Trajectories in health recovery in the 12 months following a mild traumatic brain injury in children: findings from the BIONIC Study
Kelly M. Jones 1 , Suzanne Barker-Collo 2 , Priya Parmar 1 , Nicola Starkey 3 , Alice Theadom 1 , Shanthi Ameratunga 4 , Valery L. Feigin 1 51 National Institute for Stroke and Applied Neurosciences, School of Public Health and Psychosocial Studies, Auckland University of Technology, Auckland, New Zealand
2 School of Psychology, Tamaki Campus, The University of Auckland, Auckland, New Zealand
3 School of Psychology, Faculty of Arts & Social Sciences, The University of Waikato, Hamilton, New Zealand
4 School of Population Health, Faculty of Medical & Health Sciences, The University of Auckland, Auckland, New Zealand
5 A list of members of the BIONIC team is available at: www.nisan.aut.ac.nz.
Correspondence to: Kelly M Jones, National Institute for Stroke and Applied Neurosciences, School of Public Health & Psychosocial Studies, Faculty of Health and Environmental Studies, Auckland University of Technology, AUT North Shore Campus, AA254, 90 Akoranga Drive, Northcote 0627, Private Bag 92006, Auckland 1142, New Zealand. Email: kelly.jones@aut.ac.nz
Journal of Primary Health Care 10(1) 81-89 https://doi.org/10.1071/HC17038
Published: 29 March 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: There is growing consensus that adverse child outcomes may be evident in the early recovery phase following mild traumatic brain injury (TBI). However, controversy remains around the nature of children’s longer-term recovery.
AIM: To examine child cognitive, behavioural and quality-of-life outcomes over 12 months following mild injury, and to identify prognostic factors associated with outcomes.
METHODS: A prospective sample of 222 children (aged 2–15 years at injury) with mild TBI was assessed using a cognitive testing battery and parent-report questionnaires at ≤ 14 days, 1, 6 and/or 12-months post-injury.
RESULTS: Parents reported significant improvements in their child’s behavioural adjustment between baseline and 6 months (P = 0.003), with further improvements at 12 months following injury (P = 0.001). Cognitive recovery and quality-of-life improvements were more gradual with minimal changes in the first month (P > 0.05), but significant improvements by 12-months post-injury (P = 0.03, P = 0.02, respectively). Time since injury, male gender, living rurally and parent anxiety were associated with extent of recovery beyond the acute period.
CONCLUSIONS: Children’s recovery from mild TBI continues beyond the initial 6 months following injury. Health-care providers need to be vigilant about the varying trajectories in children’s recovery from TBI. On-going monitoring of children following injury will enable timely and proactive responses to persistent difficulties, with a view to minimising longer-term adverse consequences.
KEYWORDS: Health research; paediatrics; population health; carers
| WHAT GAP THIS FILLS |
| What is already known: There is growing consensus that mild TBI may have adverse effects on children’s functioning in the initial months following the event. Longer-term management of paediatric mild TBI is a challenging area of general practice, yet longitudinal research is lacking. |
| What this study adds: This is one of few studies to show that, as a group, children’s recovery following mild TBI extends beyond the first 6 months through to at least 12-months post-injury. This evidence suggests an extended window of opportunity exists to further facilitate children’s recovery. This may be achieved via on-going monitoring and early delivery of interventions targeted at developmental domains that appear to be compromised. |
Introduction
Traumatic brain injury (TBI) during childhood affects 100–300/100,000 population each year.1,2 The most common causes of TBI in childhood are falls and motor vehicle accidents, with most injuries classified as mild in severity.1,3 Despite the ‘mild’ label, there is growing consensus that these injuries may have adverse effects on children’s functioning. While symptoms are expected to resolve shortly after injury, there is a consistent pattern of evidence suggesting full recovery does not occur for all children. Further, there is evidence to suggest that children’s patterns of recovery may differ across developmental domains.
A meta-analysis of 28 publications (from 1988 to 2007) examined children’s neurocognitive outcomes following mild to moderate TBI.4 Case-control studies revealed negligible to small differences between the mild TBI and control groups for full scale and performance IQ, working memory, problem solving and visual immediate memory and perceptual functioning. It was concluded that children with mild TBI experience few impairments in the neurocognitive domains examined. However, some studies reported increasing between-group differences at 24+ months following mild TBI. The authors concluded that there may be a subset of children who experience persistent adverse cognitive outcomes.
