Long-term membership of whale sharks (Rhincodon typus) in coastal aggregations in Seychelles and Djibouti
David Rowat A F , Katie Brooks A B , Abi March A , Ciara McCarten A , Daniel Jouannet C D , Luke Riley A , Gareth Jeffreys A E , Morgan Perri D , Michel Vely C D and Bruno Pardigon CA Marine Conservation Society, Seychelles, PO Box 1299, Victoria, Seychelles.
B Environment Department, University of York, Heslington, York, YO10 5DD, UK.
C Marine Conservation Society, Djibouti, PO Box 4476, Djibouti.
D Megaptera, 23 Rue Alexandre Dumas, 75011, Paris, France.
E Aberystwyth University, Penglais, Aberystwyth, SY23 3DA, UK.
F Corresponding author. Email: david@mcss.sc
Marine and Freshwater Research 62(6) 621-627 https://doi.org/10.1071/MF10135
Submitted: 15 June 2010 Accepted: 28 February 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
In coastal waters of several locations globally, whale sharks (Rhincodon typus) form seasonal aggregations, most of which largely comprise juvenile males of 4–8 m length. Evaluation of the period that individuals stay within these size- and age-specific groupings will clarify our understanding of the transition between life-stages in this species and how this might affect their long-term conservation. Long-term photo-identification studies in Seychelles and Djibouti provided data to evaluate this. The Seychelles aggregation had 443 individuals averaging 5.8 m identified between 2001 and 2009; however, the Djibouti aggregation comprised smaller individuals of 3.7 m mean length with 297 individuals identified between 2003 and 2010. In Seychelles, 27% of individuals identified in 2001 were seen again in 2009, while in Djibouti none of the whale sharks identified in 2003 were seen in 2010, although 13% from 2004 were. This suggests that membership periods in the Djibouti aggregation are shorter than in the other juvenile aggregations, such as in Seychelles. Continued photo-identification monitoring of other Indian Ocean aggregations might in time show the next location of these young sharks’ life-cycle and thereby allow development of informed national and regional management plans.
Additional keywords: demographic composition, photo identification.
Introduction
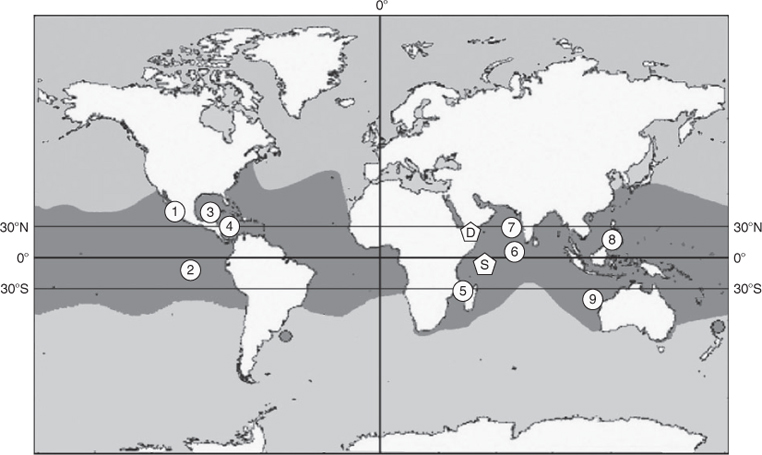
The whale shark (Rhincodon typus) is a circum-global species and, except for the Mediterranean, is found in all of the world’s tropical oceans and warm temperate seas (Compagno 2001). Most information on these sharks comes from studies conducted in coastal areas where the sharks are known to aggregate on a regular basis and where the majority of sharks have been found to be juveniles or sub-adults (<8 m), such as at Ningaloo Reef, Western Australia (Meekan et al. 2006), the Maldives (Anderson and Ahmed 1993; Riley et al. 2010), northern Mexico (Eckert and Stewart 2001) and the Philippines (Alava et al. 1997) (Fig. 1). An aggregation with similar length-class distribution has been documented in Seychelles (Rowat 1997; Rowat and Gore 2007) while another off Djibouti has been found with animals of generally smaller sizes than in these other aggregations (Rowat et al. 2007). In several Indian Ocean aggregations, and in both of the latter, many of the individual animals have been photographically identified by the spot patterns on their sides, posterior to the gill slits. These markings on this species have been shown to be stable over time (Arzoumanian et al. 2005; Speed et al. 2007) and have enabled the estimation of population size using standard catch, mark and recapture analysis (Meekan et al. 2006; Rowat et al. 2009). Many individuals in these aggregations exhibit site fidelity, having been recorded at the same site over many years (Arzoumanian et al. 2005; Meekan et al. 2006; Graham and Roberts 2007; Rowat et al. 2009). However, while movements of individuals between adjacent mainland aggregation sites have been documented through photo identification (Holmberg et al. 2009), they have not been found between any disparate sites to date.
