Phenotyping plants: genes, phenes and machines
Roland Pieruschka A B and Hendrik Poorter AA IBG-2 Plant Sciences, Forschungszentrum Jülich, D-52425, Germany.
B Corresponding author. Email: r.pieruschka@fz-juelich.de
Functional Plant Biology 39(11) 813-820 https://doi.org/10.1071/FPv39n11_IN
Published: 30 October 2012
Abstract
No matter how fascinating the discoveries in the field of molecular biology are, in the end it is the phenotype that matters. In this paper we pay attention to various aspects of plant phenotyping. The challenges to unravel the relationship between genotype and phenotype are discussed, as well as the case where ‘plants do not have a phenotype’. More emphasis has to be placed on automation to match the increased output in the molecular sciences with analysis of relevant traits under laboratory, greenhouse and field conditions. Currently, non-destructive measurements with cameras are becoming widely used to assess plant structural properties, but a wider range of non-invasive approaches and evaluation tools has to be developed to combine physiologically meaningful data with structural information of plants. Another field requiring major progress is the handling and processing of data. A better e-infrastructure will enable easier establishment of links between phenotypic traits and genetic data. In the final part of this paper we briefly introduce the range of contributions that form the core of a special issue of this journal on plant phenotyping.
Introduction
The advent of molecular genetics has drastically altered almost all research areas and applications in the field of biology. One of the fundamental principles in biology is the genotype-phenotype concept, where the genotype is considered to be all of the hereditary information that is accumulated in the individual; and the phenotype can be seen as the combination of all the morphological, physiological, anatomical, chemical, developmental and behavioural characteristics that, when put together, represent the individual organism. This concept, which was first introduced by Johanssen (1909) in his classical book on genetics, is now more than a century old. The concept was further developed by evolutionary biologists and resulted in the theory of ‘genotype-phenotype maps’ (Lewontin 1974; Suzuki et al. 1981). In these maps an individual organism occupies a certain point in the so-called ‘genotypic space’, which represents the full array of genotypes possible for that species. The position in the genotypic space, in combination with the prevailing environmental characteristics, determines the elementary phenotypic state that an organism occupies within the overall space of possible phenotypes. Additionally, an organism can also affect the environment that it or other individuals occupy, thereby creating complex feedback relationships between genes on the one hand and environments and phenotypes on the other hand (Houle 2010).
In day-to-day life, it is the phenotype that is of importance when it comes to food production in crops, health profiles in humans and husbandry or the ecological performance of organisms in nature. Therefore phenotyping – which is the process of characterising the phenotype – is as important as genotyping for establishing the relationship between genes and traits. Such relationships are relatively easy to establish in the case of monogenic traits, such as sickle cell disease. It becomes complicated if the expression is dependent on the environment, particularly in case where complex, quantitative traits are under polygenic control. Such traits are often highly relevant both from a biological and a human perspective, as they relate to growth, performance and resource use efficiencies of organisms. The techniques to investigate these relationships relate to the analysis of dedicated mapping populations, such as in the case of the quantitative trait loci (QTL) approach or in the analysis of a wide range of individuals from existing populations, e.g. in the case of genome-wide association (GWA) studies. In all cases, phenotyping forms a crucial component of the analysis. Pioneering projects where phenotyping has played an essential role are for example the Mouse Clinic (Gailus-Durner et al. 2005) and the Human Phenome Project (Freimer and Sabatti 2003). These projects have revealed the role of the genes and the environment on development, morphology, physiology, metabolism, behaviour and pathology resulting in new disease-preventing approaches (http://www.nature.com/encode, accessed September 2012). Analogous development in plant biology will provide opportunities for a revolution in understanding of plant performance, which – amongst others – will enable more efficient breeding strategies.
The origin of plant phenotyping
Plant phenotyping per se is certainly not an exclusively human activity. In so-called ‘cafeteria experiments’ herbivores such as snails, grasshoppers or deer that are offered equal amounts of leaves of different species show clear preferences for some species, whereas they disfavour others (Fig. 1; Pérez-Harguindeguy et al. 2003). This may be only a matter of taste, but we are only just beginning to discover how rich the phenotyping capacities of certain species are. For example, some grasshopper species have the capacity to probe and evaluate the herbivore defence system of an individual plant within a population (Kallenbach et al. 2012). In relation to humans, their ability to phenotype – and thereby to select for the best-yielding individuals of species for domestication – has been one of the prerequisites for the development of human civilisation (Diamond 1997).
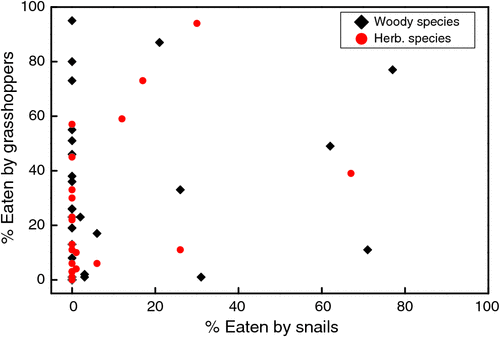
|
Agronomy and ecophysiology have a strong tradition in phenotyping. Agronomic evaluation of different genotypes, cultivars or treatments has been routinely conducted for more than a century, as can be judged from the age of various scientific journals in this field (Pearson et al. 2008). The concept of the so-called ‘genotype × environment interaction’, where the phenotypic expression of the genotype strongly depends on the environment, stems from such evaluation trials conducted in agricultural fields of various geographical locations (Fisher 1925). Breeders still rely on the evaluation of such field trials to select the appropriate genotype for new cultivars, where preferably plants are assessed in various years as well (Annicchiarico 2002).
