Platynini (Coleoptera : Carabidae) of Vanuatu: Miocene diversification on the Melanesian Arc
James K. LiebherrA Department of Entomology, John H. and Anna B. Comstock Hall, Cornell University, Ithaca, NY 14853-0901, USA.
B Email: jkl5@cornell.edu
Invertebrate Systematics 19(4) 263-295 https://doi.org/10.1071/IS04032
Submitted: 3 November 2004 Accepted: 26 June 2005 Published: 30 September 2005
Abstract
Vanuatu supports 11 resident species of the carabid beetle tribe Platynini: five indigenous species shared with other Pacific islands and Australia and six newly described precinctive species. Notagonum delaruei, sp. nov. represents a single descendant species of one colonisation event. Helluocolpodes, gen. nov. (type species Colpodes helluo Darlington of New Guinea) is proposed to accommodate a monophylum comprising the type species plus Helluocolpodes discicollis, sp. nov., H. mucronis, sp. nov., H. multipunctatus, sp. nov., H. sinister, sp. nov. and H. vanemdeni, sp. nov., all from Vanuatu. Generic assignments are informed by cladistic analysis of anatomical characters for a variety of Pacific platynine taxa. Metacolpodes Jeannel is redefined cladistically to include seven Pacific and Asian species. Biogeographic relationships among island areas housing platynine taxa on the Australian and Pacific Plates are investigated using a chrono–area cladogram, i.e. a taxon–area cladogram for which terminals are dated based on geological evidence and internal nodes based on non-reversible temporal optimisation. Conclusions reached by constraining the ages of areas within the context of phylogenetic relationships of their resident taxa include: (1) Vanuatu has supported resident platynine taxa since the Middle to Late Miocene; (2) the Hawaiian Blackburnia first colonised that archipelago in the Miocene, long before the present oldest high island, Kauai, came into existence; (3) the New Zealand Ctenognathus most likely arose from Miocene colonisation of New Zealand via Fiji; and (4) the low diversity of the Tahitian platynine fauna is due to relatively recent, Pliocene or later, colonisation of the Society Island chain by this group, also from a Fijian source.
Additional keywords: biogeography, Helluocolpodes, New Hebrides.
Acknowledgments
Much of the phylogenetic data was elucidated during the course of research supported by N.S.F. awards DEB-9208269 and DEB-9806349. Manuscript preparation was supported by N.S.F. award DEB-0315504. I thank Helène Perrin and Thierry Deuve for their forebearance in the extended loan of Aubert de la Rüe’s Vanuatuan specimens. Dan A. Polhemus provided helpful criticism and useful references for the geology of the south-west Pacific.
Ahmad I., Kamaluddin S.
(1981) A new species of the genus Catacanthus Spinola (Heteroptera: Pentatomidae: Pentatominae) from the New Hebrides with morphological notes on two other Australasian species and their relationships. Records of the South Australian Museum 18, 227–233.
for further discussion of state assignments; characters noted as (*) are new to this analysis and are discussed under Materials and methods: Characters. Bracketed character statistics [L = length or number of steps; ci = consistency index; ri = retention index] based on first of nine most parsimonious cladograms in output file (see Results: Cladistic analysis).
Character 0 (1). Apical gonocoxite 2: with 1 dorsal ensiform seta (0); lacking dorsal ensiform seta (1). [L = 2; ci = 0.50; ri = 0]
Character 1 (2). Lateral ensiform setae of apical gonocoxite 2: moderately long to elongate, > 0.12 × length gonocoxite (0); very short, peg-like, up to 0.12 × length gonocoxite (1). [L = 3; ci = 0.33; ri = 0.50]
Character 2 (4). Lateral ensiform setae of apical gonocoxite 2: short to moderately elongate (0); very long, broad, more than 0.23 × length gonocoxite (1). [L = 5; ci = 0.20; ri = 0.20]
Note: Characters 1 and 2 are related, with the plesiomorphic state for both (0,0) being ensiform setae between 0.12 × and 0.23 × length of apical gonocoxite 2.