Photo identification has also allowed estimates of the length of time individuals are resident at some sites within each season (Holmberg et al. 2009). Photo identification over consecutive years allows the evaluation of the period that individuals stay within these length- and age-specific groupings. In the present study, we compared the periods of ‘membership’ in the Djibouti aggregation, with its overall smaller size classes, with periods of membership in the more commonly found aggregations of a larger size class of sub-adults. We hypothesised that sharks in the Djibouti aggregation have a shorter membership period within that aggregation than those within the Seychelles aggregation. This information will enhance understanding of the timing and transition between life-stages in this species and how this might affect the long-term conservation of the species.
Materials and methods
Field data collection
Data on whale sharks in Seychelles and Djibouti were collected by trained observers in monitoring programs (Rowat et al. 2007, 2009). In Seychelles, these programs were annual from 2001 to 2009 and generally covered the peak months of whale shark occurrence, from the beginning of August to the end of October each year, although this period was variable. Surveys were carried out almost daily during this period and data were aggregated into annual records (Rowat et al. 2009). Unfortunately, no photo-identification data were available in 2002, so this year was omitted. In Djibouti, three short-term monitoring programs were carried out in January 2006 (Rowat et al. 2007), 2009 and 2010; from 2003 to 2008, a local non-government organisation (the Marine Conservation Society Djibouti) also gathered data from opportunistic sightings, which were made available for this analysis.
Information on each encounter with an individual shark was recorded, including date, time, location, image record, size and sex of the shark, along with notes on behaviour and any associated animals, such as pilot fish, remoras, etc. Sex was determined by the presence (in males) or absence (in females) of claspers. Total length was estimated by in-water observation with reference to an object of a known size, often a swimmer or boat, but for a proportion of sharks (Seychelles 42 of 443, Djibouti 22 of 297) actual length measurements were made using either a tape measure or a laser-metric system (Durban and Parsons 2006; Rohner et al. 2011). As there were often multiple sightings of the same individual during the course of the study period, the mean of estimated length measurements was used for the annual analysis of size if a direct measurement was not available.
Photo identification
Sharks were individually identified using the spot pattern on their sides, posterior to the gill slits, as has been validated for this species (Arzoumanian et al. 2005; Speed et al. 2007). Images were collected by trained observers (Rowat et al. 2007, 2009) but were supplemented by images gathered opportunistically from other sources. Images were sorted into year groups and then allocated to individual identities using the I3S software (Speed et al. 2007; Van Tienhoven et al. 2007).
Analysis
Data on length and identity were sorted into year groups for each site and an initial analysis of animal lengths and the maximum periods between years when individual sharks were seen in this aggregation (membership period) were established using Excel (ver. 2002.10, Microsoft, Seattle, WA). Statistical comparisons of length and membership period were made between the two aggregations using Statistica (ver. 6.0 StatSoft Inc., Tulsa, OK).
Length distributions of sharks from both areas were tested for normality by using Shapiro–Wilk tests and for homogeneity of variances by the Levene test. The hypothesis that length distributions were different between areas was tested using the Mann–Whitney U-test to compare differences in average ranks.
Results
Photo identification
In the Seychelles aggregation, 443 individuals were identified by I3S photo identification between 2001 and 2009; of the 339 individuals for which sex was determined, 279 were males (82%) and 60 were females (18%). In the Djibouti aggregation, 297 individuals were photo identified between 2003 and 2010; sex was determined for 215 of these with 182 being males (85%) and 33 being females (15%).