The ecological perspective developed during the middle of the 20th century was strongly based on (reciprocal) transplantation experiments. A classic example has been the work of Clausen et al. (1948). They grew different Achillea millefolium L. clones in common gardens at three altitudes in the Californian Mountains (Suzuki et al. 1981). The experiment was repeated several times and resulted in a wide range of phenotypes for the same genotype, indicating large phenotyping plasticity. Like the agricultural field trials, these experiments demonstrated that the plant phenotype is the unique outcome of a process that is not only dependent on the genotype, but also on the dynamic environment under which it develops. Later developments in ecology in relation to phenotyping are the trait-based approaches, in which phenotypic characteristics of a wider range of different species are evaluated either in the field (Reich et al. 1992) or under laboratory conditions (Grime and Hunt 1975; Poorter et al. 1990). They were used to derive different strategies by which the ecological niche of species could be described (Grime 1979) and to analyse the interdependence of various traits (Wright et al. 2004). The latter work is a particularly apt example that shows that constraints on fitness prevent evolution from occupying all of the phenotypic space that is, in principle, available to plants.
Genes, phenes and plants without a phenotype
The terminology in relation to phenotyping is not completely clear-cut (Mahner and Kary 1997). This is partly due to the wide scale of integration levels at which phenotyping can take place, ranging from subcellular, cellular, tissue and organ levels all the way up to that of the integrated organism in all its dimensions. Indeed, a case could even be made to extend this range to epigenetics at the one end of the scale and to populations of individuals in monocultures at the other end. Clearly, it is far more challenging to describe a phenotype than it is to characterise the genotype of a given individual, owing to the possible variation in environmental factors and fluctuations over time. Discrepancy in terminology depends partly on culture and history in the various subdisciplines of biology: ecologists traditionally define ‘traits’ when they refer to a phenotypic variable of a plant such as the specific leaf area (SLA). However, some ecologists also refer to traits in relation to characteristics of vegetation, such as the leaf area index (LAI). The relation between the genetic information and the phenotypic expression thereof then becomes a characteristic of a mixed number of individuals. If these individuals are from different species, the principle relation between genotype and phenotype becomes blurred, and it has been advocated to avoid the qualification ‘trait’ in such cases (Violle et al. 2007). An alternative to the qualifier ‘trait’ is to use ‘phene’ as the phenotypic counterpart of a gene. Using these terms, the first association could be a one-to-one relationship between gene and phene; however, this would be an oversimplification. One gene can have a range of pleiotropic effects, whereas many traits will be under polygenic control. As such, the ‘gene-phene’ tandem refers more to the general concept of a gene-trait association than to a one-to-one relationship. Related terminology uses ‘phenome’ as a counterpart to ‘genome’. If the genome is defined as the total constellation of all genes (alleles) present in an individual, then the phenome would be the aggregate of all the expressed traits of the individual. Others use these terms as the characterisation of populations. Practically speaking, the usage of these various terms may overlap as they fulfil various and different needs for different niches of the scientific community. A clear and singular definition throughout the full domain of biology is desirable but probably unreachable (Mahner and Kary 1997).
In the last two decades, a new step has been taken in plant phenotyping, by means of the study of genetically-modified organisms. These genotypes often differ from their ‘wild type’ in only one targeted gene, so form a unique system to test the relevance of that specific gene in shaping the phenotype of the plant. For a range of transformations, the genetic modifications are so dramatic that they are lethal. A non-functional hormone signalling pathway (Qin et al. 2011) could serve as an example where a genetic modification deeply interferes with the viability of the individual (Lloyd and Meinke 2012). In such cases, the effect of a given gene on the phenotype is much better studied by a moderate reduction or increase of gene transcription of few tenths of a per cent (Kruckeberg et al. 1989). In contrast with the observed lethal transformations, there is a wide range of transformations with no observable differences between wild-type plants (or more precisely, the plants that were transformed with an empty vector) and the transformant. In such cases it becomes more and more fashionable to conclude that ‘these transformants do not have a phenotype’, conclusions also made in the animal field (Crusio 2002). There are several reasons that make such a conclusion imprecise and premature. First, these plants do certainly have a phenotype, since no individual can live with only a genotype. Second, the level of macroscopic phenotyping at which this conclusion is made often pertains to relatively ‘simple’ traits such as plant size, shape of leaves or timing of development. From a theoretical perspective it is not correct to conclude that there are no black swans, just by the fact that they have hitherto not been observed (Popper 1959). Likewise, as the phenotype of the plant is much broader than its visual appearance, it may be that there are more or less marked changes at, for example, the cellular level but that they do not translate into a difference between the transformant and the wild type for the trait(s) under scrutiny. A third point of attention is that differences are often tested under one set of environmental conditions, conveniently described as ‘standard conditions’. There are clear cases indicating the importance of the environment in determining the degree of difference between transformant and wild type or between varieties within a breeding program. A particularly nice example is given by Külheim et al. (2002), who found hardly any difference in seed production between a photosynthetic mutant (npq) and the wild type grown at constant low light levels, but a considerably lower seed production at fluctuating light levels. These data, as well as some other exemplifying gene × environment interactions are shown in Fig. 2. Finally, some phenotypic differences may show up only during a particular part of the diurnal cycle (Wiese et al. 2007) or during a particular ontogenetic phase (Tanaka et al. 2008). Thus, in case of no observable differences ‘these plants do not have a discernible phenotype with respect to size or development under the specific conditions tested’ would be the formulated conclusion instead of ‘these plants do not have a phenotype’.
