of tissue tolerance of Na+ and Cl– in crop plants – the ability of organs and their component cells to maintain function in the presence of elevated tissue Na+ and Cl– concentrations. The aim is to clarify the present understanding of tissue tolerance and to identify methods that allow selection of genotypes with greater ability to tolerate high concentrations of Na+ and Cl– in their leaves, to enable tissue tolerance to be used as a trait in plant breeding for saline soils.
Three inter-related concepts: osmotic adjustment, intracellular compartmentation, and tissue tolerance
Osmotic adjustment
For a plant in saline soil, osmotic adjustment is essential. The osmotic pressure of each cell should increase to match the increase in the osmotic pressure of the soil solution, and be due to an increase of solute content, not loss of water, so turgor and volume are maintained. The increased osmotic pressure could be generated by the synthesis of organic solutes such as sugars that reach concentrations great enough to contribute a significant osmotic pressure, but this would be at the expense of growth as those solutes are no longer available for cell wall and protein synthesis (Yeo 1983; Munns 1988). If Na+ and Cl– were completely excluded, osmotic adjustment in plants growing in a soil solution of 200 mM NaCl would need increases in organic solutes of 400 mM in order to balance the osmotic pressure of the external salinity, and with a simple sugar like glucose (molecular weight 180), the amount needed would make up 36% of the plant dry mass (Munns and Gilliham 2015). The percentage would be almost double this if sucrose (molecular weight 342) were used. It would be impossible for more than half of the plant’s incoming assimilate to be diverted to pools of solutes for osmotic adjustment as normally 60–90% of the photosynthate is respired to produce energy for general maintenance of metabolic functions (Amthor 2000; Jacoby et al. 2011).
Alternatively, the required increase in osmotic pressure could come from Na+ and Cl– taken up from the soil, which demands much less ATP than the synthesis of organic solutes (Greenway and Munns 1983; Yeo 1983; Raven 1985). This energy-efficient strategy is used by halophytes (Flowers and Colmer 2008) and the more salt-tolerant non-halophytes such as barley, where the increase in both Na+ and Cl– concentration in the shoot tissues is similar to the increase in the external medium (Boyer et al. 2008). As explained below, the increases in Na+ and Cl– in the tissue are largely confined to the vacuole, so organic solutes are still needed – but only for osmotic adjustment in the cytoplasm. This is not such a big energy drain on the plant as the volume of the cytoplasm of fully expanded cells is relatively small, in the order of one tenth of the total cell volume. In leaves it is ~2% for epidermal cells (Storey et al. 1983) and parenchyma cells (Hajibagheri et al. 1984), but for cells containing chloroplasts is up to 33% for mesophyll cells of C3 plants (James et al. 2006b) and 56% for bundle sheath cells of C4 plants (Storey et al. 1983).
Intracellular compartmentation
The concept of ‘intracellular compartmentation’ of Na+ and Cl– was established in the 1970s after the finding that enzymes extracted from halophytes were significantly inhibited in vitro when NaCl concentrations increased above 80 mM, yet halophyte leaves had concentrations approximately five times this (reviewed by Flowers et al. 1977). Enzymes extracted from halophytes had a similar response to NaCl in vitro to those extracted from non-halophytes (Greenway and Osmond 1972; Flowers 1972). This led to the consensus that the tolerance to high concentrations of these ions in the tissues was determined by the ability of cells to compartmentalise most of the Na+ and Cl– within the vacuoles, rather than any special tolerance of enzymes in the cytoplasm to high Na+ or Cl–. The osmotic pressure in the cytoplasm would be balanced with organic solutes and K+ (Wyn Jones and Gorham 2002). It was argued that salt-tolerant plants would have low Na+ and Cl– in the cytoplasm and its various organelles and compartments, and so avoid toxicity, and also have low ion concentrations in the cell walls and avoid dehydration. In addition, coping with the formation of reactive oxygen species (ROS) and their removal to minimise oxidative stress (Bose et al. 2014) can also contribute to maintenance of organelle and cell functioning in salinised plants, although ROS are also involved in cellular signalling (Miller et al. 2010).
Tissue tolerance
This concerns the capacity of organs to function while their tissues or cells contain high concentrations of Na+ or Cl–. Observations by Yeo and Flowers (1983) that rice genotypes differed in the severity of chlorosis that was not simply related to differences in tissue concentrations of Na+ resulted in the concept of ‘tissue tolerance’. Intracellular compartmentation is the main component of tissue tolerance, but other mechanisms contribute, as discussed below.
Tissue tolerance – definition and component parts
Our definition of tissue tolerance is the ability of an organ to maintain function in the presence of elevated tissue Na+ and Cl– concentrations. The concentration should be as high as necessary for the purpose of osmotic adjustment, but toxicity in the cytoplasm must be avoided.
Another definition of tissue tolerance could be a relative concept that has a practical application for breeding within a given species: the ability of one genotype to tolerate leaf Na+ and Cl– concentrations above that in another; ‘tolerance’ being defined as functional normality, that is no loss of chlorophyll, no dehydration, and sufficient photosynthetic activity to sustain growth (the growth rate being set by the osmotic stress of the soil solution).
It would be satisfying to have a more precise definition, such as ‘the ability of a cell or tissue to tolerate internal Na+ or Cl– above x mM’, that is, a concentration that is toxic to the cytoplasm, or a specific component of it, and therefore indicates that these ions are preferentially compartmentalised in the vacuole. However, we do not know exactly what this potentially toxic concentration of Na+ or Cl– is (Cheeseman 2013). Increases in tissue Na+ are usually accompanied by decreases in tissue K+, and it is possible that regulation of cytosolic K+ concentrations at an optimum level or ‘homeostasis’ might be critical for tolerance (Shabala and Pottosin 2014). It is also possible that the toxic effect of Na+ might be related to its competition with K+ for K+-requiring enzymes so that the cytoplasmic Na+/K+ ratio might be more significant than the Na+ concentration itself (Shabala and Cuin 2008). We note that some in vitro studies comparing the effects of NaCl and KCl on enzymes involved in cellular metabolism or photosynthesis have found the inhibitory effects of higher concentrations to be identical (e.g. Greenway and Osmond 1972; Osmond and Greenway 1972; Besford and Maw 1976). This means that further work is needed to understand exactly where and how Na+ and Cl– are damaging or toxic.
Component parts are defined as a range of factors acting together that are likely to confer tissue tolerance. These factors are shown in Fig. 1 and can be broadly separated into two main categories; (1) those involved in the transport of Na+ or Cl–; and (2) those involved in maintaining the functional water status of the leaf.
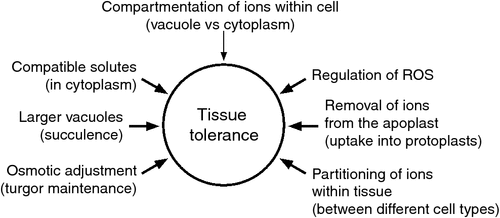
|
Fig. 1 indicates the component parts of tissue tolerance to Na+ and Cl–. These ions can be partitioned between different cell types, and Cl– is often reported to be higher in epidermal cells than mesophyll cells (Conn and Gilliham 2010). In wheat and barley, Na+ was similar in the mesophyll and epidermis; however, K+ accumulated at much higher concentrations in the mesophyll compared with the epidermis so that the K+/Na+ ratio was more favourable in these key cells (James et al. 2006b). Ideally, Na+ and Cl– concentrations should be high in the vacuole as these ions are a ‘cheap’ source of osmotic adjustment, but at the same time be below potentially toxic concentrations in the cytoplasm. Vacuolar compartmentation is required to maintain low cytoplasmic concentrations of Na+ and Cl. Once the vacuolar storage capacity of cells is reached, and these ions are still entering the leaf via the xylem, concentrations in cytoplasmic compartments will increase substantially and approach concentrations that induce cell damage. Alternatively, Na+ and Cl– could build up in the apoplast, leading to cellular dehydration (Oertli 1968; Flowers and Yeo 1986). Preventing oxidative stress from ROS can be a factor when plants are exposed to salinity (Miller et al. 2010).