Length estimation
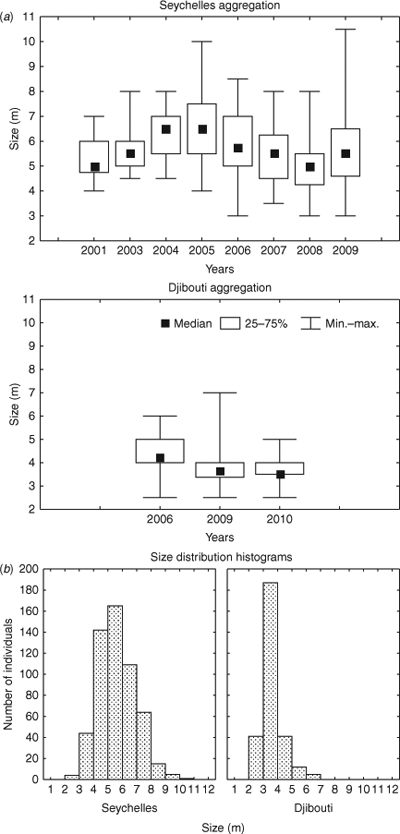
Within both the Seychelles and Djibouti data, median lengths varied between the years (Fig. 2a) and in both cases were normally distributed (P < 0.001); however, the two groups had unequal variance around their respective means. Length distribution analysis showed that there was a difference in the lengths of animals between the two aggregations (Fig. 2b), with a mean of 5.8 m ± 1.3 m s.d. (n = 549) in Seychelles and 3.7 m ± 0.6 m s.d. (n = 232) in Djibouti (Mann–Whitney U = 6868.0, P < 0.001).
|
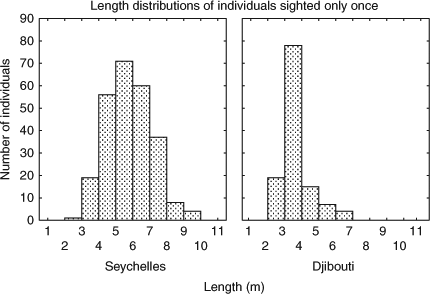
An analysis of the distribution of lengths of individuals from both aggregations that were only ever seen once and were never resighted again shows that in Djibouti the mean length was smaller (3.8 m ± 0.9 m s.d.) than at Seychelles (6.04 m ± 1.3 m s.d.; Fig. 3). This distribution of lengths resembled that of the individuals resighted in subsequent years: 3.8 m ± 0.7 m s.d. in Djibouti compared with 5.97 m ± 1.1 m s.d. in Seychelles.
|
Aggregation membership period
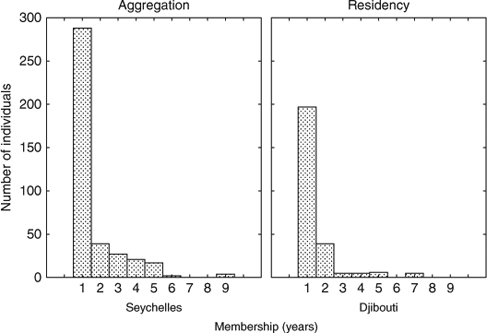
The periods of membership of individual sharks within each aggregation were derived from the presence or absence of each individual at any time in that aggregation for each year. In Seychelles, of the 443 individuals identified, after removing individuals that were seen for the first time in the last year and therefore could not be resighted, 398 individuals remained. Of these, 288 were only ever seen in 1 year while of the 110 resighted individuals, 39 (35.5%) were seen in two consecutive years, 27 (24.5%) over three (not necessarily consecutive) years and the remaining 44 (40%) were seen a maximum of 3–9 years apart, ignoring sightings in intervening years. The mean period of membership was 3.4 years, with a median of 3.0 years. In the Djibouti aggregation, 257 individuals were available for multiple year sightings; of these 197 individuals were seen only once, while of the 60 that were resighted, 39 (65%) were seen 2 years apart and the other 21 (35%) seen 3–7 years apart (Fig. 4). The mean period of membership was 2.9 years, with a median of 2.0 years.