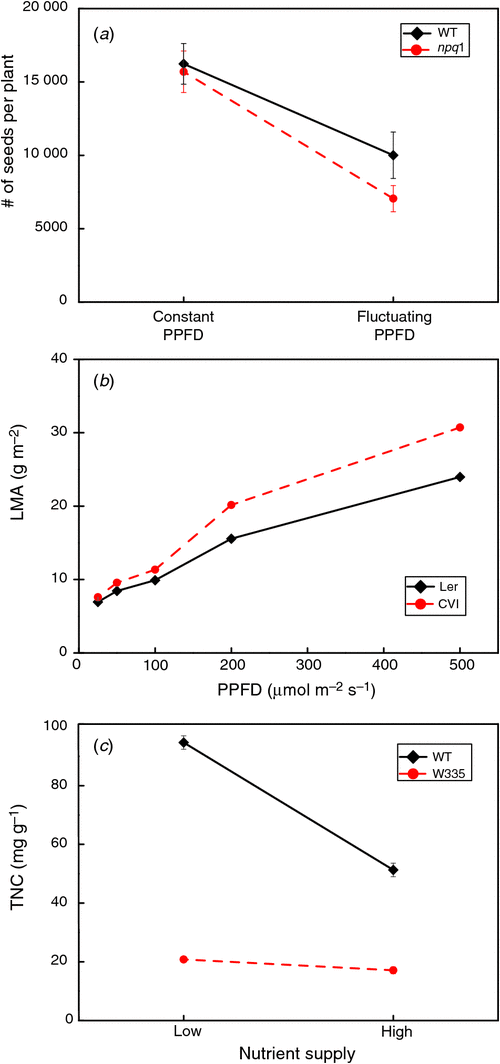
|
Phenotyping machines
The sequencing of the genome of the model plant Arabidopsis thaliana (L.) Heynh. (Meinke et al. 1998) has represented a landmark in plant genomics. Subsequently, the genomes of many economically important crops such as rice and maize have been sequenced and increasing amount of information on genomics is becoming available in databases such as, e.g. GABI DB (Riano-Pachon et al. 2009) or the TAIR DB (Huala et al. 2001). High-definition genotyping can now be performed with thousands of plants in robotised platforms, which allows for an increasing speed of genotype selection in breeding programs (Langridge and Fleury 2011). Efforts to extend these high-throughput techniques to aspects of protein interactions or metabolism have substantially increased our ability to detect genetic influences of subcellular phenotypes. However, when considering multicellular organisms whose phenotype includes traits related to anatomy, morphology, physiology and development, our capabilities are less advanced (Houle 2010; Kolukisaoglu and Thurow 2010). The prospects for application of phenotypic data are more rewarding than ever, especially in the case of complex traits, such as growth and yield, we could profit from large-scale phenotyping of mapping populations to establish QTL locations. This easily requires evaluation of >300 genetically different lines. GWA studies preferably require an even larger number of genotypes to be measured. Given that some replication within lines is required as well, organising the growth and evaluation of such a large number of plants becomes daunting. Clearly, our phenotyping capabilities are currently a bottleneck for such studies (Furbank and Tester 2011).
Automation and establishment of high-throughput systems in the life sciences has significantly progressed within the last decade, mainly in the field of drug discovery and development (Mayr and Bojanic 2009) and in the field of animal behaviour (Noldus et al. 2001). Plant sciences have benefited from this development but mostly at the molecular and cellular scale (Gibon et al. 2004; Delseny et al. 2010). Essentially, research at the macroscopic level still relies to a large extent on human measurements and assessment (Kolukisaoglu and Thurow 2010). However, over the last few years there has been significant developments made towards automated phenotyping platforms employed in growth chambers and glasshouses (Granier et al. 2006; Jansen et al. 2009; Furbank and Tester 2011). These platforms are usually built to move plants (individually or in groups) to a sensing station. An argument in favour of these plant-to-sensor systems is that repeatable imaging conditions can be assured at the location of the measurement, often a chamber shielded from outside light. The effect of moving may not be beneficial per se, but it may minimise micro-environmental differences between positions within the growth facility that could otherwise affect growth specifically (Poorter et al. 2012a). In contrast, sensor-to-plant systems could provide significant advantages, especially when assays for phenotyping require increased complexity of environmental conditions during plant growth. Systematic approaches to address benefits and drawbacks of both alternatives are presently missing.