Another systematic review of 11 studies in 2012 found that 56% reported poor quality of life (QOL) in children aged 3-months to 5-years post-injury.5 Poor QOL outcomes were often not evident in the first 6-months post injury, but were reported later, highlighting the need for repeated-measurement approaches.
A further systematic review of 30 studies examining children’s (< 19 years) psychological, psychiatric and behavioural outcomes following mild TBI found higher rates of hyperactivity and inattention.6 The authors concluded that most children recover quickly, but a small proportion have persistent problems. Prospective longitudinal studies evaluating children’s recovery over time were recommended.6
With increasing numbers of children with mild TBI presenting for treatment,7 continuing controversy surrounding the nature of children’s recovery8 has done little to alleviate the challenges faced by community-based, healthcare providers. These challenges can be ameliorated by accurate information about paediatric outcomes, and identifying factors associated with recovery.6 Using a population-based sample of children with mild TBI, this study aimed to determine the pattern of children’s generalised cognitive function, parent-reported behavioural adjustment and QOL over the year following mild TBI, and to identify prognostic factors associated with outcomes at 12 months.
Methods
Ethical approval was obtained from the Northern Y Health and Disability Ethics Committee (NTY/09/09/095 and NTY/11/02/016) and the Auckland University of Technology Ethics Committee (09/265). Parents gave informed written consent, with written assent sought from children aged ≥ 8 years.
Design
This prospective longitudinal cohort study examined children with mild TBI over the first 12 months following their injury. Children were identified from a population-based incidence study, the Brain Injury Incidence and Outcomes New Zealand in the Community (BIONIC) study. Assessment scores were compared to normative data. Full details of the methodology of the BIONIC study have been published separately.9 All confirmed cases were invited to participate in baseline (within 14 days of injury) and follow-up assessments at 1, 6, and 12-months post injury.
Participants
The current analysis includes children aged 2–15 years at the time of injury, who resided in the study area and sustained a confirmed mild TBI between March 2010 and February 2011. TBI was defined as an acute brain injury resulting from mechanical energy to the head from external physical forces. Mild TBI was defined as a Glasgow Coma Score of 13–15 or Post Traumatic Amnesia (< 24 h)10 and the presence of one or more of the operational criteria, consistent with the World Health Organization (WHO) definition of mild traumatic brain injury (TBI): (1) confusion or disorientation; (2) loss of consciousness; (3) post-traumatic amnesia; and (4) other neurological abnormalities (eg seizure).11 Given the difficulties in applying TBI criteria to children (eg determining confusion in young children), evidence of a head injury accompanied by medical or behavioural changes immediately following the injury was required to confirm TBI (eg vomiting, persistent crying).
Additional inclusion criteria were the availability of outcome data at baseline, 1, 6, and/or 12-month time points. As many people with mild TBI do not seek medical attention following their injury, recruitment was undertaken via multiple sources, including general practices, Accident and Medical centres, Accident Compensation Corporation (ACC) records, community health-care services, concussion clinics, sports clubs and self-referrals. This approach aimed to minimise skewing of findings of longer-term affect due to focusing solely on families seeking medical treatment following injury.
Eligibility criteria were confirmed by medical records review. In the absence of medical records, self-reported details of each TBI were obtained and reviewed by a diagnostic team of neurologists, clinicians and neuropsychologists to determine eligibility for the study. Children with mild TBI were excluded from the current analysis if they were aged < 2 years due to measurement limitations.
Procedure
Baseline assessments captured child injury characteristics and demographic details about each participating family. Each outcome measure was administered at baseline to capture children’s functioning shortly after injury, and re-administered at 1, 6 and 12-months post-injury.