|
Identification rates
The cumulative total of the first identification of individual sharks was similar in both aggregations, rising rapidly before levelling off; in Seychelles this was at around 350 individuals while in Djibouti it was at just over 280 (Fig. 5). The ‘net’ population curves, estimated from the cumulative total for each year less the number of animals recorded as having left the aggregation (i.e. those that were last seen more than 12 months previously, irrespective of whether they were seen once or several times), showed rapid declines. This reached a plateau in the Seychelles aggregation at around 90 individuals, whereas the Djibouti population did not achieve a new asymptote. However, looking at the average maximum time periods between consecutive sightings for individual sharks, it was apparent that 12 months was too short an interval for exclusion, as the average in Seychelles was 1.9 years (max. 8 years) and in Djibouti was 1.8 years (max. 6 years). Using a 2-year absence to indicate emigration showed a more gradual decline in the Seychelles population, with no new asymptote, while in Djibouti the ‘net’ population appeared to still be in its discovery phase, with an increasing net total (Fig. 5).
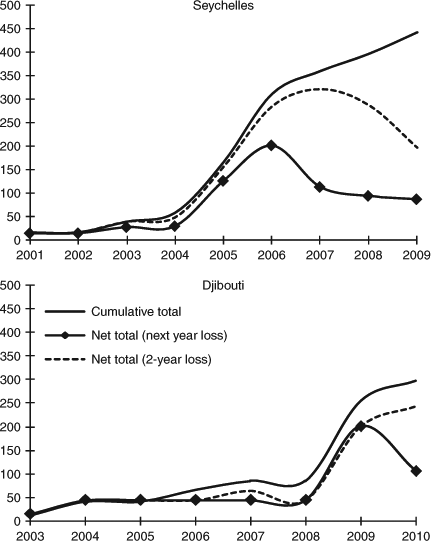
|
Discussion
The whale shark aggregations off Seychelles and Djibouti are two of the most thoroughly photo-identified aggregations globally, with 443 and 297 individuals, respectively; the highest published number of identities was at Ningaloo in 2008 with 420 individuals (Meekan 2008). The largest reported aggregation was off Holbox in the Mexican Caribbean, with aggregations of over 100 individuals recorded by aerial survey (Hueter et al. 2008), but as of October 2010 only 412 individuals from this area had been identified in the EcOcean database (www.whaleshark.org). Our present study thus encompasses over 26% of the global number of identified whale sharks (2800 identified sharks in the EcOcean database as of October 2010).
Length ranges and sex ratios
The length range of the Seychelles aggregation (5.8 m ± 1.3 m s.d.) and sex ratios (82 male : 18 female) are similar to those reported in several other aggregations. Meekan et al. (2006) reported 118 males (82.5%) to 25 females (17.5%), with male sharks averaging 6.8 m and females 6.3 m at Ningaloo. Graham and Roberts (2007) found a mean length of 6.3 m ± 1.7 m s.d. for whale sharks in Belize, and for the 163 sharks for which they determined sex, 154 (94%) were males compared with just 9 (6%) females. In the Maldives, 63 individuals were identified (95% immature males) with a mean length of 5.9 m ± 1.5 m s.d. (Riley et al. 2010), while in Mozambique 300 individuals (81% males) were identified with a mean length of 6.7 m (Pierce et al. 2008). By comparison, the Djibouti aggregation, while similar in sex ratio to these other aggregation areas with 85% males to 15% females, had a much smaller mean length than whales sharks in any other aggregations (3.7 m ± 0.6 m s.d.).
Whale sharks in two other aggregations also differ from the general pattern of length and sex composition, in the Philippines the majority of sharks were female with a mean length of 5.9 m ± 1.0 m s.d. (Quiros 2008) and in the Gulf of Baja, California, whale sharks found in the north were apparently small juvenile males while those in the south were mainly large mature females, many of which were gravid (Eckert and Stewart 2001). Although these reports differ from the general pattern for length and sex composition, both indicate a marked segregation by length and sex, and in both the lengths of the sharks reported were larger than those found in Djibouti.