There is a consistent development in the establishment and implementation of non-destructive imaging approaches of plants into phenotyping platforms (Furbank and Tester 2011; Fiorani et al. 2012). Estimations of plant size and leaf area of large germplasm collections based on 2D series of colour images is performed routinely at most platforms. They are generally accurate when plants are young, but become more challenging when plants get larger, as there is more overlap between leaves. For example, in an experiment where projected leaf area and shoot weight were followed over time in the rosette species Plantago major L. (Poorter et al. 1988), the relationship between shoot DW and projected leaf area seems reasonably close-to-linear in a log-log plot to use projected leaf area as an estimator of shoot mass (Fig. 3a, b). However, further analysis shows that in young plants true shoot weight was 35 g for each m2 of projected leaf area, whereas it was 4-fold higher at the end of the experiment. The problem may be less severe in species with an erect growth form, yet, with an analysis that should be sensitive enough to measure differences between genotypes of, say, 10%, 2D analyses are likely to underperform. 3D image analyses may mitigate this problem (Dornbusch et al. 2012). Alternatively, the structure of a dense canopy can be quantitatively resolved with approaches such as magnetic resonance imaging (MRI), be it with a penalty of low throughput.
Apart from structural traits it is necessary to also characterise leaves and roots at the physiological or chemical level. Fluorescence and hyperspectral analysis are good steps forward, particularly as they allow evaluation of various plant traits in a fast and non-destructive manner. However, only specific aspects of plant functioning can be evaluated in this way. An exciting new development is the robotised sensor-actor for destructive sampling of relevant plant parts (Alenyà et al. 2012). This method may enable automated measurement of cellular processes and or gene expression at specific time points, thus, dramatically widening our phenotyping capabilities.
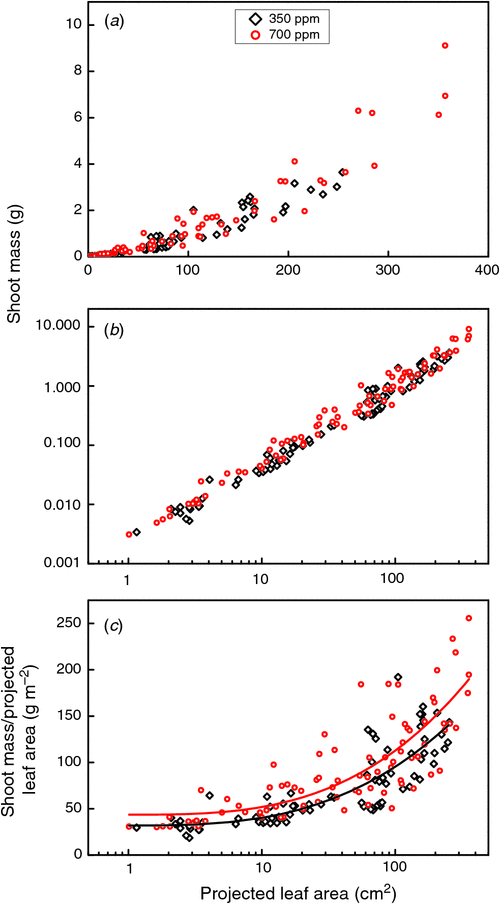
|
Phenotyping in the field is the next essential step in the evaluation chain from well-defined and controlled conditions in the laboratory and greenhouse to the heterogeneous natural environment. Field measurements represent a significant test for the relevance of the laboratory and greenhouse approaches. Traits considered critical in the greenhouse may be less important in the field, since the canopy of a stand and not a single plant is the relevant unit under field conditions. A very basic requirement for plant screening under field conditions is the delivery of sensors to the canopy, which can be performed with various systems and different levels of detail. Positioning systems can be used for mechanistic field phenotyping with high accuracy and repeatability of measurements in given plots, mobile platforms such as a tractor equipped with specific sensors enable larger spatial flexibility, whereas drones or airborne platforms can cover vast agricultural areas. Most field sensors have to rely on passive measurement principle and the most promising is the measurement of reflected sunlight by multi- and hyperspectral technologies (Rascher and Pieruschka 2008; Comar et al. 2012). Active approaches induce a certain response of a plant, for example, by application of a defined light pulse to probe specific physiological processes. These approaches are employed in the laboratory and there are only few robust techniques using an active measurement principle in the field such as the laser-induced fluorescence transient (LIFT) approach to estimate photosynthetic efficiency (Pieruschka et al. 2010). Dedicated field sensors are already applied in precision agriculture for nutrient management (Scotford and Miller 2005) and may become important tools for sensing of plant disease in the near future (Mahlein et al. 2012). Establishment of wireless sensor networks enables continuous monitoring of the environment and crop properties and will provide valuable information for agricultural management (Ruiz-Garcia et al. 2009).