Outcome measures
Parent-reported behavioural adjustment: Suitable for ages 2–25 years and repeated administration, age-appropriate parent rating scales of the Behavioural Assessment System for Children - Second Edition12 (BASC) were used to assess children’s behaviour in their home and community. Parent-report subscales include hyperactivity, aggression, conduct problems, anxiety, depression, somatisation, attention problems, atypicality, and withdrawal clinical subscales, and the adaptability, leadership and social skills adaptive subscales. Related clinical composite scales include externalising and internalising behaviours, and adaptive functioning. Parents responded to each item using a scale from ‘never’ to ‘almost always’. BASC scores are standardised for age and sex, and provide composite and subscale t-scores (mean 50 ± s.d. 10). Scores range from 20 to 120 for externalising, internalising and behavioural symptoms, and 10–90 for adaptive skills. All scoring was completed using scoring software (BASC 2 PRQ ASSISTTM, Pearson Assessments, USA). Total problems across all subscales are reflected in the behavioural symptoms index score (used in the current analysis), with higher scores indicating more behavioural problems. The BASC has good internal consistency, test–retest reliability, content and construct validity, and convergent discriminate validity.13
Parent-reported quality of life (QOL): The 23-item parent-report Paediatric Quality of Life (PedsQL) 4.0 Generic Core Scales14 is suitable for use with children aged 2–16 years. This measure systematically assesses children’s physical health (eight items), social functioning (five items), emotional functioning (five items) and school functioning (five items). Using an age-appropriate version (2–4 years (toddler), 5–7 years (young child), 8–12 years (child), and 13–18 years (adolescent)), parents were asked how much of a problem each item has been in the past month. The five-level response scale ranges from 0 = never a problem to 4 = almost always a problem. Items are reverse-scored and linearly transformed to a 0 – 100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). The total score used in the current analysis is the mean score of all items. Higher scores indicate better QOL. The PedsQL has proven reliability and validity as a parent proxy-report for children aged 2–16 years.15
Cognitive function assessment: An additional measure, the Central Nervous System Vital Signs (CNS-VS) test battery, was administered only to children aged ≥ 8 years.16 This 30-min computerised and age-standardised test captures objective scores on nine cognitive domains: verbal memory, visual memory, composite memory, psychomotor speed, reaction time, complex attention, cognitive flexibility, processing speed, executive functioning. These tests generate an overall neurocognition index (mean 100 ± s.d. 15) that was used in the current analysis. Using age-standardised scores and automated scoring to eliminate variability, this measure was designed for repeated administration (ie content of subtests alter across repeat administrations). This measure has extensive norms available (N = 8000), established concurrent16 and discriminant validity,17,18 and sensitivity within TBI populations.19 Lower scores indicate poor cognitive ability.