Aggregation membership period
The length of time that sharks are present in an aggregation on an interannual basis is largely unreported for other areas. In the Ningaloo aggregation, 33 sharks were re-identified in different years, the longest period between sightings (ignoring intervening sightings) was 12 years with a mean of 2.7 years and a median of 2 years (Meekan et al. 2006). This is a longer maximum period of membership than either Seychelles or Djibouti, which may be because the monitoring program has been running for longer at Ningaloo. However, the mean period of membership would appear to be lower in Ningaloo than in either Seychelles (3.4 years) or in Djibouti (2.9 years) and may reflect variability in the number of photo identities captured over the years. In recent years, all images from tourism activities are being processed for photo identification and this may affect the numbers of resightings and thus the mean membership period.
The estimated periods of membership in the two populations were not statistically analysed at this time, primarily because there is potentially a big difference between our working definition of membership period in this paper, and the true membership period. Sampling issues that need to be explicitly recognised include: (1) the initial sighting is unlikely to be when the individuals first join the aggregation and, in many (most) cases, the individual would have been in the aggregation for some time before first sighting; (2) detection rates are probably less than 100% in most years, so some of the sharks would have resided, or will continue to reside, beyond the last reported sighting; and (3) sighting effort has increased over time, such that there are many recent short-residence observations that will be resighted again with continued monitoring. All these issues bias the estimate of membership time downward. There is also a disparity in the period of sampling between the two sites and between years. At both sites, survey intensity was less during the first few years, as shown by the slow rise in the cumulative totals. During the later years of monitoring, there is a short but intensive program in Djibouti compared with that in Seychelles, which covers nearly 3 months. This might also affect the results, although as similar numbers of sharks have been identified at each site over the last few years and the discovery curves are flattening off in both areas, the survey effort would appear to adequately sample both aggregations.
An attempt to model the population dynamics of these aggregations has been initiated in which the size/age-dependent processes of joining and leaving the aggregation are estimated, along with the effects of interannual variability in search effort on sighting and resighting probabilities. Initial results suggest that the data (from the Seychelles aggregation in particular) are potentially consistent with a large range of membership time estimates and could be longer than those estimated here.
Analysis of the cumulative and ‘net’ population numbers in the two aggregations is also based on several assumptions, including that all individuals have an equal opportunity of being sighted each season if present in the aggregation, and that once individuals leave the population they do not rejoin it. These assumptions are not necessarily valid, as indicated by the maximum time between consecutive sightings: 8 years in Seychelles and 6 years in Djibouti. Formal catch, mark and recapture estimates have been developed for the Seychelles aggregation based purely on capture histories (Rowat et al. 2009) and are in preparation for Djibouti; however, these approaches currently lack demographic information including the length and maturity of the individuals concerned.
As the two study aggregations are segregated by length and sex, the length of time the sharks are present within them is likely to depend to a great extent on the rate of growth exhibited by the species. However, data on growth rates of whale sharks are limited and come primarily from a few reported observations made on whale sharks in captivity. The first of these was based on neonates being reared in aquaria (n = 2), which exhibited growth rates of from 0.98 to 2.34 m year–1 to around a length of 3 m; juveniles >3.5 m (n = 5) showed rates from 0.21 to 0.5 m year–1 with a mean of 0.29 m year–1 (Chang et al. 1997; Uchida et al. 2000). More variable results were reported from juveniles >4 m at the Georgia aquarium, where rates varied from 0.25 to 1.22 m year–1 (Carlson 2008). Data for wild population growth rates have been inferred from stranded specimens by the linear relationship between vertebral growth rings to body length (Wintner 2000); growth rates so calculated indicated growth of 0.22 m year–1 for juveniles >3.5 m, which is comparable to the slowest of the rates seen in aquaria. Using this growth rate of 0.22 m year–1 and based on the minimum length shark found and the median of the largest sharks in each aggregation, membership in the Djibouti aggregation (2.5–6.0 m) would be ~16 years and in Seychelles (3.0–8.0 m) would be 23 years, supporting our working hypothesis that individuals use these aggregation sites for different lengths of time.