e-Infrastructure and phenotyping
Full potential of the newly developing phenotyping approaches will enfold when physiological and morphological data can be linked to genomic information. This requires a dedicated e-infrastructure to handle the surge of data from automated phenotyping platforms, which are collecting more and more information at an increasingly fine time scale. These data have to be processed in a standardised way to allow analysis (and re-analyses). The overarching goal is to grasp and understand the whole phenome of the plant under a wide range of environmental conditions. This understanding will allow us to simulate and predict plant properties in particular of complex traits such as yield or biomass, the most important challenge to address future needs of a growing human population. How can we achieve these ambitious objectives? One way forward is to develop databases such as the plant meta-phenomics database (Poorter et al. 2010) or the Plant Trait database TRY (http://www.try-db.org, accessed September 2012), which bring together phenotypic responses to the environment for a wide range of plant traits and parameters. They allow for the establishment of dose–response curves, which form the knowledge base for further modelling efforts. A possible next step on this route may be a development that has started in the field of medicine. In this approach, called ‘evidence-based practice’, where the aim is to apply the best possible evidence obtained from scientific insights for the care of individual patients. The evidence is based on meta-analyses such as the Cochrane Reviews (http://www.cochrane.org/, accessed September 2012). Evidence-based practice identifies the best treatment for a patient, as depending on medical expertise, expert judgement as well as the patient’s state of being, needs and preferences (Leach 2006). In plant phenotyping, a development analogous to this evidence-based practice may provide a substantial step forward in targeting special genotypes to be used in breeding programs. This would allow the breeder to select the best possible parental lines based on broad knowledge of the genotypic and phenotypic characteristics of these lines and their range of interactions with the environment.
Content of this special issue
This special issue focusses on several aspects centred around plant phenotyping. Proper choice and description of the environment is paramount to any phenotyping effort and Poorter et al. (2012a) provide some general guidelines. Attention is paid to the experimental design of experiments and appropriate application of experimental conditions. In a more specialised paper, Poorter et al. (2012b) focus on the effect of pot size on plant growth and performance. They conclude that the effect of pot size is rather large, with – averaged over a range of experiments published in the literature – a 43% increase in biomass for every doubling in pot size. In a further analysis of the data they show that pot size is particularly strongly impacting plant size when more than 1 g of total plant dry mass was present per litre of rooting volume. The design of drought tests is the focus of work by Passioura (2012). Many studies have applied drought conditions to plants grown under laboratory conditions in a way that is rather irrelevant to the type of drought stress experienced by crop plants in the field. Passioura draws attention to the fact that such experiments are generally of little use to plant breeders.
Phenotyping of plants can gain from a better 3D representation of the shoot. Dornbusch et al. (2012) describe a method by which a laser-scanning device comes close to a 3D image of an Arabidopsis plant. Leaf size and angle can be followed over time without additional effort. Another development is 3D phenotyping of Capsicum plants growing in a glasshouse at a density that is characteristic for commercial growers. This poses strong challenges upon the analysis, especially when it comes to computationally separating leaves from each other in an environment with natural variation in light levels. Van der Heijden et al. (2012) combine traditional red-green-blue (RGB) cameras with a time-of-flight (TOF) camera, which can gauge depth by measuring the distance from the camera to the object. The information from both systems is linked to differentiate between different leaves. The system is a noteworthy step towards a 3D reconstruction of a canopy. Functional plant traits such as assessment of chlorophyll fluorescence were used by Sharma et al. (2012) to screen for heat tolerant wheat cultivars with a standardised chlorophyll fluorescence protocol. Repeated screening with increased selection pressure led to identification of a set of cultivars with differences in capacity of photosynthetic efficiency of photosystem II under heat treatments. A step out into the field by Comar et al. (2012) showed the use of hyperspectral measurements in combination with RGB cameras to test different vegetation indices. Green area per unit ground area derived from RGB imaging and vegetation indices for estimation of green biomass and pigment content were measured in micro-plots of different wheat cultivars in the field during the growth cycle. The plant dynamics were described with a model and its parameters were used to evaluate heritability of these indices with plant traits obtained during harvest. Hyperspectral data are also in the focus of the study by Römer et al. (2012). These authors used an unsupervised classification approach called simplex volume maximisation (SiVM) to analyse hyperspectral data by calculating how similar each spectrum is to observed typical spectra and by that identify stress. SiVM provided better results than established vegetation indices when analysing drought and nutrient stressed plants.
After due consideration of the aboveground parts of plants, further work in this issue considered the belowground parts of plants: the roots. Accessibility of roots is difficult although there are examples in this issue of techniques to estimate the 3D distribution of root mass in pots (Poorter et al. 2012b) by means of nuclear magnetic resonance (NMR) imaging. This method provides very detailed information about root structure but is time-consuming and expensive. An alternative approach based on 2D imaging is described by Nagel et al. (2012), who developed a unique robotised system with plants growing in rhizotrons, large but relatively thin containers, where one side is transparent. Root structure and development can then be followed over time for those roots that grow along the transparent surface. A study by De Sousa et al. (2012) linked root architecture with phosphorus uptake. The study was performed under field conditions and in root pouches linking genes involved in phosphorus acquisition efficiency to root structural properties.
Most of the commercially-available automated platforms can be acquired only at considerable cost. Pereyra-Irujo et al. (2012) developed such a low cost platform, which would allow it to be used by academic users and breeders. They tested the platform for measurement of water use efficiency of two different soybean genotypes under different water availability scenarios. Almost all increases in automation imply that more plants can be measured, often at a finer timescale and with more methodologies. Data-analysis often forms an indispensable part of such work. This poses challenges on informatics as well, in particular, when the projects become large scale with respect to the number of people that are involved. Additional issues of what data will be available to whom and how to ensure access over extended time period with a guarantee of data integrity and consistency are introduced. Billiau et al. (2012) faced this problem and describe how they solved the challenge. A laboratory information management system (LIMS) was already in place and augmented with another shell of programs that handled data storage, retrieval and accessibility. As such, safe access and fast data handling could be achieved, ensuring access to all scientists and stakeholders in the project.