An increase in succulence (measured as water per unit leaf area) is a common response of dicotyledonous halophytes to external salinity (Polle and Chen 2015). Succulence leads to larger cells with a resultant dilution of salt without an increase in leaf area. Increased succulence also occurs in many dicot non-halophytes. In four species of Brassica, the water per unit leaf area increased by 50–70% when plants were grown in 200 mM NaCl; for example the succulence of canola (Brassica napus L.) increased from 176 to 306 g m–2 (Ashraf and McNeilly 1990). Development of succulence is due to changes in orientation of cellulose fibrils in enlarging cell walls. The cell wall enzymes xyloglucan endotransglucosylase/hydrolases (XTHs) are likely involved in salt-elicited leaf succulence in higher plants (Polle and Chen 2015).
Tissue tolerance is therefore physiologically and genetically complex, a result of the coordinated contribution of a range of components. We now discuss ways to screen for tissue tolerance and how these various approaches might be used in a breeding program.
Genetic variation in tissue tolerance for the purpose of plant breeding
Measurement of genetic variation of salt tolerance in rice (Oryza sativa L.) by Flowers and Yeo (1981) showed that genotypes differed in survival of a period in NaCl that was not simply related to differences in leaf tissue concentrations of Na+. Examination of the possible reasons underlying the variation in tolerance showed that several traits could be involved (Yeo et al. 1990). Apart from a low shoot Na+ accumulation, the other traits were partitioning of salt into older rather than younger leaves, plant vigour and the tolerance to salt within the leaves. This latter trait could be quantified by the rate at which chlorophyll was lost from the leaves with increase in leaf Na+ concentration following salinisation of seedlings and resulted in the concept of ‘tissue tolerance’ (Yeo and Flowers 1983; Fig. 2).
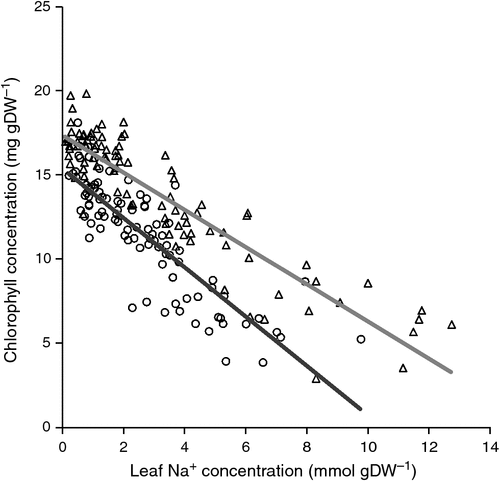
|
Experiments on durum wheat (Munns and James 2003) resulted in similar findings to rice. Fig. 3 compares the effect of salinity on the growth of nine durum genotypes with different rates of Na+ transport to leaves. The figure is drawn with leaf 3 concentration Na+ on the x-axis, and with biomass as the dependent variable, as leaf 3 was the major photosynthesising leaf at that stage of plant development and would be supplying the bulk of assimilate to the growing leaves. In Fig. 3 it can be seen that two durum wheat genotypes had a leaf Na+ concentration of 1.5 mmol g–1 DW (equivalent to 300 mM in tissue water), but one had twice the biomass production of the other (as a % of control) indicating a higher tolerance of Na+ in the leaf tissue. If these data can be replicated in different experiments and in different environments, then there is good grounds for assuming that these two genotypes differ in tissue tolerance.
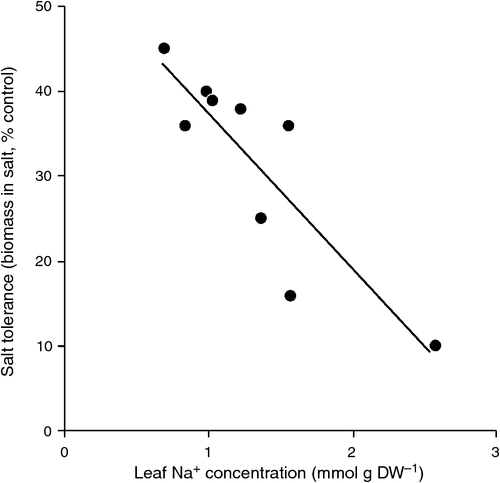
|
Barley also appears to vary in tissue tolerance. A study of 187 Tibetan wild barley (Hordeum vulgare L. ssp. spontaneum) accessions by Qiu et al. (2011) showed a significant correlation (r = 0.455) between Na+ accumulation and salt tolerance (% biomass after 27 days at 300 mM NaCl) but there were many accessions with the same Na+ concentration and with different salt tolerance. A similar finding can be seen in the study by Chen et al. (2005) for seven barley cultivars grown at 320 mM NaCl. This type of analysis indicates that variation in tissue tolerance exists, but does not allow it to be quantified.
Similar to toxicological approaches, and presuming that the chlorosis in rice was a response to (and therefore a useful indicator of) the toxic effect of the Na+ in the tissues, Yeo and Flowers (1983) suggested a tissue tolerance index using the concentration of Na+ in the leaf at which the chlorophyll concentration was reduced by 50%. These authors sought to establish a quantitative screening method to enable quantitative trait loci (QTL) analysis and marker-assisted selection of this trait for breeding salt tolerant rice. Further work showed a five-fold difference in tissue tolerance trait between genotypes. However, it was not correlated positively with survival (Yeo et al. 1990), rather it was a contributory trait, along with other traits such as low leaf Na+ concentration, sequestering Na+ in older rather than younger leaves, and plant vigour, which all contributed to salt tolerance. Only plant vigour was strongly correlated with survival (Yeo et al. 1990).
The concept of tissue tolerance first proposed as a potential trait for rice breeding by Yeo and Flowers (1986) has regrettably not yet progressed to utilisation in plant breeding.
Efforts at quantifying genetic variation in tissue tolerance
A study on genetic variation in salt tolerance of tetraploid wheat (i.e. durum wheat and its relatives) had identified an outstanding landrace, ssp. polonicum (Line 455), with high Na+ concentrations in leaves coupled with better maintenance of green leaf area and biomass production (described in Munns and James 2003). To quantify its tissue tolerance, a method similar to that proposed in rice by Yeo and Flowers (1983) was used to compare Line 455 with the salt-sensitive Australian durum cultivar Wollaroi (James et al. 2002). The results, shown in Fig. 4, indicated that cv Wollaroi had a greater degree of chlorosis for a given Na+ concentration above 1 mmol g DW–1 (above 200 mM on a leaf tissue water basis) than Line 455. The Na+ concentration at 50% loss of chlorophyll was higher in Line 455 than Wollaroi, indicating it had greater tissue tolerance; however, at this stage turgor was very low, and photosynthesis was undetectable. At this stage, increases in Na+ concentration were rapid, having been steady for the previous 17 days (the circled data).