The four main issues regarding the potential sampling bias can all be remedied to some extent by continued long-term monitoring. Although in the first seasons of monitoring the initial sighting of individuals was unlikely to be when they first joined the aggregation, as monitoring continues the chances of new individuals being recognised during their first season increases as the discovery curve flattens out. By the same token, if monitoring can be maintained at the current rates, the probability of detecting most sharks becomes much higher. Assuming that the sighting effort is maintained, comparisons between these and other areas should be more robust and should enable differentiation between new-comers that will become members of the aggregation, and transients that are only ever seen once. The final issue was the disparity in sampling between the two aggregations; while it may not be possible to monitor the two sites for the same length of time each year, the shorter intensive program in Djibouti has been capturing a large proportion of the sharks, as indicated by the inter-seasonal capture histories and is therefore likely to be sufficient to compare with the longer monitoring period.
Further analysis
Estimations of the length of whale sharks in the wild, through the standard methods of comparison to an object of a known length by experienced observers (Graham and Roberts 2007) or by use of a tape measure or measuring rope between two swimmers (Meekan et al. 2006) all have a variability >0.5 m, which, as such, is greater than the potential annual rate of growth. The use of accurate length-measurement devices such as stereo-photogrammetry (Klimley and Brown 1983; Spitz et al. 2000) or laser metrics (Durban and Parsons 2006; Rohner et al. 2011) will allow a more precise evaluation to be made. As the latter technique is now being employed on both the Seychelles and Djibouti aggregations, this will open up avenues for more detailed analysis of membership periods and annual population estimates. It is expected that these accurate length data will be very valuable in the modelling framework mentioned previously for improving the estimates of the length- and age-dependent processes that control when individuals join and leave aggregations.
The current variability of length measurement limits the inferences that can be made from these data about the period of membership within the Djibouti and Seychelles aggregations with respect to the age and maturity of the individual sharks. However, the individuals found in the Djibouti aggregation are smaller and appear to stay in this aggregation for shorter periods compared with those in the Seychelles aggregation. As there have been no other juvenile whale shark aggregations reported from the Djibouti area and with no other reported populations or aggregations of <4 m whale sharks in the Indian Ocean region, it is possible that once the sharks leave this aggregation, they may appear in other regional aggregations that have the more usual (larger) juvenile length-classes. This reinforces the need for continued monitoring and photo identification in all known regional aggregation sites, as well as the importance of finding as yet undiscovered aggregations. Currently, there are no known aggregations of adult whale sharks and only one area has reported regular occurrence of adult (and gravid) females, the south of Baja (Eckert and Stewart 2001). Similarly, there are no confirmed pupping grounds, with only 15 reports of neonatal pups recorded (Wolfson 1983; Rowat et al. 2008; Aca and Schmidt 2011).
As such, the aggregation found off Djibouti appears to be unique in having much smaller (and thus younger) individuals than in any other known aggregation. The reason for its occurrence may be linked to the topography of the area (the land-locked end of the Gulf of Tadjourah, a deep inlet of the Indian Ocean into North East Africa formed by the fault between the East African and Arabian continental plates that extends into Africa as the East African Rift Valley). The sea-bed slopes steeply to depths of 1100 m (Dauteuil et al. 2001) and strong local winds develop daily all year, caused by rapid heating of the air over the inshore land masses and funnelled by the coastal mountains through the Gulf. This appears to cause an upwelling of plankton in the study area, with biomass of up to 12.7 g m–3 (Marine Conservationn Society Seychelles, unpubl. data), and the arrival of the whale shark aggregation in the area. This ‘sub-juvenile’ aggregation may thus be being revealed by fortuitous environmental and physical conditions, rather than the area having any specific ecological benefits, apart from abundant food.
Conclusions
While both the Seychelles and Djibouti whale shark aggregations are segregated by sex, as are all the other known aggregations, and are similar to most in terms of being male-dominated, our study has shown that the sharks in the Djibouti aggregation are much smaller. As there are very few larger juvenile sharks, it may be that the population off Djibouti serves as a ‘staging group’ for other regional aggregations. If this is the case, it would be prudent to adopt a precautionary approach to its management and conservation as it may prove to be pivotal in the recruitment process of the ‘older’ regional aggregations. Similarly, populations of smaller, younger juveniles must exist in other ocean basins and accurate monitoring of the length composition of other aggregations may provide clues about their locations.