Character 3 (5). Apical gonocoxite 2 tip: acuminate (0); rounded (1). [L = 3; ci = 0.33; ri = 0]
Character 4 (6). Apical gonocoxite 2: triangular (0); subparallel and narrow (1). [L = 7; ci = 0.14; ri = 0.57]
Character 5 (19). Bursa copulatrix: symmetrical or with left lobe (0); with right lobe (1). [L = 3; ci = 0.33; ri = 0]
Character 6 (20). Bursa copulatrix: with medial band of spike-like microtrichia (0); band of mixed spikes and cristae (1). [L = 2; ci = 0.50; ri = 0.50]
Character 7 (21). Bursa copulatrix: with medial band of spikes or mixed spikes and cristae (0); totally with cristae (1). [L = 2; ci = 0.50; ri = 0]
Note: Characters 6 and 7 are part of a two-step transformation series.
Character 8 (22). Bursal microtrichia: dense (0); sparse to glabrous (1). [L = 9; ci = 0.11; ri = 0.42]
Character 9 (*). Bursal microtrichia: present (0); absent (1). [L = 7; ci = 0.14; ri = 0.33]
Character 10 (23). Dorsal bursal pouch: absent (0); present (1). [L = 7; ci = 0.14; ri = 0.68]
Note: The dorsal bursal pouch can be distinguished from the basal bursal pouch, as the former is situated at the position in which the lumenal band of microtrichia is observed when it is present. The basal dorsal pouch is much closer to the base of the bursa, dorsad the bases of the gonocoxites.
Character 11 (24). Dorsal bursal pouch: broad, rounded, unsclerotised (0); narrow, keyhole-shaped (1). [L = 5; ci = 0.20; ri = 0.20]
Character 12 (28). Dorsal bursal pouch intima: unsclerotised (0); sclerotised with microsculpture (1). [L = 6; ci = 0.16; ri = 0.54]
Character 13 (30). Dorsal bursal pouch: unsclerotised (0); sclerotised in lip-like or collar-like shape (1). [L = 3; ci = 0.33; ri = 0]
Character 14 (31). Basal bursal pouch: absent (0); present (1). [L = 1; ci = 1.0; ri = 1.0]
Character 15 (32). Basal bursal pouch: broadly rounded, unsclerotised (0); heavily sclerotised, keyhole or pocket shaped (1). [L = 1; ci = 1.0; ri = 1.0]
Note: These characters are linked in this analysis. The more generalised condition of the basal pouch (1, 0) is observed in Ctenognathus parabilis and C. bidens, where a broad membranous lobe occurs across the dorsal bursal base. In Metacolpodes spp., the pouch at this position is highly sclerotised (1, 1).
Character 16 (33). Bursa copulatrix: subequal to 1.5 × length common oviduct (0); 1.6–2.5 × length common oviduct (1). [L = 6; ci = 0.16; ri = 0.70]
Character 17 (34). Bursa copulatrix: less than 2.5 × length common oviduct (0); more than 2.6 length common oviduct (1). [L = 3; ci = 0.33; ri = 0.80]
Note: Characters 16 and 17 define a two-step transformation series, with the plesiomorphic state (0,0) being bursal length less than 1.6 × the common oviduct length.
Character 18 (41). Spermatheca reservoir: with 12–20 constrictions (0); more than 30 constrictions (1). [L = 7; ci = 0.14; ri = 0.33]
Character 19 (42). Spermathecal reservoir: apical (0); basal, apical filament present (1). [L = 3; ci = 0.33; ri = 0]
Character 20 (43). Vaginal setae: not pediculate (0); pediculate (1). [L = 10; ci = 0.10; ri = 0.52]
Character 21 (44). Bursa copulatrix: membranous (0); leathery (1). [L = 2; ci = 0.50; ri = 0.90]
Character 22 (45). Aedeagal median lobe: gracile (0); stout (1). [L = 6; ci = 0.16; ri = 0.37]
Character 23 (46). Aedeagal median lobe: evenly curved euventrally (0); straight (1). [L = 9; ci = 0.11; ri = 0.55]
Character 24 (47). Aedeagal median lobe: curved or straight (0); recurved (1). [L = 2; ci = 0.50; ri = 0]
Note: Characters 23 and 24 define a two-step transformation series.