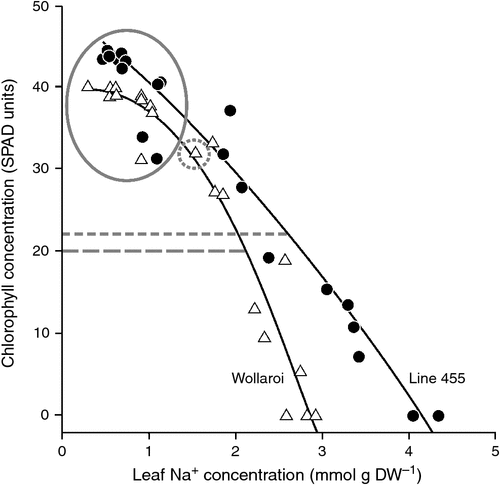
|
The differences between genotypes in leaf Na+ concentration at 50% loss of chlorophyll were relatively small, so follow-up experiments were done to confirm these values. This proved difficult as the relationship between loss of chlorophyll versus tissue Na+ concentration was different for leaves 1, 2 and 3, indicating there was no absolute relationship between the degree of leaf chlorosis and the Na+ concentration in that leaf. Key lessons learnt from these experiments were that measurements should not be made on dying or dead tissues, and that the focus should be on still-healthy functional leaf tissue to determine the highest Na+ concentration tolerated before injury occurs or decline in function such as photosynthetic capacity.
The study also indicated that measurements of injury such as membrane leakage or lipid peroxidation, even at the early onset of tissue damage, would be too late to provide a quantitative measure of tissue tolerance, as would be Na+ measurements after visible injury. Na+ concentrations, which increase rapidly after any loss of chlorophyll (e.g. James et al. 2002), can rise because of increased import (for instance, if the storage capacity of the lower part of the leaf or stem is exceeded), decreased export (if retranslocation processes are significant), or loss of water. Measurements that would predict the time at which injury occurred or when Na+ concentrations might start to escalate were investigated. It was found that chlorophyll concentration (as measured with a SPAD meter) were as good as the chlorophyll fluorescence parameter Fv/Fm in detecting when irreversible damage occurs to photosystem II and that the only fluorescence parameter that predicted this injury was NPQ, a measurement not rapid or feasible for a large number of genotypes (James et al. 2002).
Salinity stress, like other abiotic stresses, is associated with changes in levels of ROS and the various antioxidants that detoxify them or regulate their concentration (Miller et al. 2010). However, such measurements have not yet proved to be of diagnostic use for screening for salt tolerance. For example, consistent correlations with antioxidant activity and salt tolerance across genotypes were not found with barley (Maksimovic et al. 2013) or halophytes (Bose et al. 2014).
Another approach is to look for genetic variation in K+ concentration in leaves of plants in saline conditions, as maintaining the optimal K+ concentration in the cytosol is critical for cell viability. A lower K+ efflux from leaves in the presence of NaCl treatment may indicate a greater ability to maintain a higher K/Na+ ratio in the cytoplasm. A positive correlation was found between the ability of salt-treated leaves to retain K+ and the salt tolerance of whole plants in wheat and barley (Wu et al. 2013).
‘Tissue tolerance’ therefore should be broadly defined as the capacity of the cells or tissues to continue to function without injury despite high internal Na+ or Cl– concentrations, and in the face of a significant osmotic stress caused by the external NaCl concentration in the root zone. Thus, quantification of ‘tissue tolerance’ requires a measure of metabolic or physiological functioning in the face of increasing internal Na+ or Cl– concentrations (high concentrations experienced over a prolonged period of time). As one example, the relationship between photosynthesis and leaf Na+ concentration differed between two chickpea genotypes of known differences in tolerance to saline soil. The salt-sensitive one had a greater reduction in photosynthesis per increase in tissue Na+ (Khan et al. 2016). The same salt-sensitive genotype also suffered a greater reduction in photosynthesis via non-stomatal limitations and greater assessed damage to photosystem II at similar leaf ion concentrations as the more tolerant genotype (Khan et al. 2015), indicating that these two chickpea genotypes differ in leaf tissue tolerance of Na+.
Genes involved in tissue tolerance – can these provide a molecular approach to screening?
At the cellular level tissue tolerance is achieved by compartmentation of Na+ and Cl– in the vacuole, together with the synthesis of compatible solutes and their location within the cytoplasm to balance the osmotic pressure of the ions in the vacuole. Previous reviews have covered the tonoplast-located Na+ and Cl– transporters and the H+-pumps likely to be involved in compartmentation (Hedrich 2012; Shabala 2013). Compatible solutes have been reviewed for halophytes (Slama et al. 2015) and glycophytes (Deinlein et al. 2014; Roy et al. 2014).
Briefly, transporters traditionally thought to be of primary importance in vacuolar Na+ sequestration are tonoplast-localised H+-ATPases and H+-PPiases, which generate the membrane potential and proton motive force needed to drive Na+ uptake into the vacuole via tonoplast-localised Na+/H+ exchangers such as NHX1 and NHX2. The greater ability of halophytes than non-halophytes to sequester Na+ in their vacuoles may be related to the constitutive expression of tonoplast Na+/H+ antiporters and the further stimulation of their activity under saline conditions (Barkla et al. 1995; Glenn et al. 1999), whereas in non-halophytes tonoplast Na+/H+ antiporters are salt-inducible (summarised by Shabala 2013). The activity of Na+/H+ antiporters is energised by the vacuolar H+ pumps, so increased activity of Na+/H+ antiporters should be accompanied by increased activity of tonoplast H+-ATPases or H+-PPiases. This activity, however, would place an additional demand on the ATP (or PPi in the case of the H+-PPiase) pool and on respiratory activity. Knockouts of both H+ pumps and NHX proteins have salt-sensitive phenotypes (Krebs et al. 2010). However, the exact role of NHX proteins in salt tolerance and plant development is unclear. The NHX1 proteins may operate principally as K+ rather than Na+ exchangers (Bassil et al. 2011; Barragan et al. 2012), with their major role being the regulation of cytosolic K+ or pH. Furthermore, transcriptional changes in NHX1 and NHX2 expression were not correlated with salt tolerance in three barley cultivars (Adem et al. 2014), and overexpression of AtNHX1 in barley did not improve its salt tolerance (Adem et al. 2015). This may be because these proteins are regulated post-translationally. It is also perplexing that a quadruple knockout of vacuolar and Golgi NHX proteins in Arabidopsis thaliana (L. Heynh.) had a reduced salt sensitivity, although root growth was slower under non-saline conditions (McCubbin et al. 2014). However, the knockouts of Golgi-localised NHX proteins are salt sensitive and these proteins are suggested to be involved in pH homeostasis of vesicle trafficking compartments (Bassil et al. 2011), and possibly of secretion of Na+ into the vacuole via these (Reguera et al. 2015).
A chloride-cation-cotransporter (CCC) that is present on the Golgi may also provide a mechanism of Na+ and Cl– loading of vesicles: examples have been reported in grapevine and Arabidopsis (Henderson et al. 2015). In rice, a similar transporter (OsCCC1) was found to transport Na+, K+ and Cl– into roots and shoots. It was speculated to be on the plasma membrane, and is thought to have a role in maintaining the osmotic potential of plant cells required for elongation (Chen et al. 2016).