Plant phenotyping is rapidly changing into a highly integrated and industrial approach in which the growth of plants, actual measurements and subsequent analysis of the data are highly co-ordinated. This special issue focuses on several aspects related to these issues. As the resulting platforms, equipment and e-infrastructure are maturing, it is now time to harvest the fruits from all these investments.
Acknowledgements
This special issue on plant phenotyping was based on the contributions to the 2nd International Plant Phenotyping Symposiums in 2011. We thank Fabio Fiorani, Nikolaos Ntagkas and Koen Verhoeven for commenting on a previous version of this manuscript, Carsten Külheim and Oscar Nagel for providing additional data for the figures and all the reviewers involved in this special issue for their valuable suggestions and evaluations.
References
Alenyà G, Dellen B, Foix S, Torras C (2012) Leaf segmentation from ToF data for robotized plant probing. IEEE Robotics & Automation Magazine in press.Annicchiarico P (2002) Genotype × environment interaction: challenges and opportunities for plant breeding and cultivar recommendations. FAO Plant Production and Protection Paper 174.
Billiau K, Sprenger H, Walther D, Koehl K (2012) Data management pipeline for plant phenotyping in a multisite project. Functional Plant Biology 39, 948–957.
| Data management pipeline for plant phenotyping in a multisite project.Crossref | GoogleScholarGoogle Scholar |
Clausen J, Keck DD, Hiesey, WM (1948) Experimental studies on the nature of plant species. The effect of varied environments on western North American plants. Carnegie Institution of Washington Publication 520.
Comar A, Burger PH, de Solan B, Baret F, Daumard F, Hanocq JF (2012) A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results. Functional Plant Biology 39, 914–924.
| A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results.Crossref | GoogleScholarGoogle Scholar |
Crusio WE (2002) ‘My mouse has no phenotype’. Genes Brain & Behavior 1, 71
| ‘My mouse has no phenotype’.Crossref | GoogleScholarGoogle Scholar |
De Sousa SM, Clark RT, Mendes FF, de Oliveira AC, de Vasconcelos MJV, Parentoni SN, Kochian LV, Guimarães CT, Magalhães JV (2012) The role of root morphology and related candidate genes in P acquisition efficiency in maize. Functional Plant Biology 39, 925–935.
| The role of root morphology and related candidate genes in P acquisition efficiency in maize.Crossref | GoogleScholarGoogle Scholar |
Delseny M, Han B, Hsing YI (2010) High throughput DNA sequencing: the new sequencing revolution. Plant Science 179, 407–422.
| High throughput DNA sequencing: the new sequencing revolution.Crossref | GoogleScholarGoogle Scholar |
Diamond J (1997) ‘Guns, germs, and steel: the fates of human societies.’ (Norton & Company Inc.: New York, USA)
Dornbusch T, Lorrain S, Kuznetsov D, Fortier A, Liechti R, Xenarios I, Fankhauser C (2012) A phenotyping method to measure diurnal leaf movements on Arabidopsis plants in the early rosette growth stage. Functional Plant Biology 39, 860–869.
| A phenotyping method to measure diurnal leaf movements on Arabidopsis plants in the early rosette growth stage.Crossref | GoogleScholarGoogle Scholar |
Fiorani F, Rascher U, Jahnke S, Schurr U (2012) Imaging plants dynamics in heterogenic environments. Current Opinion in Biotechnology 23, 227–235.
| Imaging plants dynamics in heterogenic environments.Crossref | GoogleScholarGoogle Scholar |
Fisher RA (1925) ‘Statistical methods for research workers.’ (Oliver & Boyd: Edinburgh, UK)
Freimer N, Sabatti C (2003) The human phenome project. Nature Genetics 34, 15–21.
| The human phenome project.Crossref | GoogleScholarGoogle Scholar |
Furbank RT, Tester M (2011) Phenomics – technologies to relieve the phenotyping bottleneck. Trends in Plant Science 16, 635–644.
| Phenomics – technologies to relieve the phenotyping bottleneck.Crossref | GoogleScholarGoogle Scholar |
Gailus-Durner V, Fuchs H, Becker L, Bolle I, Brielmeier M, Calzada-Wack J, Elvert R, Ehrhardt N, Dalke C, Franz TJ, et al (2005) Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nature Methods 2, 403–404.
| Introducing the German Mouse Clinic: open access platform for standardized phenotyping.Crossref | GoogleScholarGoogle Scholar |
Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. The Plant Cell 16, 3304–3325.
| A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness.Crossref | GoogleScholarGoogle Scholar |
Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux JJ, Rolland G, Bouchier-Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist 169, 623–635.
| PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1979) ‘Plant strategies and vegetation processes.’ (John Wiley & Sons: Chichester, UK)
Grime JP, Hunt R (1975) Relative growth rate: its range and adaptive significance in a local flora. Journal of Ecology 63, 393–422.
| Relative growth rate: its range and adaptive significance in a local flora.Crossref | GoogleScholarGoogle Scholar |
Houle D (2010) Numbering the hairs on our heads: the shared challenge and promise of phenomics. Proceedings of the National Academy of Sciences of the United States of America 107, 1793–1799.