Character 25 (49). Aedeagal median lobe: without euventral excavation (0); with excavation (1). [L = 9; ci = 0.11; ri = 0.20]
Character 26 (50). Aedeagal median lobe apex: evenly curved (0); angled ventrally (1). [L = 6; ci = 0.16; ri = 0]
Character 27 (52). Aedeagal median lobe apex: curved or angled (0); straight (1). [L = 6; ci = 0.16; ri = 0]
Note: Characters 25 and 26 define a two-step transformation series.
Character 28 (53). Aedeagal median lobe tip: tightly rounded to broad (0); acuminate (1). [L = 6; ci = 0.16; ri = 0.68]
Character 29 (54). Aedeagal median lobe tip: not downturned (0); downturned (1). [L = 6; ci = 0.16; ri = 0.72]
Character 30 (55). Aedeagal median lobe tip: tightly rounded or acuminate (0); broad, bottlenose (1). [L = 3; ci = 0.33; ri = 0.50]
Note: Characters 28 and 30 define a divergent transformation series, with a tightly rounded tip the plesiomorphic state for both.
Character 31 (56). Aedeagal median lobe tip: less than 2 × long as deep (0); more than 2 × (1). [L = 7; ci = 0.14; ri = 0.62]
Character 32 (*). Aedeagal tip: extended beyond membranous base of internal sac (0); extremely short, not extended beyond sac insertion (1). [L = 2; ci = 0.50; ri = 0.66]
Character 33 (57). Aedeagal median lobe tip: not hooked (0); hooked (1). [L = 1; ci = 1.0; ri = 1.0]
Character 34 (59). Apical half of median lobe, euventral view: evenly narrowed to tip (0); slightly pinched before tip (1). [L = 7; ci = 0.14; ri = 0.57]
Character 35 (60). Apical half of median lobe, euventral view: slightly pinched before tip (0); attenuate (1). [L = 3; ci = 0.33; ri = 0.88]
Character 36 (61). Apical half of median lobe, euventral view: evenly narrowed to attenuate (0); strongly attenuate, nipple-like (1). [L = 5; ci = 0.20; ri = 0.66]
Note: Characters 34–36 define a three-step transformation series.
Character 37 (63). Aedeagal median lobe shaft: evenly narrowed from base (0); constricted medially (1). [L = 2; ci = 0.50; ri = 0]
Character 38 (64). Aedeagal median lobe shaft: of moderate breadth, euventral view (0); narrow, needle-like (1). [L = 1; ci = 1.0; ri = 1.0]
Character 39 (65). Aedeagal median lobe shaft: normal depth, lateral view (0); slender (1). [L = 3; ci = 0.33; ri = 0.71]
Character 40 (67). Aedeagal internal sac surface: with only small, pale spicules (0); with fields of darker, sclerotised spicules (1). [L = 5; ci = 0.20; ri = 0.69]
Character 41 (*). Aedeagal internal sac surface: without large spines (0); with 1 or more large spines (1). [L = 6; ci = 0.16; ri = 0.50]
Character 42 (*). Testis configuration: bilateral, two (0); unilateral, one on right side (1). [L = 1; ci = 1.0; ri = 1.0]
Character 43 (68). Ocular ratio: 1.38 to 1.61 (0); more than 1.63 (1). [L = 5; ci = 0.20; ri = 0.55]
Character 44 (71). Eye diameter: large relative to depth (0); small, eyes protruding, bugeyed (1). [L = 1; ci = 1.0; ri = 1.0]
Character 45 (73). Labral apex: straight medially (0); broadly, shallowly emarginate (1). [L = 9; ci = 0.11; ri = 0.52]
Character 46 (74). Labral apex: straight to shallowly emarginate (0); broadly, moderately emarginate (1). [L = 4; ci = 0.25; ri = 0]
Note: Characters 45 and 46 define a two-step transformation series.
Character 47 (76). Frons microsculpture: isodiametric, shiny (0); granulate (1). [L = 2; ci = 0.50; ri = 0.66]
Character 48 (77). Frons microsculpture: isodiametric, shiny (0); less developed isodiametric (1). [L = 4; ci = 0.25; ri = 0.83]
Character 49 (78). Frons microsculpture: present (0); reduced, surface shiny (1). [L = 8; ci = 0.12; ri = 0.56]
Note: Characters 47–49 define a divergent three-step transformation series, with shiny isodiametric microsculpture the plesiomorphic state.
Character 50 (79). Neck: with strong dorsal impression (0); with slight impression (1). [L = 1; ci = 1.0; ri = 1.0]
Character 51 (81). Pedicel: with 1 outer apical seta (0); with outer seta plus 1 or 2 others (1). [L = 1; ci = 1.0; ri = 1.0]
Character 52 (85). Mandibles: moderate, acuminate (0); elongate (1). [L = 3; ci = 0.33; ri = 0.33]
Character 53 (89). Mentum tooth: triangular to rounded (0); truncate to slightly bifid (1). [L = 3; ci = 0.33; ri = 0.33]
Character 54 (91). Pronotal marginal gutter: narrow to broad, edge upturned (0); very broad (1). [L = 3; ci = 0.33; ri = 0.75]
Character 55 (92). Pronotal marginal gutter: broad to very broad, edge upturned (0); moderate, edge upturned (1). [L = 7; ci = 0.14; ri = 0.45]
Character 56 (93). Pronotal marginal gutter: broad to moderate, edge upturned (0); moderate, edge beaded (1). [L = 2; ci = 0.50; ri = 0.50]
Character 57 (94). Pronotal marginal gutter: broad, moderate, edge upturned or beaded (0); narrow, edge upturned (1). [L = 2; ci = 0.50; ri = 0.50]
Note: Characters 54–57 are related. A moderately broad marginal gutter with an upturned edge is plesiomorphic. Derived from that condition are an expanded gutter with upturned ege on one hand, and progressive narrowing, and development of a marginal bead on the other.
Character 58 (97). Anterior pronotal setae: present (0); absent (1). [L = 3; ci = 0.33; ri = 0.33]
Character 59 (*). Anterior pronotal seta: not directly at margin of lateral explanation (0); set directly on margin (1). [L = 2; ci = 0.50; ri = 0.75]
Character 60 (98). Posterior pronotal seta: present (0); absent (1). [L = 3; ci = 0.33; ri = 0.33]
Character 61 (99). Pronotal basal bead: complete (0); effaced medially (1). [L = 7; ci = 0.14; ri = 0.40]
Character 62 (100). Pronotal basal bead: present at least laterally (0); effaced medially and laterally (1). [L = 2; ci = 0.50; ri = 0.50]
Note: Characters 61 and 62 define a two-step transformation series.
Character 63 (103). Pronotal basolateral margin: straight (0); expanded posteriorly (1). [L = 7; ci = 0.14; ri = 0.64]
Character 64 (107). Pronotal laterobasal depressions: smooth (0); punctate (1). [L = 11, ci = 0.09; ri = 0.41]
Character 65 (112). Pronotal hind angles: sharp (0); obtuse-rounded (1). [L = 9; ci = 0.11; ri = 0.38]
Character 66 (115). Pronotal basolateral margins: sinuate before hind angles (0); straight or convex (1). [L = 3; ci = 0.33; ri = 0]
Character 67 (116). Pronotal disc microsculpture: transverse mesh (0); granulate (1). [L = 2; ci = 0.50; ri = 0.50]
Character 68 (117). Pronotal disc microsculpture: transverse mesh (0); reduced transverse mesh (1). [L = 9; ci = 0.11; ri = 0.61]
Character 69 (118). Pronotal disc microsculpture: evident mesh (0); obsolete, surface shiny (1). [L = 2; ci = 0.50; ri = 0]
Note: Characters 66–68 are related, with transverse mesh microsculpture the plesiomorphic condition, and either granulate microsculpture, or reduced microsculpture two types of specialisation.