Potential predictor variables
Potential predictive factors included child characteristics, injury factors and aspects of the post-injury environment. Child characteristics were age at injury, gender and ethnicity. Injury factors were mild TBI severity (low-, medium- or high-risk for complications), number of parent-reported prior TBIs, intentional injury (ie youth versus youth assault) and mechanism of injury. There were no suspected cases of child abuse in this sample. Post-injury environment factors were family socioeconomic status,20 and parental anxiety and depression.21 Family socioeconomic status was assessed using the Australia and New Zealand Standard Classification of Occupations20 and based on the highest skill level of either parent occupation. Parent anxiety and depression at 1-month post child TBI was assessed using the 14-item self-report Hospital Anxiety and Depression scale, with scores ranging from 0 to 21 (normal 0–7; mild 8–10; moderate 11–14; severe 15–21).21
Statistical analysis
Linear mixed-effects models were used to examine children’s recovery at 1-, 6- and 12-months post-injury compared to functioning in each respective domain at baseline; and to identify predictive factors of recovery over the year following injury from the variables listed in Table 1. Potential outcome predictors significantly correlated with each outcome were examined more closely following the addition of time since injury, age at injury, gender and ethnicity to each model. Injury factors were added to each model before the addition of any post-injury environmental factors. P < 0.05 denoted statistical significance. All analyses were performed using the R statistical package.22
|
Results
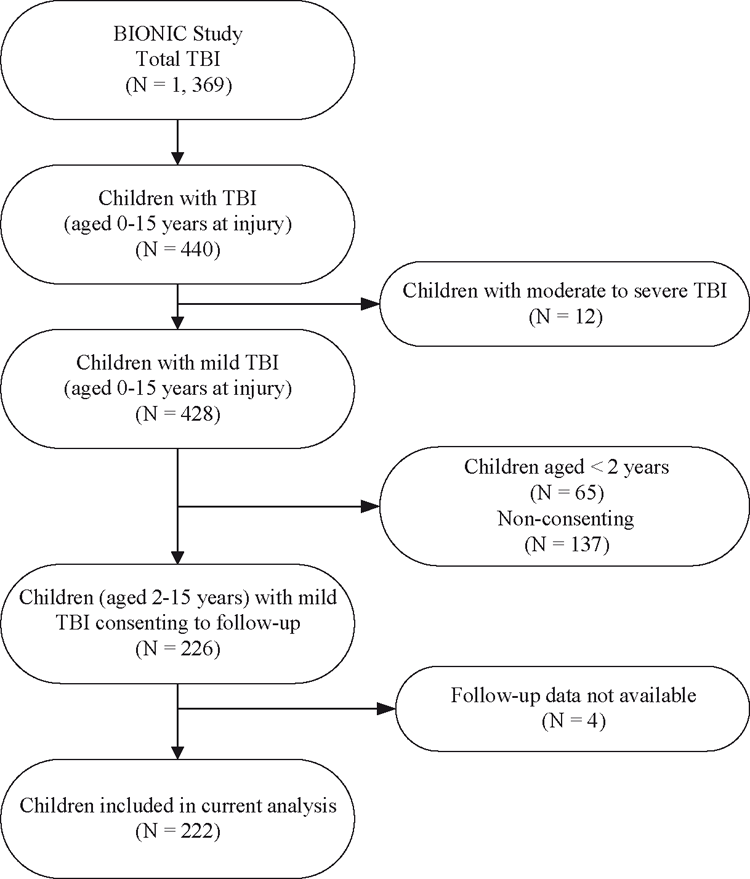
In total, 222 children with mild TBI (52% of the total child mild TBI incidence sample) were included in the current analysis (Fig. 1). Similar to current New Zealand population data,23 75% of children were urban residents. Over one-third of the sample sought treatment from community-based, healthcare providers.
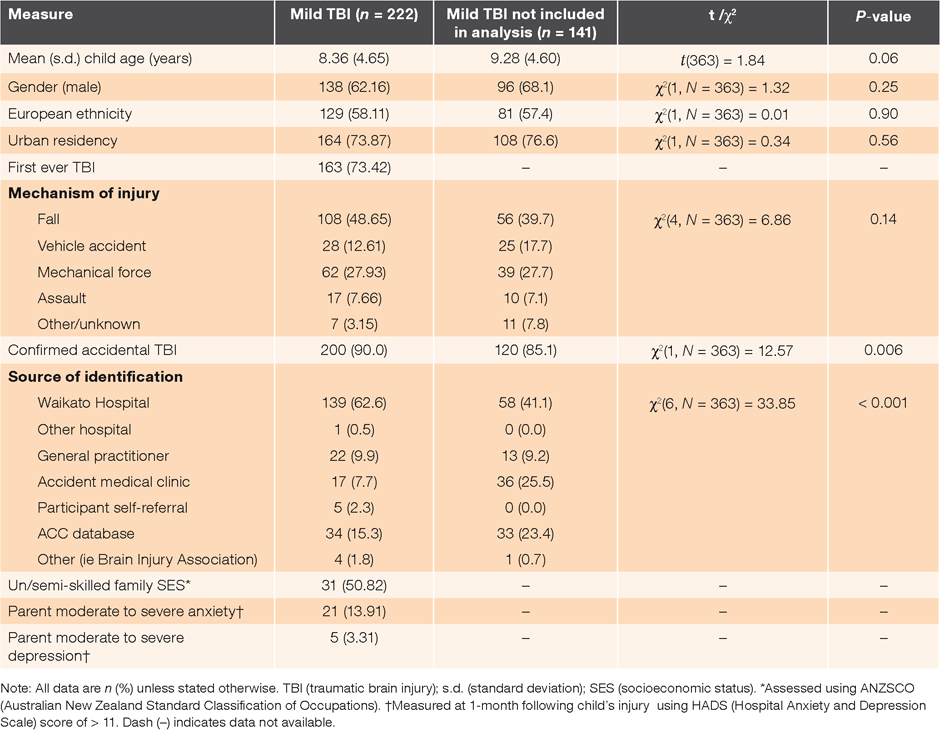
Comparative analyses examined children with mild TBI aged 2–15 years at injury (inclusive) who were included in the current analysis (n = 222) compared to all other children aged 2–15 years with mild TBI who were identified in the incidence sample but not included in this study (n = 141). Groups did not differ significantly in terms of age, gender, urban or rural residence, or mechanism of injury. Incidence cases not included in the current analysis were more likely to have been identified via sources outside of hospital (P < 0.001) and were less likely to have a confirmed accidental TBI (85% vs. 90% respectively, P = 0.006) than cases included in the current analysis (Table 1).