| Numbering the hairs on our heads: the shared challenge and promise of phenomics.Crossref | GoogleScholarGoogle Scholar |
Huala E, Dickerman AW, Garcia-Hernandez M, Weems D, Reiser L, LaFond F, Hanley D, Kiphart D, Zhuang M, Huang W, et al (2001) The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Research 29, 102–105.
| The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant.Crossref | GoogleScholarGoogle Scholar |
Jansen M, Gilmer F, Biskup B, Nagel K, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, De Jaeger I, Metzlaff M, Schurr U, Scharr H, Walter A (2009) Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Functional Plant Biology 36, 902–914.
| Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants.Crossref | GoogleScholarGoogle Scholar |
Johanssen W (1909) ‘Elemente der exakten Erblichkeitslehre.’ (Gustav Fischer: Jena, Germany)
Kallenbach M, Bonaventure G, Gilardoni PA, Wissgott A, Baldwin IT (2012) Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proceedings of the National Academy of Sciences of the United States of America 109, E1548–E1557.
| Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations.Crossref | GoogleScholarGoogle Scholar |
Kolukisaoglu U, Thurow K (2010) Future and frontiers of automated screening in plant sciences. Plant Science 178, 476–484.
| Future and frontiers of automated screening in plant sciences.Crossref | GoogleScholarGoogle Scholar |
Kruckeberg AL, Neuhaus HE, Feil R, Gottlieb LD, Stitt M (1989) Decreased-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast of Clarkia xantiana. Impact on mass-action ratios and fluxes to sucrose and starch, and estimation of flux control coefficients and elasticity coefficients. Biochemical Journal 261, 457–467.
Külheim C, Ågren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297, 91–93.
| Rapid regulation of light harvesting and plant fitness in the field.Crossref | GoogleScholarGoogle Scholar |
Langridge P, Fleury D (2011) Making the most of ‘omics’ for crop breeding. Trends in Biotechnology 29, 33–40.
| Making the most of ‘omics’ for crop breeding.Crossref | GoogleScholarGoogle Scholar |
Leach MJ (2006) Evidence-based practice: a framework for clinical practice and research design. International Journal of Nursing Practice 12, 248–251.
| Evidence-based practice: a framework for clinical practice and research design.Crossref | GoogleScholarGoogle Scholar |
Lewontin RC (1974) ‘The genetic basis of evolutionary change.’ (Columbia University Press: New York)
Lloyd J, Meinke D (2012) A comprehensive dataset of genes with a loss-of-function mutant phenotype in Arabidopsis. Plant Physiology 158, 1115–1129.
| A comprehensive dataset of genes with a loss-of-function mutant phenotype in Arabidopsis.Crossref | GoogleScholarGoogle Scholar |
Mahlein AK, Oerke EC, Steiner U, Dehne HW (2012) Recent advances in sensing plant diseases for precision crop protection. European Journal of Plant Pathology 133, 197–209.
| Recent advances in sensing plant diseases for precision crop protection.Crossref | GoogleScholarGoogle Scholar |
Mahner M, Kary M (1997) What exactly are genomes, genotypes and phenotypes? And what about phenomes? Journal of Theoretical Biology 186, 55–63.
| What exactly are genomes, genotypes and phenotypes? And what about phenomes?Crossref | GoogleScholarGoogle Scholar |
Mayr LM, Bojanic D (2009) Novel trends in high-throughput screening. Current Opinion in Pharmacology 9, 580–588.
| Novel trends in high-throughput screening.Crossref | GoogleScholarGoogle Scholar |
Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M (1998) Arabidopsis thaliana: a model plant for genome analysis. Science 282, 662–682.
| Arabidopsis thaliana: a model plant for genome analysis.Crossref | GoogleScholarGoogle Scholar |
Nagel OW, Konings H, Lambers H (2001) Growth rate and biomass partitioning of wildtype and low-gibberellin tomato (Solanum lycopersicum) plants growing at a high and low nitrogen supply. Physiologia Plantarum 111, 33–39.
| Growth rate and biomass partitioning of wildtype and low-gibberellin tomato (Solanum lycopersicum) plants growing at a high and low nitrogen supply.Crossref | GoogleScholarGoogle Scholar |
Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Blossfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert AK, Galinski A, Scharr H, Fiorani F, Schurr U (2012) GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39, 891–904.
| GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons.Crossref | GoogleScholarGoogle Scholar |
Noldus LPJJ, Spink AJ, Tegelenbosch RAJ (2001) Etho vision: a versatile video tracking system for automation of behavioral experiments. Behavior Research Methods 3, 398–414.