Character 70 (119). Pronotal laterobasal depression microscuplture: transverse mesh (0); isodiametric mesh (1). [L = 9; ci = 0.11; ri = 0.33]
Character 71 (121). Prosternal process: not margined (0); dorsal triangle on posterior face (1). [L = 6; ci = 0.16; ri = 0.73]
Character 72 (126). Pronotal hind seta: at hind angle (0); 0.02–0.21 × median pronotal length before angle (1). [L = 3; ci = 0.33; ri = 0.50]
Character 73 (130). Dorsal elytral setae: 3–4 (0); 4–5 (1). [L = 3; ci = 0.33; ri = 0]
Character 74 (132). Dorsal elytral setae: not in foveae, or setae absent (0); set in foveae (1). [L = 2; ci = 0.50; ri = 0]
Character 75 (*). Elytral apical margin: smooth (0); with irregular granulate serrations (1). [L = 1; ci = 1.0; ri = 1.0]
Character 76 (*). Elytral subapical sinuation: smooth (0); with irregular granulate serrations (1). [L = 1; ci = 1.0; ri = 1.0]
Character 77 (135). Sutural apex: rounded, non-denticulate (0); short tooth present (1). [L = 7; ci = 0.14; ri = 0.40]
Character 78 (136). Sutural apex: non-denticulate or with short tooth (0); with longer spine (1). [L = 4; ci = 0.25; ri = 0.40]
Note: Characters 77 and 78 define a two-step transformation series.
Character 79 (137). Elytral subapical tooth: absent (0); present as prominence (1). [L = 8; ci = 0.12; ri = 0.61]
Character 80 (139). Elytral striae: smooth. continuous (0); punctate at least partially (1). [L = 7; ci = 0.14; ri = 0.50]
Character 81 (141). Eytral strial punctures: fine (0); moderate, expanding striae laterally (1). [L = 3; ci = 0.20; ri = 0]
Character 82 (143). Elytral strial punctures: even throughout length (0); strongest in basal 1/2 to 2/3 (1). [L = 5; ci = 0.20; ri = 0]
Character 83 (146). Elytral intervals: without costae (0); weakly rounded costae on intervals 1, 5, 7 (1). [L = 2; ci = 0.50; ri = 0]
Character 84 (151). Elytral intervals: slightly convex to costate (0); nearly flat (1). [L = 2; ci = 0.20; ri = 0.33]
Character 85 (152). Elytral intervals: slightly convex to costate (0); broadly convex (1). [L = 4; ci = 0.25; ri = 0.25]
Note: Characters 84 and 85 define a divergent two-step transformation series, with slightly convex to costate intervals the plesiomorphic state.
Character 86 (154). Humeri: broad (0); slightly narrowed (1). [L = 6; ci = 0.16; ri = 0.44]
Character 87 (156). Humeral angle: rounded (0); tightly rounded or angulate (1). [L = 6; ci = 0.16; ri = 0.44]
Character 88 (157). Humeri: rounded to tightly rounded (0); angulate (1). [L = 3; ci = 0.33; ri = 0.33]
Note: Characters 87 and 88 define a two-step transformation series.
Character 89 (163). Lateral elytral setae: 16–32 (0); 8–16 (1). [L = 3; ci = 0.33; ri = 0.66]
Character 90 (169). Elytral microsculpture: transverse mesh 2–3 × broad as long (0); fine microlines without mesh (1). [L = 8; ci = 0.12; ri = 0.56]
Character 91 (170). Elytral microsculpture: transverse mesh or lines (0); transverse isodiametric mesh (1). [L = 7; ci = 0.14; ri = 0.57]
Character 92 (171). Elytral microsculpture: transverse (0); isodiametric (1). [L = 7; ci = 0.14; ri = 0.33]
Character 93 (172). Elytral microsculpture: transverse or isodiametric (0); granulate (1). [L = 5; ci = 0.20; ri = 0.33]
Note: Characters 90–94 are related. Transverse mesh microsculpture is plesiomorphic, with derivation occurring in two manners; 1, to microsculpture composed of fine transverse lines, and 2, progressive evolution of first isodiametric microsculpture, and then rough, granulate isodiametric microsculpture.
Character 94 (173). Elytral microsculpture: present (0); reduced but traceable (1). [L = 2; ci = 0.50; ri = 0.50]
Character 95 (*). Dorsal body surface: glabrous except for fixed macrosetae (0); with fine pelage of fine microsetae (1). [L = 1; ci = 1.0; ri = 1.0]
Character 96 (177). Apical female abdominal segment: 2 setae each side (0); unilaterally or bilaterally 3 for a total of 5 or 6 setae, or bilaterally 5 setae for a total of 10 (1). [L = 2; ci = 0.50; ri = 0]
Character 97 (178). Apical female abdominal segment: 2 setae each side (0); bilaterally 4 for a total of 8 (1). [L = 1; ci = 1.0; ri = 1.0]
Note: Characters 96 and 97 define three means of increase for apical abdominal setae in the females from the 4-setose plesiomorphic condition. The autapomorphous 10-setose B. mandibularis is grouped with with 5–6 setose taxa, whereas the 8-setose taxa are viewed as an independent doubling of the 4-setose condition.
Character 98 (179). Profemur with: 0–1 anteroventral setae (0); 2 anteroventral setae (1). [L = 3; ci = 0.33; ri = 0]
Character 99 (180). Mesofemur with: 2–3 anteroventral setae (0); 3–7 anteroventral setae (1). [L = 4; ci = 0.25; ri = 0.72]
Character 100 (181). Metacoxa with: 3 setae (0); inner seta absent, outer 2 present (1). [L = 5; ci = 0.20; ri = 0.42]
Character 101 (182). Metafemur with: 1 or more dorsoapical setae (0); dorsoapically glabrous (1). [L = 7; ci = 0.14; ri = 0.60]
Character 102 (183). Metatarsi: gracile (0); broadened apically (1). [L = 5; ci = 0.20; ri = 0.60]
Character 103 (184). Metatarsi: gracile or broadened apically (0); broad over all of length (1). [L = 2; ci = 0.50; ri = 0]
Note: Characters 102 and 103 define a two-step transformation series.
Character 104 (186). Ventral tarsal vestituture: sparse on metatarsomeres 2–4 (0); thicker, densely packed (1). [L = 6; ci = 0.16; ri = 0.73]
Character 105 (187). Ventral tarsal vestituture: sparse to dense with central space (0); densely setose, central space reduced (1). [L = 2; ci = 0.50; ri = 0]
Note: Characters 104 and 105 define a two-step transformation series.
Character 106 (188). Metatarsomeres 1–3 with: moderate inner and outer sulci (0); narrow inner and outer sulci (1). [L = 5; ci = 0.20; ri = 0.55]
Character 107 (189). Metatarsomeres 1–3 with: inner and outer sulci present (0); outer sulcus fine, inner obsolete (1). [L = 3; ci = 0.33; ri = 0.50]
Character 108 (191). Metatarsomeres 1–3 with: sulci present or absent (0); sulci so deep to produce medially carinate tarsomeres (1). [L = 5; ci = 0.20; ri = 0.75]
Note: Characters 106–108 are related. Plesiomorphically inner and outer dorsal sulci are present. These may be reduced (106–107), or enhanced (108).
Character 109 (192). Metatarsomere 4 outer lobe: less than length tarsal base (0); 1.1–1.75 × length tarsal base (1). [L = 5; ci = 0.20; ri = 0.20]
Character 110 (195). Metarsomere 4 outer lobe: 1–1.5 × length inner lobe (0); more than 1.5 × length inner lobe (1). [L = 6; ci = 0.16; ri = 0.73]
Note: Characters 109–110 define a two-step transformation series.
Character 111 (196). Metatarsomere 5 ventral surface: apparently glabrous to setae 1/2 depth of metatarsomere 5 (0); with 4–6 setae subequal in length to depth of metatarsomere 5 (1). [L = 5; ci = 0.20; ri = 0.33]
Character 112 (198). Metatarsomere 5 ventral surface: apparently glabrous or with 4–6 setae (0); with 4–18 setae (1). [L = 4; ci = 0.25; ri = 0.25]
Note: Characters 111–112 define a two-step transformation series.
Character 113 (199). Metatarsomere 4 apical setae: set apically (0); set subapically (1). [L = 6; ci = 0.16; ri = 0.68]
Character 114 (200). Flight wing ratio: 2.9–5.0 (0); 1.4–2.3 (1). [L = 4; ci = 0.25; ri = 0.57]
Character 115 (201). Flight wing ratio: 1.4–5.0 (0); 0.4–1.1 (1). [L = 4; ci = 0.25; ri = 0.40]
Character 116 (203). Flight wings: full or brachypterous, WR more than 0.4 (0); vestigial, extending slightly beyond metanotum (1). [L = 2; ci = 0.50; ri = 0.66]
Note: See Liebherr & Zimmerman (1998) for a full discussion of wing reduction in these beetles.
Character 117 (205). Body length: 4.6–10.9 mm (0); 10.9–16.0 mm (1). [L = 5; ci = 0.20; ri = 0.71]
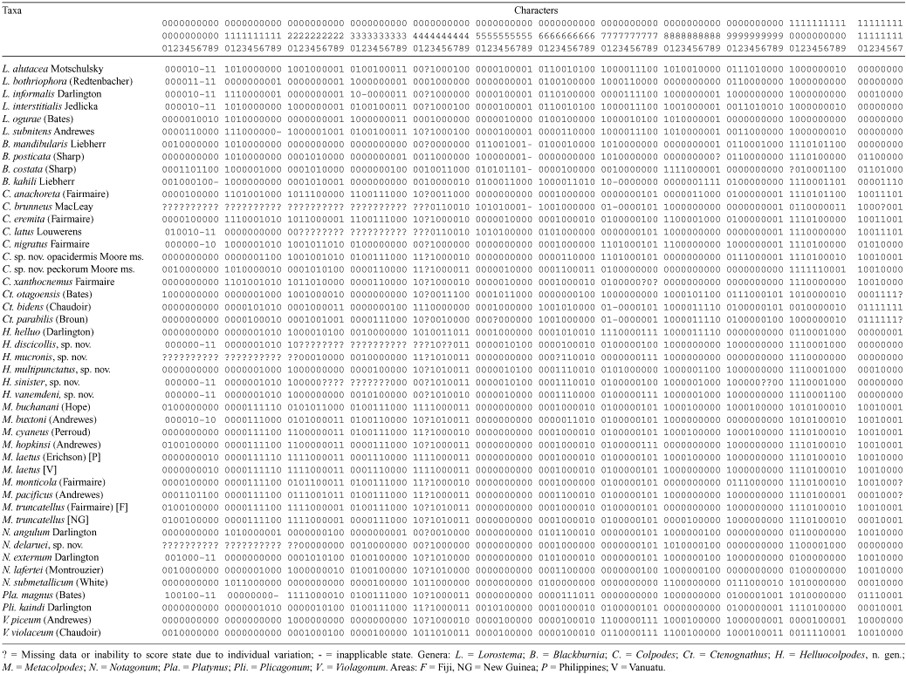
|