Successful Na+ sequestration into vacuoles requires both efficient loading and retention, i.e. very low back-leakage of Na+, which is of particular importance for halophytes with very high internal Na+ concentrations (Leach et al. 1990). This may be due to slow vacuolar (SV) and fast vacuolar (FV) channels with particularly low rates of leakage (Shabala 2013). These channels were studied in the mesophyll cells of the salt tolerant Chenopodium quinoa Willd. and it was found that the numbers of SV and FV channels differed between different genotypes and correlated with differences in salt tolerance (Bonales-Alatorre et al. 2013). Polyamines and choline, a precursor of the compatible solute glycine betaine, may also play a role in prevention of Na+ leakage out from the SV channel as these compounds in the cytosol inhibit SV channel activity (Pottosin and Shabala 2014; Pottosin et al. 2014). In addition to avoiding possible toxicity in the cytoplasm, prevention of Na+ and Cl− back-leakage would also diminish the need for excessive expenditure of energy to maintain ion compartmentation (Shabala and Mackay 2011).
A study with three barley cultivars with contrasting salt tolerance looked at expression of five candidate genes mentioned above that are considered to be involved in cellular tissue tolerance (Adem et al. 2014). The study confirmed that tissue tolerance was a dominating component of the overall plant responses to salinity. However, it was not possible to infer which cultivars were salinity tolerant based solely on expression profiling of candidate genes at one specific time point.
Many of the genes mentioned above are not transcriptionally regulated by salt, and approaches that have searched for differential transcriptional responses in crop cultivars that differ in salt tolerance have not revealed any clear patterns that could be used to reveal marker genes for tolerance (Roy et al. 2014). So, at present there does not seem to be a reliable or feasible molecular-based method for screening for tissue tolerance, even at the cellular level. The molecular basis of other components of tissue tolerance (Fig. 1) are even more complex.
We now comment on experimental approaches and protocols that are most appropriate for screening genotypes for tissue tolerance, and measurements that can be employed.
Future approaches to measure tissue tolerance
Tissue tolerance is the result of the coordinated contribution of several physiological components (summarised in Fig. 1). It would be beneficial to assess the relative contribution of each of these components to overall tissue tolerance, and their contribution to energy-efficient growth and key yield determinants. However, energy costs associated with complex physiological processes are difficult to measure. A solution would be to measure the overall impact of these processes on net carbon availability for growth and development. Tissue tolerance resulting from effective intracellular compartmentation and osmotic adjustment using Na+ and Cl– rather than organic solutes would increase carbon availability for growth in terms of overall biomass, and for development in terms of more lateral shoots (or tillers in the case of cereals), more fertile florets or flowers, and larger seeds.
Physiological indicators
Physiological indicators of tolerance of a given soil salinity that indicate sustained growth rate and functional integrity are most useful when non-destructive. Stomatal conductance is a non-destructive measurement of leaf health and functional integrity, and measurements of stomatal conductance with a porometer are rapid and reliable (James et al. 2008). Leaf temperature provides a high-throughput alternative to measuring stomatal conductance (Sirault et al. 2009). Modern phenotyping methods using image analysis coupled with physiological measurements such as photosynthesis (fluorescence) and conductance (infrared) were summarised by Walter et al. (2015). These methods are also non-destructive, and can reveal mechanisms when accompanied by specific physiological measurements.
Stomatal conductance is more sensitive to salinity than rates of photosynthesis per unit leaf area, as leaves that develop after the soil becomes saline are smaller in area but considerably thicker with a higher concentration of chlorophyll per unit area, so that photosynthesis per unit area may not decrease (e.g. James et al. 2002). The downside of these measurements of stomatal conductance or CO2 assimilation is that they vary with time of day and with leaf age, as well as ambient conditions, so measurements need to be confined to controlled environments. They must be done on a defined leaf with known-age, and Na+ concentrations must be measured at the same time the functional index is measured.
Turgor maintenance using Na+ and Cl– rather than organic solutes for osmotic adjustment is the successful result of tissue tolerance, and perhaps its best indicator, but measurements of turgor require special expertise in the use of thermocouple psychrometers (Boyer et al. 2008). Osmotic pressure of the leaf sap is a simple measurement and an indicator of osmotic adjustment if normalised for changes in water content of the tissue (e.g. for wheat, Colmer et al. 1995). It should be noted that measurements of ‘relative water content’ for tissues that have undergone osmotic adjustment can lead to underestimates of turgor (Boyer et al. 2008), because of cellular swelling and leakage when leaves from salt-stressed plants are transferred to distilled water. Measurement of the water content of leaves without transfer to distilled water (water : dry weight ratio), avoids this artefact but is subject to diurnal changes in dry weight. Measurement of succulence (water : area ratio), an important adaptation in dicotyledonous species, avoids diurnal changes in DW. However, both water content per unit area and per unit dry weight decrease as a leaf ages under control conditions as well as accelerating under stress, so matching control tissue is needed.
Screening a large number of genetically diverse genotypes with different intrinsic growth rates or early vigour is very challenging and resource-intensive if the protocol relies on comparisons of plants grown under control conditions as well as under salinity stress. The glasshouse or growth cabinet space required to grow control plants under optimum conditions of light and pot size is prohibitive for a large number of genotypes; yet pot size matters and plants with restricted root volume will grow more slowly and have different biomass allocation to roots than those in large pots (Poorter et al. 2012). As much as possible, protocols should avoid the need to compare biomass production of plants in salt versus control conditions, as providing optimum control conditions for the latter is so difficult to achieve in experiments lasting more than several weeks.
Protocols should also avoid the need for repeated tissue Na+ measurements, which again can be time consuming. Ideally they are done only at the point of loss-of-function or incipient damage. However this is hard to predict. A protocol that exposes all genotypes to the same apoplastic Na+ or rate of Na+ influx to leaves would aid assessments of leaf tissue tolerance.
Protocols that minimise the need for Na+ measurements
A novel method involving leaves detached from salt-stressed plants of wheat and barley was proposed by Wu et al. (2015). The plants had been grown at 300 mM NaCl and 50 mM NaCl was fed in dilute nutrient solution to detached leaves over 48 h. The benefit of this method is that all leaves were receiving the same concentration of salt, whereas in intact plants there could be genetic differences in exclusion by the roots which complicates the interpretation of results. Internal Na+ or Cl– concentrations do not need to be measured if transpiration (i.e. salt uptake rates) are the same. However one drawback of this method is that the change in water relations: leaves are no longer under osmotic stress. The osmotic stress is now very low as the tissue is at a water potential the same as the fed solution instead of the saline soil. In barley, this protocol indicated tissue tolerance correlated with whole plant salt tolerance as assessed in saline soil. With wheat, however, the detached leaf injury did not correlate with whole plant injury, probably because the wheat was also grown at 300 mM NaCl which was a lethal treatment and the fed solution of 50 mM was far too high. The method of feeding detached leaves or any part of the shoot should be mindful that the concentration in the xylem to a transpiring leaf is normally less than 10% of the soil solution. In barley it was ~5% of the soil solution (Munns 1985) and less in wheat (James et al. 2006a). Feeding higher concentration may cause salt to build up in the apoplast outside the cells and result in dehydration. This may explain the lack of correlation with wheat.