Passioura JB (2012) Phenotyping for drought tolerance in grain crops: when is it useful to breeders? Functional Plant Biology 39, 851–859.
| Phenotyping for drought tolerance in grain crops: when is it useful to breeders?Crossref | GoogleScholarGoogle Scholar |
Pearson CH, Ernst SM, Barbarick KA, Hatfield JL, Peterson GA, Buxton DR (2008) Agronomy Journal turns one hundred. Agronomy Journal 100, 1–8.
| Agronomy Journal turns one hundred.Crossref | GoogleScholarGoogle Scholar |
Pereyra-Irujo GA, Gasco ED, Peirone LS, Aguirrezábal LAN (2012) A low-cost automatic platform for phenotyping plant growth and water use. Functional Plant Biology 39, 905–913.
| A low-cost automatic platform for phenotyping plant growth and water use.Crossref | GoogleScholarGoogle Scholar |
Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen JHC, Gurvich DE, Cabido M (2003) Leaf traits and herbivore selection in the field and in cafeteria experiments. Austral Ecology 28, 642–650.
| Leaf traits and herbivore selection in the field and in cafeteria experiments.Crossref | GoogleScholarGoogle Scholar |
Pieruschka R, Klimov D, Kolber Z, Berry JA (2010) Continuous measurements of the effects of cold stress on photochemical efficiency using laser induced fluorescence transient (LIFT) approach. Functional Plant Biology 37, 395–402.
| Continuous measurements of the effects of cold stress on photochemical efficiency using laser induced fluorescence transient (LIFT) approach.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Pot CS, Lambers H (1988) The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major. Physiologia Plantarum 73, 553–559.
| The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology 94, 621–627.
| Carbon and nitrogen economy of 24 wild species differing in relative growth rate.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Niinemets Ü, Walter A, Fiorani F, Schurr U (2010) A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. Journal of Experimental Botany 61, 2043–2055.
| A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin OK, Tardieu F, Pons TL (2012a) The art of growing plants for experimental purposes; a practical guide for the plant biologist. Functional Plant Biology 39, 821–838.
| The art of growing plants for experimental purposes; a practical guide for the plant biologist.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Bühler J, Van Dusschoten D, Climent J, Postma J (2012b) Pot size matters: a meta-analysis on the effects of rooting volume on plant growth. Functional Plant Biology 39, 839–850.
| Pot size matters: a meta-analysis on the effects of rooting volume on plant growth.Crossref | GoogleScholarGoogle Scholar |
Popper K (1959) ‘The logic of scientific discovery.’ (Harper & Row: New York)
Qin F, Kodaira KS, Maruyama J, Tran LSP, Fujita Y, Morimoto K, Shinozaki K, Yamaguchi-Shinozaki K (2011) SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Cell 157, 1900–1913.
Rascher U, Pieruschka R (2008) Spatio-temporal variations of photosynthesis: the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems. Precision Agriculture 9, 355–366.
| Spatio-temporal variations of photosynthesis: the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems.Crossref | GoogleScholarGoogle Scholar |
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs 62, 365–392.
| Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems.Crossref | GoogleScholarGoogle Scholar |
Riano-Pachon DM, Nagel A, Neigenfind J, Wagner R, Basekow R, Weber E, Mueller-Roeber B, Diehl S, Kersten B (2009) GabiPD: the GABI primary database – plant integrative ‘omics’ database. Nucleic Acids Research 37, D954–D959.
| GabiPD: the GABI primary database – plant integrative ‘omics’ database.Crossref | GoogleScholarGoogle Scholar |
Römer C, Wahabzada M, Ballvora A, Pinto F, Rossini M, Panigada C, Behmann J, Léon J, Thurau C, Bauckhage C, Kersting K, Rascher U, Plümer L (2012) Early drought stress detection in cereals: simplex volume maximization for hyperspectral image analysis. Functional Plant Biology 39, 878–890.
| Early drought stress detection in cereals: simplex volume maximization for hyperspectral image analysis.Crossref | GoogleScholarGoogle Scholar |
Ruiz-Garcia L, Lunadei L, Barreiro P, Robla JI (2009) A review of wireless sensor technologies and applications in agriculture and food industry: state of the art and current trends. Sensors (Basel, Switzerland) 9, 4728–4750.
| A review of wireless sensor technologies and applications in agriculture and food industry: state of the art and current trends.Crossref | GoogleScholarGoogle Scholar |
Scotford IM, Miller PCH (2005) Applications of spectral reflectance techniques in northern European cereal production: a review. Biosystems Engineering 90, 235–250.
| Applications of spectral reflectance techniques in northern European cereal production: a review.Crossref | GoogleScholarGoogle Scholar |
Sharma DK, Andersen SB, Ottosen C.-O, Rosenqvist E (2012) Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Functional Plant Biology 39, 936–947.
| Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence.Crossref | GoogleScholarGoogle Scholar |
Suzuki DT, Griffiths AJF, Lewontin RC (1981) ‘An introduction to genetic analysis.’ (Freeman: San Francisco)
Tanaka M, Kikuchi A, Kamada H (2008) The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiology 146, 149–161.
| The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination.Crossref | GoogleScholarGoogle Scholar |
Van der Heijden G, Song Y, Horgan G, Polder G, Dieleman A, Bink M, Palloix A, Van Eeuwijk F, Glasbey C (2012) SPICY: towards automated phenotyping of large pepper plants in the greenhouse. Functional Plant Biology 39, 870–877.
| SPICY: towards automated phenotyping of large pepper plants in the greenhouse.Crossref | GoogleScholarGoogle Scholar |
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116, 882–892.
| Let the concept of trait be functional!Crossref | GoogleScholarGoogle Scholar |
Wiese A, Christ MM, Virnich O, Schurr U, Walter A (2007) Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle. New Phytologist 174, 752–761.
| Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle.Crossref | GoogleScholarGoogle Scholar |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar |