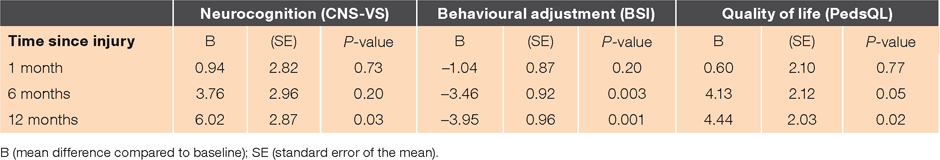
Table 2 reports the findings of linear mixed models examining parent-reported child behaviour and QOL at baseline, 1-, 6-, and 12-months post-injury. Significant improvements in overall behaviour were observed between baseline and 6 months (P < 0.001), and between baseline and 12 months (P < 0.001). The extent of these changes did not reach significance between baseline and 1 month (P > 0.05). Improvements in generalised cognitive function and QOL were significant between baseline and 12 months (P = 0.02 and P = 0.02 respectively), but did not reach significance between baseline and 1 month (P = 0.73, P = 0.77) or baseline and 6 months (P = 0.20, P = 0.05).
|
Predictors of outcomes
Low family socioeconomic status, male gender, living rurally and experiencing a non-accidental injury (P < 0.0001, P < 0.01, P = 0.001, P = 0.03 respectively) placed children at increased risk of poor generalised cognitive function at 12 months (Table 3). Similarly, low family socioeconomic status (P = 0.008) and moderate–severe parental anxiety at 1 month (P = 0.04) placed children at risk of poor behavioural adjustment over the year following mild TBI. Time since injury was the only significant predictor of QOL outcomes over the year following injury, with improvements reported over time.

|
Discussion
This study examined patterns of recovery over the year following mild TBI using a population-based sample of children, and examined predictors of outcome. We found that improvements in children’s generalised cognitive function, parent-reported behavioural adjustment and QOL extend over the year following injury, at least. We also identified opportunities to support children facing persistent difficulties, such as assisting parents to develop coping strategies to reduce anxiety and promote children’s behavioural recovery. Our findings also suggest an extended period for children’s on-going natural recovery, up until at least 12 months following mild TBI.
Consistent with the findings of a recent systematic review,6 improvements in child behaviour were evident during the first 6 months following injury. However, our findings go further to show that on-going improvements in children’s behaviour were evident 6- to 12-months post injury. This suggests that the window for supporting children’s behavioural recovery following mild TBI extends further than previously thought. Likewise, significant improvements in cognitive function and QOL were evident across the year following injury, although not significant until 12 months. This finding supports evidence from a review by Babikian and Asarnow (2009) suggesting that there may be a subset of children who experience long-term cognitive deficits in some domains.4
In terms of QOL, moderate-to-severe TBI has been associated with impairment in up to 40% of children at 12 months, 24 months, and beyond.24,25 Our findings suggest that mild TBI is also associated with risk for impaired QOL. This finding aligns with previous evidence suggesting that 13% of children with mild TBI have poor QOL at 12 months,26 higher than reports of 6% of healthy control children aged 6–12 years having poor QOL.27 Consistent with previous research,5 our findings reflect the changing nature of QOL over time, and suggest that repeated assessment is needed to obtain a more complete picture of the true affect of mild TBI on children and their families.
The current study did not include a control group free from TBI. However, all outcome measures were age-standardised and suitable for repeat administration. Therefore, we can be confident that the observed changes in scores over time reflect actual changes in children’s functioning rather than being due to children’s development over time.