A second novel method for assessing tissue tolerance as distinct from Na+ exclusion in rice was suggested by the studies on submerged intact seedlings in aerated solution which measured the growth of very young plants at NaCl concentrations up to 200 mM (Kurniasih et al. 2013). Again this has the advantage of all plants being exposed to the same concentration of salt, and that initially at least, internal salt concentrations need not be measured (but to date only one genotype has been studied in this way). An advantage over the detached leaf method is that water relations are as normal, i.e. the osmotic stress has not been relieved. A drawback is that it might be applicable only to submergence-tolerant species such as rice seedlings.
Suggested treatments and measurements
For salt-tolerant species like barley, the treatment should be at least 200 mM NaCl in the root-zone. Osmotic adjustment demands a total of 400 mM solutes in the leaf tissue, and we know that 200 mM Na+ and Cl– in the cytoplasm is toxic, so if the concentration of Na+ or Cl– in the tissue as a whole is 200 mM, there must be intracellular compartmentation of these ions. It would be sufficient to measure some indicator of growth rate or health such as osmotic adjustment, photosynthesis, or chlorophyll (with a SPAD meter), and this could be done over time at one NaCl treatment or at increasing NaCl treatments up to 350 mM NaCl. Na+ measurements could be restricted to selected, contrasting genotypes. For salt-sensitive species, the treatment should be lower than 200 mM, such as 50, 100 or 150 mM NaCl depending on species, e.g. chickpea, rice and wheat respectively.
A given fully-expanded leaf should be measured rather than the shoot as a whole for two reasons. One that Na+ concentration is not uniform across the shoot (Wolf et al. 1991) and the second that other factors (such as the osmotic pressure of the soil solution) may have affected the shoot growth rate and hence the concentration of Na+ in the growing regions, as concentration depends not only on the delivery rate to the shoot in the xylem but is inversely related to the relative growth rate of the shoot. (Note that it is also influenced by the root : shoot ratio and the uptake rate of the root).
An alternative approach is to grow plants for a long period of time at a lower salinity, or for shorter periods in gradually increasing salinity, and measure genotype variation in final biomass, % survival, or degree of injury.
Screening in the field
For genetically complex salt tolerance traits such as tissue tolerance, would screening be carried out most effectively in the field in saline soil using grain yield as the fundamental indicator of salt tolerance? Yield encapsulates the complexity of all the physiological processes involved in tissue tolerance into a quantifiable product that is both recognisable and relevant to breeders. For example, efficient osmotic adjustment through effective cellular and sub-cellular compartmentation of salt ions would result in a greater allocation of carbon to key yield determinants such as number of productive tillers, and grain number and size. Screening in the field for tissue tolerance could be feasible if two key factors were either regulated or measured: phenology and the spatial variability in soil salinity.
Flowering time is a key factor that determines yield of wheat and barley in saline fields in a rain-fed environment where water limits yield (Setter et al. 2016). Genotypes that flower earlier have an advantage through avoiding an increase in soil salinity that typically increases later in the season as the soil dries out. To deal with this issue, breeding populations or germplasm collections for screening for salt tolerance per se would need to be truncated to limited range in flowering time to no more than 1 week, preferably even shorter.
Well characterised field trial sites with appropriate spatial designs and adequate replication are required to account for the spatial heterogeneity of salinity, and also other abiotic and biotic factors that may influence yield. Variation of salinity within the field can be exploited if soil conductivity readings with an electromagnetic induction meter such as EM38 (preferably calibrated against soil cores from the same site) are taken for each plot and used as a covariate in the statistical analysis (James et al. 2012; Setter et al. 2016). Only then can yield differences between genotypes be reliably detected.
Field trials are unpredictable. Unusual climatic patterns can be disastrous or serendipitous. Even with the best agronomic management, unseasonal rain, hail or frost can ruin a trial, as can a severe drought. These issues mean that field trials need replicating at different sites and in different years. However, these issues can throw up unexpected and useful results, as found by Setter et al. (2016). Barley showed the benefit of the tissue tolerance trait, as it yielded more than wheat, which could have been due to its early flowering, but it yielded more in a saline than a dry soil indicating that it could use Na+ and Cl– for osmotic adjustment.
Field validation of putatively tissue-tolerant germplasm selected from controlled environment screening protocols is essential for uptake by breeders and incorporation into breeding programs. Typically, breeders will not incorporate novel traits in supplied germplasm into breeding programs unless there is convincing proof of trait value (improved grain yield relative to current varieties under the appropriate saline field conditions), that the traits are present in well adapted germplasm, and additionally, that there is no yield penalty - that the germplasm yields as well as current cultivars under non-saline conditions.
Concluding remarks
In summary we have suggested that ‘tissue tolerance’ should be broadly defined as the capacity of the cells or tissues to continue to function without injury, with a high internal Na+ and/or Cl– concentration, in the face of a significant osmotic stress caused by the external NaCl concentration in the root zone. Tissue tolerance is physiologically and genetically complex, and so far there is no evidence that any single gene or molecular marker is a quantitative predictor of this complex trait.
What would be the exact benefit of a greater degree of tissue tolerance to a crop like barley? A higher turgor is not expected to make any single shoot grow faster as turgor itself is not limiting the rate of leaf growth (Termaat et al. 1985; Munns et al. 2000). However, a more energy-efficient osmotic adjustment using Na+ and Cl– rather than organic solutes is expected to stimulate an increase in lateral shoot development. It is well known in dry or saline soils that the number of tillers or lateral branches are reduced and recent research has shown that outgrowth of lateral buds is determined by sugar supply (Kebrom et al. 2012; Mason et al. 2014). Another expectation is that plants with greater tissue tolerance will be able to grow in, or at least survive, a higher salinity.
The challenge ahead lies in the development and validation of high throughput methods that can be used to screen germplasm, and can be validated in the field. Only then will useful genetic variation be harnessed for plant breeding.
Acknowledgements
This work was supported through funding by the Australian Research Council to RM and MG (CE140100008 and FT130100709), and by the Grains Research and Development Corporation to RAJ, MG and TDC (UA00145). We thank Hank Greenway and Steve Tyerman for many stimulating discussions. We remember André Läuchli who passed away in January 2015, for his seminal and sustained contributions over decades to the literature on mechanisms of salt tolerance and nutrient interactions, from microscopy to whole-plant physiology, and for being an inspirational colleague and friend. This paper is dedicated to André Läuchli.
References
Adem GD, Roy SJ, Plett DC, Zhou M, Bowman JP, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biology 14, 113| Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley.Crossref | GoogleScholarGoogle Scholar | 24774965PubMed |
Adem GD, Roy SJ, Plett DC, Zhou M, Bowman JP, Shabala S (2015) Expressing AtNHX1 in barley (Hordeum vulgare L.) does not improve plant performance under saline conditions. Plant Growth Regulation 77, 289–297.
| Expressing AtNHX1 in barley (Hordeum vulgare L.) does not improve plant performance under saline conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmt12itL0%3D&md5=b2043f16359a8d71d49cc16584c3db34CAS |
Amthor JS (2000) The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Annals of Botany 86, 1–20.
| The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktlSntL8%3D&md5=98213a0b80a421904fb5cbead41685bfCAS |
Ashraf M, McNeilly T (1990) Responses of four Brassica species to sodium chloride. Environmental and Experimental Botany 30, 475–487.
| Responses of four Brassica species to sodium chloride.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXpvVGlug%3D%3D&md5=94b4c39e9a27d0130f7b964075e2548cCAS |
Ayers AD, Brown JW, Wadleigh CH (1952) Salt tolerance of barley and wheat in soil plots receiving several salinization regimes. Agronomy Journal 44, 307–310.
| Salt tolerance of barley and wheat in soil plots receiving several salinization regimes.Crossref | GoogleScholarGoogle Scholar |
Barkla BJ, Zingarelli L, Blumwald E, Smith JAC (1995) Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiology 109, 549–556.
Barragan V, Leidi EO, Andres Z, Rubio L, De Luca A, Fernandez JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. The Plant Cell 24, 1127–1142.
| Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmsl2jtL4%3D&md5=0386eaab23396a0b8dd28e181caaeb7cCAS | 22438021PubMed |
Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. The Plant Cell 23, 224–239.
| The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFCmsrw%3D&md5=2e0d7b958f204d2cbd717ead7cb87ab1CAS | 21278129PubMed |
Besford RT, Maw GA (1976) Effect of potassium nutrition on some enzymes from tomato plant. Annals of Botany 40, 461–471.
Bonales-Alatorre E, Shabala S, Chen ZH, Pottosin I (2013) Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, Quinoa. Plant Physiology 162, 940–952.
| Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, Quinoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Oqs7g%3D&md5=0b5144f02b96923d169e3d3c9d1a6647CAS | 23624857PubMed |
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context salinity stress tolerance. Journal of Experimental Botany 65, 1241–1257.
| ROS homeostasis in halophytes in the context salinity stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks12htbY%3D&md5=75f259e49c111146271aa61a36df1a47CAS | 24368505PubMed |
Boyer JS, James RA, Munns R, Condon AG, Passioura JB (2008) Osmotic adjustment may lead to anomalously low estimates of relative water content in wheat and barley. Functional Plant Biology 35, 1172–1182.
| Osmotic adjustment may lead to anomalously low estimates of relative water content in wheat and barley.Crossref | GoogleScholarGoogle Scholar |
Cheeseman JM (2013) The integration of activity in saline environments: problems and perspectives. Functional Plant Biology 40, 759–774.
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell & Environment 28, 1230–1246.
| Screening plants for salt tolerance by measuring K+ flux: a case study for barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGitbnE&md5=8de0795123844635e118c78e34e66cecCAS |
Chen ZC, Yamaji N, Fujii-Kashino M, Ma JF (2016) A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiology 171, 494–507.
| A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice.Crossref | GoogleScholarGoogle Scholar | 26983995PubMed |
Colmer TD, Epstein E, Dvorak J (1995) Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat × Lophopyrum elongatum (Host) A. Love amphiploid. Plant Physiology 108, 1715–1724.
Conn S, Gilliham M (2010) Comparative physiology of elemental distribution in plants. Annals of Botany 105, 1081–1102.
| Comparative physiology of elemental distribution in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVOqt74%3D&md5=6b56342ced31d012bc508a5418234999CAS | 20410048PubMed |
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JL (2014) Plant salt-tolerance mechanisms. Trends in Plant Science 19, 371–379.
| Plant salt-tolerance mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXksVWmsb8%3D&md5=0e72eb61fed69684d0e9b09a857d0643CAS | 24630845PubMed |
Flowers TJ (1972) Salt tolerance in Suaeda maritima (L.) Dum. The effect of sodium chloride on growth respiration and soluble enzymes in a comparative study with Pisum sativum L. Journal of Experimental Botany 23, 310–321.
| Salt tolerance in Suaeda maritima (L.) Dum. The effect of sodium chloride on growth respiration and soluble enzymes in a comparative study with Pisum sativum L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XksVGmurY%3D&md5=3945b52846c484ecffbb23bb7c6a741bCAS |
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytologist 179, 945–963.
| Salinity tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFWqur%2FE&md5=ead9e0f52d5a777986f79fa03e42e1abCAS | 18565144PubMed |
Flowers TJ, Yeo AR (1981) Variability of sodium chloride resistance within rice (Oryza sativa L.) varieties. New Phytologist 88, 363–373.
| Variability of sodium chloride resistance within rice (Oryza sativa L.) varieties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXlt12ksL8%3D&md5=07f6f660ac61f4f12e7ba55373004434CAS |
Flowers TJ, Yeo AR (1986) Ion relations of plants under drought and salinity. Australian Journal of Plant Physiology 13, 75–91.
| Ion relations of plants under drought and salinity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XitVeku7g%3D&md5=9d8e84bf15f60ba6ae57adde2fe770a9CAS |
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology 28, 89–121.
| The mechanism of salt tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXksFSisb8%3D&md5=ff9530ae4f1e1531c9e3b6fb259b818cCAS |
Flowers TJ, Colmer TD, Munns R (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Annals of Botany 115, 419–431.
| Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar | 25466549PubMed |
Fricke W, Leigh RA, Tomos AD (1994) Epidermal solute concentrations and osmolality in barley leaves studied at the single-cell level – changes along the leaf blade, during leaf ageing and NaCl stress. Planta 192, 317–323.
| Epidermal solute concentrations and osmolality in barley leaves studied at the single-cell level – changes along the leaf blade, during leaf ageing and NaCl stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhtVajt7Y%3D&md5=f581fac279422bdb83b0e5950383d0b6CAS |
Glenn EP, Brown JJ, Blumwald E (1999) Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences 18, 227–255.
| Salt tolerance and crop potential of halophytes.Crossref | GoogleScholarGoogle Scholar |
Gorham J, Wyn Jones RG, Bristol A (1990) Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta 180, 590–597.
| Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXitFequr4%3D&md5=a0316c00d06fc03d6853d8ebf9f4f0c0CAS | 24202105PubMed |
Greenway H, Munns R (1983) Interactions between growth and uptake of Cl– and Na+, and water relations of plants in saline environments. Plant, Cell & Environment 6, 575–589.
| Interactions between growth and uptake of Cl– and Na+, and water relations of plants in saline environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXitVeltQ%3D%3D&md5=b4840be89b6cb894cd3b315876cb1806CAS |
Greenway H, Osmond CB (1972) Salt responses of enzymes from species differing in salt tolerance. Plant Physiology 49, 256–259.
| Salt responses of enzymes from species differing in salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38Xps1amtg%3D%3D&md5=cef8be26b31d81dbfeff75bdc64bed68CAS | 16657936PubMed |
Hajibagheri MA, Hall JL, Flowers TJ (1984) Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum. Journal of Experimental Botany 35, 1547–1557.
| Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum.Crossref | GoogleScholarGoogle Scholar |
Hedrich R (2012) Ion channels in plants. Physiological Reviews 92, 1777–1811.
| Ion channels in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhsl2lsbnF&md5=323dbe2187f22cff95fc90b8177b6c85CAS | 23073631PubMed |
Henderson SW, Wege S, Qiu J, Blackmore DH, Walker AR, Tyerman SD, Walker RR, Gilliham M (2015) Grapevine and Arabidopsis cation-chloride cotransporters localise to the Golgi and trans-Golgi network and indirectly influence long-distance ion homeostasis and plant salt tolerance. Plant Physiology 169, 2215–2229.
Husain S, von Caemmerer S, Munns R (2004) Control of salt transport from roots to shoots of wheat in saline soil. Functional Plant Biology 31, 1115–1126.
| Control of salt transport from roots to shoots of wheat in saline soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVWjt7fJ&md5=ce9800c4f8e778e8ef08e7684edcb178CAS |
Jacoby RP, Taylor NL, Millar AH (2011) The role of mitochondrial respiration in salinity tolerance. Trends in Plant Science 16, 614–623.
| The role of mitochondrial respiration in salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVaqtLnM&md5=43bb52b3e312ebbcf8d498cd6c5c5551CAS | 21903446PubMed |
James RA, Rivelli AR, Munns R, von Caemmerer S (2002) Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Functional Plant Biology 29, 1393–1403.
| Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovFWhsA%3D%3D&md5=885d4c0f8d5d4ec7c124bc9bce60fc4fCAS |
James RA, Davenport RJ, Munns R (2006a) Physiological characterisation of two genes for Na+ exclusion in durum wheat: Nax1 and Nax2. Plant Physiology 142, 1537–1547.
| Physiological characterisation of two genes for Na+ exclusion in durum wheat: Nax1 and Nax2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCns7vI&md5=24874c800e7fa59e5b8491eb9de532ebCAS | 17028150PubMed |
James RA, Munns R, von Caemmerer S, Trejo C, Miller C, Condon AG (2006b) Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant, Cell & Environment 29, 2185–2197.
| Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVaisA%3D%3D&md5=3f8359f2f3ee6ce9cf6301a981d3f7b8CAS |
James RA, von Caemmerer S, Condon AG, Zwart AB, Munns R (2008) Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat. Functional Plant Biology 35, 111–123.
| Genetic variation in tolerance to the osmotic stress component of salinity stress in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVKktbY%3D&md5=59992d44908cac0c061aadb434fac6e3CAS |
James RA, Blake C, Zwart AB, Hare RA, Rathjen AJ, Munns R (2012) Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Functional Plant Biology 39, 609–618.
| Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVCmtL7N&md5=7e0fab933147882418360922728a2c41CAS |
Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W (2012) Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiology 160, 308–318.
| Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlOmtLzK&md5=39c12262848f922b57fe6ecaea2384bcCAS | 22791303PubMed |
Khan HA, Siddique KHM, Munir R, Colmer TD (2015) Salt sensitivity in chickpea: growth, photosynthesis, seed yield components and tissue ion regulation in contrasting genotypes. Journal of Plant Physiology 182, 1–12.
| Salt sensitivity in chickpea: growth, photosynthesis, seed yield components and tissue ion regulation in contrasting genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXptVSmurk%3D&md5=9d3ae4874475a689be9bb4f5bfdf7b53CAS | 26037693PubMed |
Khan HA, Siddique KHM, Colmer TD (2016) Salt sensitivity in chickpea is determined by sodium toxicity. Planta 244, 623–637.
| Salt sensitivity in chickpea is determined by sodium toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XmslOit7w%3D&md5=12aa5401285ed00f237da0220240dc01CAS | 27114264PubMed |
Krebs M, Beyhl D, Gorlich E, Al-Rasheid KAS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis VATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proceedings of the National Academy of Sciences of the United States of America 107, 3251–3256.
| Arabidopsis VATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Cgurw%3D&md5=db46065ed63941e1c4310cdf015a0df8CAS | 20133698PubMed |
Kronzucker HJ, Britto DT (2011) Sodium transport in plants: a critical review. New Phytologist 189, 54–81.
| Sodium transport in plants: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltlGhug%3D%3D&md5=a025ecb1a12065fcec42b5c5667cfc23CAS | 21118256PubMed |
Kurniasih B, Greenway H, Colmer TD (2013) Tolerance of submerged germinating rice to 50–200 mM NaCl in aerated solution. Physiologia Plantarum 149, 222–233.
| Tolerance of submerged germinating rice to 50–200 mM NaCl in aerated solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslCqtLbK&md5=e93da53f64e04571ea2d9eabc8a24763CAS | 23379468PubMed |
Leach RP, Wheeler KP, Flowers TJ, Yeo AR (1990) Molecular markers for ion compartmentation in cells of higher plants. II Lipid composition of the tonoplast of the halophyte Suaeda maritima (L.) Dum. Journal of Experimental Botany 41, 1089–1094.
| Molecular markers for ion compartmentation in cells of higher plants. II Lipid composition of the tonoplast of the halophyte Suaeda maritima (L.) Dum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtVyns7g%3D&md5=f13c1e3c1d27349b3eb5c30b5f39ff99CAS |
Ledesma F, Lopez C, Ortiz D, Chen P, Korth KL, Ishibashi T, Zeng A, Orazaly M, Florez-Palacios L (2016) Simple greenhouse method for screening salt tolerance in soybean. Crop Science 56, 585–594.
| Simple greenhouse method for screening salt tolerance in soybean.Crossref | GoogleScholarGoogle Scholar |
Maksimovic JD, Zhang JY, Zeng FR, Živanović BD, Shabala L, Zhou MX, Shabala S (2013) Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant and Soil 365, 141–155.
| Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkslantLs%3D&md5=d8618b2f2afff7a4a1b4748d21440febCAS |
Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America 111, 6092–6097.
| Sugar demand, not auxin, is the initial regulator of apical dominance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtlWksbY%3D&md5=ae13739e7a191ad7f64b74e3f58102c8CAS | 24711430PubMed |
McCubbin T, Bassil E, Zhang S, Blumwald E (2014) Vacuolar Na+/H+ NHX-type antiporters are required for cellular K+ homeostasis, microtubule organization and directional root growth. Plants-Basel 3, 409–426.
| Vacuolar Na+/H+ NHX-type antiporters are required for cellular K+ homeostasis, microtubule organization and directional root growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFKnsLbN&md5=1b0164398b85a273dd016e2a54d48e76CAS | 27135511PubMed |
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment 33, 453–467.
| Reactive oxygen species homeostasis and signalling during drought and salinity stresses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltV2hur8%3D&md5=42f2cbcf2143d1c8e9db6fbe95dfa198CAS |
Munns R (1985) Na+, K+ and Cl– in xylem sap flowing to shoots of NaCl-treated barley. Journal of Experimental Botany 36, 1032–1042.
| Na+, K+ and Cl– in xylem sap flowing to shoots of NaCl-treated barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXkvVGisb4%3D&md5=1223fcfa9e5ec7e06dc2d91c63a4bc76CAS |
Munns R (1988) Why measure osmotic adjustment? Australian Journal of Plant Physiology 15, 717–726.
| Why measure osmotic adjustment?Crossref | GoogleScholarGoogle Scholar |
Munns R, Gilliham M (2015) Salinity tolerance of crops – what is the cost? New Phytologist 208, 668–673.
| Salinity tolerance of crops – what is the cost?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs12lsrzF&md5=2f335d2d51fd319d9cf3ddcd06668140CAS | 26108441PubMed |
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil 253, 201–218.
| Screening methods for salinity tolerance: a case study with tetraploid wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVemsbc%3D&md5=7e726238b806bda9e38e996ba14ece64CAS |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=dd68a82a6d2c6414e1266213f98edac3CAS | 18444910PubMed |
Munns R, Guo J, Passioura JB, Cramer GR (2000) Leaf water status controls day-time but not daily rates of leaf expansion in salt-treated barley. Australian Journal of Plant Physiology 27, 949–957.
| Leaf water status controls day-time but not daily rates of leaf expansion in salt-treated barley.Crossref | GoogleScholarGoogle Scholar |
Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D, Gilliham M (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotechnology 30, 360–364.
| Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtlOgu7w%3D&md5=37d8f5975a0ce922abb720ad8cf03d8fCAS | 22407351PubMed |
Oertli JJ (1968) Extracellular salt accumulation, a possible mechanism of salt injury in plants. Agrochimica 12, 461–469.