Beyond allowing time for recovery, low socioeconomic status and elevated parental anxiety are modifiable factors that were associated with worse outcomes. Associations between socioeconomic disadvantage and adverse sequelae are commonly reported in normative and TBI samples, and are likely multifactorial in nature.28–31 In the family stress model, financial pressures exacerbate emotional and behavioural challenges for parents, with these difficulties then adversely affecting parenting and children’s outcomes.32 However, it remains unclear whether children following mild TBI face any greater risk of adverse outcomes due to socioeconomic disadvantage than any other group of children.
Similarly, poor mental health in parents is a recognised contributor to child outcomes in normative samples,32 and also following mild-to-moderate–severe TBI.33,34 A 2013 study of 132 children (aged 12–17 years) found parent psychiatric symptoms were the only consistent predictor of child internalising behaviour problems following complicated mild-to-severe TBI.34 A 2013 study of 150 children with mild TBI and their parents found greater parental distress was significantly associated with post-concussion symptoms at 18 months.35 Such links may be mediated by the development of maladjusted parenting practices. Alternatively, based on the depression–distortion hypothesis,36 parents with poor mental health may over-report their child’s behavioural problems. However, given the lack of association between parent anxiety and parent-reported QOL, it seems unlikely that reporting bias accounts for the associations found in the current study. There were no interaction effects between low socioeconomic status and parent anxiety, suggesting that each make unique and important contributions to children’s recovery after mild TBI.
Our findings highlight parental anxiety as a potentially modifiable factor that may be addressed by interventions aiming to promote children’s behavioural recovery post-mild TBI as well as parents’ coping mechanisms. For example, emerging evidence suggests that group parenting interventions incorporating programs such as Stepping Stones, Triple P and Acceptance and Commitment Therapy may be effective in improving outcomes for children and their parents.37
Our study extends earlier research in several ways. This study prospectively examined a population-based sample using a longitudinal data analysis. Standard, well-validated measures appropriate for repeat administration were used to examine children’s recovery across several areas of functioning. A broad range of potential predictive factors were examined in this population-based sample that was found to be representative of children with mild TBI in the study region, enhancing the generalisability of findings. A limitation of this study was not having controlled for pre-injury function, an inherent difficulty in paediatric TBI studies. However, our use of age-standardised measures alongside evidence of continuing improvements in scores over time suggest that children are not likely to return to their pre-injury level of functioning until 12-months post-injury, at least. It is possible that repeated administration led to some form of practice effects, although the CNS-VS was designed to reduce the chances of these occurring by altering the administration slightly across tests. A further limitation was the use of a cognitive assessment that did not allow inclusion of the entire sample in some aspects of the analysis. Measurement limitations are a common challenge in studies examining such an extensive age range in children.
With increasing numbers of children with mild TBI presenting for treatment in primary care, these findings are relevant to general practice training and for the monitoring of children’s recovery. While direct links between cause and effect cannot be determined by our data, taken together, our findings suggest that the recovery period for children with mild TBI extends beyond an initial 6-month period through to at least 12-months post-injury. Links found between rural residency, low socioeconomic status, intentional injury, parental anxiety and poor outcomes from mild TBI clearly show a range of psychosocial factors influence recovery.
Together, these findings suggest that some children may benefit from closer, longer-term monitoring following mild TBI, and may require different types of intervention to reach developmental milestones. Our findings highlight potentially malleable prognostic factors (eg parent anxiety) associated with poor outcomes for children. While the pathways implicated in children’s recovery and parental anxiety are complex and likely to be inter-related, these factors should be considered when training clinicians in supporting the recovery of children experiencing persistent difficulties following mild TBI. For example, intervention efforts directed towards the optimisation of positive parenting behaviour may be helpful in addressing parental anxiety levels, as well as promoting children’s recovery.
We used a measure of generalised cognitive function in the current analysis, so further work is needed to examine sub-components of cognitive function that follow different developmental trajectories, and therefore may be differentially affected by mild TBI. Further, the potential for longer-term associations between childhood mild TBI and poor developmental outcomes, and the identification of predictive factors beyond the first year of recovery are yet to be determined. These are needed to inform understanding of the full manifestation of childhood mild TBI, and to enable more accurate identification of children at-risk of unrelenting and potentially cumulative difficulties.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the children and their families, researchers and community-based, health-care providers involved in this study. Funding was provided by the Health Research Council of New Zealand (grant number 09/063A). The funders had no role in the study design, data collection and analysis, preparation of the manuscript or the decision to submit for publication.