Osmond CB, Greenway H (1972) Salt responses of carboxylation enzymes from species differing in salt tolerance. Plant Physiology 49, 260–263.
| Salt responses of carboxylation enzymes from species differing in salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38Xot1OntA%3D%3D&md5=6a8b625419b989aa4460c7841c257e1fCAS | 16657937PubMed |
Polle A, Chen S (2015) On the salty side of life: molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats. Plant, Cell & Environment 38, 1794–1816.
| On the salty side of life: molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1yqsLnO&md5=0981fedbbaa58d0782a7d2f2a4200599CAS |
Poorter H, Buehler J, van Dusschoten D, Climent J, Postma JA (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology 39, 839–850.
| Pot size matters: a meta-analysis of the effects of rooting volume on plant growth.Crossref | GoogleScholarGoogle Scholar |
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Frontiers in Plant Science 5, 154
| Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling.Crossref | GoogleScholarGoogle Scholar | 24795739PubMed |
Pottosin I, Bonales-Alatorre E, Shabala S (2014) Choline but not its derivative betaine blocks slow vacuolar channels in the halophyte Chenopodium quinoa: Implications for salinity stress responses. FEBS Letters 588, 3918–3923.
| Choline but not its derivative betaine blocks slow vacuolar channels in the halophyte Chenopodium quinoa: Implications for salinity stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsF2murvP&md5=63ed8c59ffc6f3bb6341f3739ec94becCAS | 25240200PubMed |
Qiu L, Wu D, Ali S, Cai S, Dai F, Jin X, Wu F, Zhang G (2011) Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley. Theoretical and Applied Genetics 122, 695–703.
| Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvVCmurs%3D&md5=fbe7a6fb7c6f1ec9688919f1f7a25c3bCAS | 20981400PubMed |
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular plants – a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytologist 101, 25–77.
| Regulation of pH and generation of osmolarity in vascular plants – a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXmtFWjt7g%3D&md5=149c3f276cef7adc596af8a92bf66928CAS |
Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E (2015) pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. The Plant Cell 27, 1200–1217.
| pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFSjtbc%3D&md5=be123f11e0d2d087a49e144f13350d08CAS | 25829439PubMed |
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Current Opinion in Biotechnology 26, 115–124.
| Salt resistant crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXlt1Cns7c%3D&md5=7f9cbeb695826e06a98f986e76327527CAS | 24679267PubMed |
Setter TL, Waters I, Stefanova K, Munns R, Barrett-Lennard EG (2016) Salt tolerance, date of flowering and rain affect the productivity of wheat and barley on rainfed saline land. Field Crops Research 194, 31–42.
| Salt tolerance, date of flowering and rain affect the productivity of wheat and barley on rainfed saline land.Crossref | GoogleScholarGoogle Scholar |
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany 112, 1209–1221.
| Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops.Crossref | GoogleScholarGoogle Scholar | 24085482PubMed |
Shabala S, Cuin T (2008) Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669.
| Potassium transport and plant salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit70%3D&md5=e3b7086a1c0639a11d71b2068d781bd9CAS | 18724408PubMed |
Shabala S, Mackay A (2011) Ion transport in halophytes. Advances in Botanical Research 57, 151–199.
| Ion transport in halophytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1Sit74%3D&md5=0be98b691971716a3ef0e5235d525e71CAS |
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257–279.
| Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXps1OjtL0%3D&md5=a0acade96ec4a194c130a751673c8ed0CAS | 24506225PubMed |
Sirault XRR, James RA, Furbank RT (2009) A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Functional Plant Biology 36, 970–977.
| A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7rI&md5=e1ddc0bd079fca8d9a056b6b41c68b74CAS |
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Annals of Botany 115, 433–447.
| Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress.Crossref | GoogleScholarGoogle Scholar | 25564467PubMed |
Storey R, Pitman MG, Stelzer R, Carter C (1983) X-ray microanalysis of cells and cell compartments of Atriplex spongiosa. Journal of Experimental Botany 34, 778–794.
| X-ray microanalysis of cells and cell compartments of Atriplex spongiosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXlslart7c%3D&md5=e0557bd2ae8fea77625ef3d86837d98fCAS |
Termaat A, Passioura JB, Munns R (1985) Shoot turgor does not limit shoot growth of NaCl-affected wheat and barley. Plant Physiology 77, 869–872.
| Shoot turgor does not limit shoot growth of NaCl-affected wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXitFymsrs%3D&md5=8db6b000c8e902a7d59877bd879a8a96CAS | 16664152PubMed |
Walker RR, Blackmore DH, Clingeleffer PR, Correll RL (2004) Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) 2. Ion concentrations in leaves and juice. Australian Journal of Grape and Wine Research 10, 90–99.
| Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana) 2. Ion concentrations in leaves and juice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVyku7c%3D&md5=9765a40fe57b3981628002cd2ed9e19bCAS |
Walter A, Liebisch F, Hund A (2015) Plant phenotyping: from bean weighing to image analysis. Plant Methods 11, 14
| Plant phenotyping: from bean weighing to image analysis.Crossref | GoogleScholarGoogle Scholar | 25767559PubMed |
Wolf O, Munns R, Tonnet ML, Jeschke WD (1991) The role of the stem in the partitioning of Na+ and K+ in salt-treated barley. Journal of Experimental Botany 42, 697–704.
| The role of the stem in the partitioning of Na+ and K+ in salt-treated barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXkvV2gur8%3D&md5=f983a97b8cc0f511e570f53c551f03c8CAS |
Wu H, Shabala L, Barry K, Zhou M, Shabala S (2013) Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiologia Plantarum 149, 515–527.
| Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslyhtLnL&md5=1665f79029b20a93683151c26f65a4c2CAS | 23611560PubMed |
Wu H, Shabala L, Zhou M, Stefano G, Pandolfi C, Mancuso S, Shabala S (2015) Developing and validating a high-throughput assay for salinity tissue tolerance in wheat and barley. Planta 242, 847–857.
| Developing and validating a high-throughput assay for salinity tissue tolerance in wheat and barley.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVGls7%2FP&md5=efede3ebb7e196041bbb49f51b02a0fcCAS | 25991439PubMed |
Wyn Jones G, Gorham J (2002) Intra- and inter-cellular compartmentation of ions – a study in specificity and plasticity. In ‘Salinity: environment – plants – molecules’. (Ed. A Lauchli, U Luttge) pp. 159–180. (Springer: Dordrecht, The Netherlands)
Yeo AR (1983) Salinity resistance: physiologies and prices. Physiologia Plantarum 58, 214–222.
| Salinity resistance: physiologies and prices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXktlGqsLw%3D&md5=59d2b07bf963eb2738661517bdcd6d3aCAS |
Yeo AR, Flowers TJ (1983) Varietal differences in the toxicity of sodium ions in rice leaves. Physiologia Plantarum 59, 189–195.
| Varietal differences in the toxicity of sodium ions in rice leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXmtV2qtLg%3D&md5=7b3223acafd3694309fa3f0f2ef691c2CAS |
Yeo AR, Flowers TJ (1986) Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. Australian Journal of Plant Physiology 13, 161–173.
| Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils.Crossref | GoogleScholarGoogle Scholar |
Yeo AR, Yeo ME, Flowers SA, Flowers TJ (1990) Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theoretical and Applied Genetics 79, 377–384.
| Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c7mt1Whug%3D%3D&md5=b55fd772da32967952e08ec886c37ee9CAS | 24226357PubMed |


 ).
).