References
[1] Feigin VL, Theadom A, Barker-Collo S, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013; 12 53–64.| Incidence of traumatic brain injury in New Zealand: a population-based study.Crossref | GoogleScholarGoogle Scholar |
[2] Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004; 43 28–60.
| Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[3] Bazarian JJ, McClung J, Shah MN, et al. Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 2005; 19 85–91.
| Mild traumatic brain injury in the United States, 1998–2000.Crossref | GoogleScholarGoogle Scholar |
[4] Babikian T, Asarnow RF. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychol Rev. 2009; 23 283–96.
[5] Battista AD, Soo C, Catroppa C, Anderson V. Quality of life in children and adolescents post-TBI: a systematic review and meta-analysis. J Neurotrauma. 2012; 29 1717–27.
| Quality of life in children and adolescents post-TBI: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[6] Emery CA, Barlow KM, Brooks BL, et al. A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can J Psychiatry. 2016; 61 259–69.
| A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents.Crossref | GoogleScholarGoogle Scholar |
[7] Bowman SM, Bird TM, Aitken ME. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics. 2008; 122 988–93.
| Trends in hospitalizations associated with pediatric traumatic brain injuries.Crossref | GoogleScholarGoogle Scholar |
[8] McKinlay A. Controversies and outcomes associated with mild traumatic brain injury in childhood and adolescence. Child Care Health Dev. 2010; 36 3–21.
| Controversies and outcomes associated with mild traumatic brain injury in childhood and adolescence.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c%2FjsVOltw%3D%3D&md5=55649acef3fd81d2640c2d4dddc69890CAS |
[9] Theadom A, Barker-Collo S, Feigin V, et al. The spectrum captured: a methodological approach to studying incidence and outcomes of traumatic brain injury on a population level. Neuroepidemiology. 2012; 38 18–29.
| The spectrum captured: a methodological approach to studying incidence and outcomes of traumatic brain injury on a population level.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC387ntVSisw%3D%3D&md5=a98be673b516c2ff650080210dab134dCAS |
[10] Carroll LJ, Cassidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004; 43 113–25.
| Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury.Crossref | GoogleScholarGoogle Scholar |
[11] Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004; 43 84–105.
| Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury.Crossref | GoogleScholarGoogle Scholar |
[12] Reynolds CR, Kamphaus RW. Behavior assessment system for children (BASC). Circle Pines, MN: American Guidance Service, Inc.; 1992.
[13] Reynolds C, Kamphaus RW. Behavior assessment system for children. Circle Pines, MN: American Guidance Service, Inc.; 1998.
[14] Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 Generic core scales in healthy and patient populations. Med Care. 2001; 39 800–12.
| PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 Generic core scales in healthy and patient populations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvitVGgtw%3D%3D&md5=4a0d10867f20798f66cf183ac1ed5381CAS |
[15] Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007; 5 2
| Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL™ 4.0 Generic Core Scales.Crossref | GoogleScholarGoogle Scholar |
[16] Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006; 21 623–43.
| Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs.Crossref | GoogleScholarGoogle Scholar |
[17] Gualtieri CT, Johnson LG. Efficient allocation of attentional resources in patients with ADHD: maturational changes from age 10 to 29. J Atten Disord. 2006; 9 534–42.
| Efficient allocation of attentional resources in patients with ADHD: maturational changes from age 10 to 29.Crossref | GoogleScholarGoogle Scholar |
[18] Gualtieri CT, Johnson LG. Antidepressant side effects in children and adolescents. J Child Adolesc Psychopharmacol. 2006; 16 147–57.
| Antidepressant side effects in children and adolescents.Crossref | GoogleScholarGoogle Scholar |
[19] Gualtieri CT. A computerized test battery sensitive to mild and severe brain injury. Medscape J Med. 2008; 10 90
[20] Trewin D, Pink B. Australian and New Zealand standard classification of occupations. Canberra, ACT, Australia: Australian Bureau of Statistics; 2006.
[21] Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67 361–70.
| The hospital anxiety and depression scale.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s3nvFWjug%3D%3D&md5=5d6e0c2352a7630fc23839003e338ab5CAS |
[22] Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
[23] Statistics New Zealand. Urban and rural migration tables 1-17. Wellington: Statistics New Zealand; 2006. [cited 2017 February 24]. Available from: http://www.stats.govt.nz/browse_for_stats/population/migration/internal-migration/urban-rural-migration.aspx
[24] McCarthy ML, MacKenzie EJ, Durbin DR, et al. Health-related quality of life during the first year after traumatic brain injury. Arch Pediatr Adolesc Med. 2006; 160 252–60.
| Health-related quality of life during the first year after traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[25] Stancin T, Drotar D, Taylor HG, et al. Health-related quality of life in children and adolescents after traumatic brain injury. Pediatrics. 2002; 109 e34
| Health-related quality of life in children and adolescents after traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[26] Zonfrillo MR, Dennis DR, Koepsell T, et al. Prevalence of and risk factors for poor functioning after isolated mild traumatic brain injury in children. J Neurotrauma. 2014; 31 722–7.
| Prevalence of and risk factors for poor functioning after isolated mild traumatic brain injury in children.Crossref | GoogleScholarGoogle Scholar |
[27] Khairy SA, Eid SR, El Hadidy LM, et al. The health-related quality of life in normal and obese children. Egypt Pediatr Assoc Gaz. 2016; 64 53–60.
| The health-related quality of life in normal and obese children.Crossref | GoogleScholarGoogle Scholar |
[28] Mangeot S, Armstrong K, Colvin AN, et al. Long-term executive function deficits in children with traumatic brain injuries: assessment using the Behavior Rating Inventory of Executive Function (BRIEF). Child Neuropsychol. 2002; 8 271–84.
| Long-term executive function deficits in children with traumatic brain injuries: assessment using the Behavior Rating Inventory of Executive Function (BRIEF).Crossref | GoogleScholarGoogle Scholar |
[29] Anderson VA, Catroppa C, Rosenfeld J, et al. Recovery of memory function following traumatic brain injury in pre-school children. Brain Inj. 2000; 14 679–92.
| Recovery of memory function following traumatic brain injury in pre-school children.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7gvFejsg%3D%3D&md5=6aacf6da18858ebb2b817766347c9d1eCAS |
[30] Crowe LM, Catroppa C, Babl FE, Anderson V. Executive function outcomes of children with traumatic brain injury sustained before three years. Child Neuropsychol. 2013; 19 113–26.
| Executive function outcomes of children with traumatic brain injury sustained before three years.Crossref | GoogleScholarGoogle Scholar |
[31] Loher S, Fatzer ST, Roebers CM. Executive functions after pediatric mild traumatic brain injury: a prospective short-term longitudinal study. Appl Neuropsychol Child. 2014; 3 103–14.
| Executive functions after pediatric mild traumatic brain injury: a prospective short-term longitudinal study.Crossref | GoogleScholarGoogle Scholar |
[32] Masarik AS, Conger RD. Stress and child development: a review of the family stress model. Current Opinion in Psychology. 2017; 13 85–90.
[33] Raj SP, Wade SL, Cassedy A, et al. Parent psychological functioning and communication predict externalizing behavior problems after pediatric traumatic brain injury. J Pediatr Psychol. 2014; 39 84–95.
| Parent psychological functioning and communication predict externalizing behavior problems after pediatric traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[34] Peterson RL, Kirkwood MW, Gerry Taylor H, et al. Adolescents’ internalizing problems following traumatic brain injury are related to parents’ psychiatric symptoms. J Head Trauma Rehab. 2014; 28 E1–E12.
[35] Olsson KA, Lloyd OT, LeBrocque RM, et al. Predictors of child post-concussion symptoms at 6 and 18 months following mild traumatic brain injury. Brain Inj. 2011; 27
| Predictors of child post-concussion symptoms at 6 and 18 months following mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[36] Richters JE. Depressed mothers as informants about their children: A critical review of the evidence for distortion. Psychol Bull. 1992; 112 485–99.
| 1:STN:280:DyaK3s%2Fms1yhtQ%3D%3D&md5=30bb511f378cf409c077dfd6ec12fed8CAS |
[37] Brown FL, Whittingham K, Boyd RN, et al. Improving child and parenting outcomes following paediatric acquired brain injury: a randomised controlled trial of Steeping Stones Triple P plus Acceptance and Commitment Therapy. J Child Psychol Psychiatry. 2014; 55 1172–13.