Acknowledgements
Studies in Seychelles and Djibouti were supported with grant aid from the Save Our Seas Foundation. Our thanks to local non-government organisations DECAN (DECouvrir et Aider la Nature), the Marine Conservation Society Djibouti, and Megaptera for their support in the organisation of Djibouti field studies, to Dolphin Services for their logistical support in Djibouti and to all the volunteers who assisted on both sites. We are particularly grateful to Mauvis Gore and Dale Kolody for their advice on analysis and to the Editor, Guest Editors and two anonymous reviewers for their comments, which have improved the manuscript.
References
Aca, E. Q., and Schmidt, J. V. (2011). Revised size limit for viability in the wild: neonatal and young of the year whale sharks identified in the Philippines. Asia Life Sciences 20, 361–367.Alava, M. N. R., Yaptinchay, A. A., Acogido, G., Dolar, M., Louella, L., et al. (1997). Fishery and trade of whale shark (Rhincodon typus) in the Philippines. In ‘13th Meeting of the American Elasmobranch Society (Pennsylvania, USA)’. Available at http://elasmo.org/abst1997.php [Accessed October 2010]. [Abstract]
Anderson, R. C., and Ahmed, H. (1993). ‘Shark Fisheries of the Maldives.’ (Ministry of Fisheries and Agriculture: Maldives.)
Arzoumanian, Z., Holmberg, J., and Norman, B. (2005). An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. Journal of Applied Ecology 42, 999–1011.
| An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus.Crossref | GoogleScholarGoogle Scholar |
Carlson, B. (2008). Whale shark research at the Georgia aquarium. In ‘Proceedings of the 2nd International Whale Shark Conference, Holbox, Quintana Roo, Mexico, 15–20 July 2008’. Available at http://www.domino.conanp.gob.mx/doc_conf/Bruce.pdf [Accessed October 2010].
Chang, W. B., Leu, M. Y., and Fang, L. S. (1997). Embryos of the whale shark, Rhincodon typus, early growth and size distribution. Copeia 2, 444–446.
| Embryos of the whale shark, Rhincodon typus, early growth and size distribution.Crossref | GoogleScholarGoogle Scholar |
Compagno, L. J. V. (2001). ‘Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date.’ (Food and Agriculture Organization of the United Nations: Rome.)
Dauteuil, O., Huchon, P., Quemeneur, F., and Souriot, T. (2001). Propagation of an oblique spreading centre: the western Gulf of Aden. Tectonphysics 332, 423–442.
| Propagation of an oblique spreading centre: the western Gulf of Aden.Crossref | GoogleScholarGoogle Scholar |
Durban, J. W., and Parsons, K. M. (2006). Laser-metrics of free-ranging killer whales. Marine Mammal Science 22, 735–743.
| Laser-metrics of free-ranging killer whales.Crossref | GoogleScholarGoogle Scholar |
Eckert, S., and Stewart, B. (2001). Telemetry and satellite tracking of whale sharks, Rhincodon typus, in the Sea of Cortez, Mexico, and north Pacific Ocean. Environmental Biology of Fishes 60, 299–308.
| Telemetry and satellite tracking of whale sharks, Rhincodon typus, in the Sea of Cortez, Mexico, and north Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Graham, R., and Roberts, C. M. (2007). Assessing the size, growth rate and structure of a seasonal population of whale sharks (Rhincodon typus Smith 1828) using conventional tagging and photo identification. Fisheries Research 84, 71–80.
| Assessing the size, growth rate and structure of a seasonal population of whale sharks (Rhincodon typus Smith 1828) using conventional tagging and photo identification.Crossref | GoogleScholarGoogle Scholar |
Holmberg, J., Norman, B., and Arzoumanian, Z. (2009). Estimating population size, structure, and residency time for whale sharks Rhincodon typus through collaborative photo-identification. Endangered Species Research 7, 39–53.
| Estimating population size, structure, and residency time for whale sharks Rhincodon typus through collaborative photo-identification.Crossref | GoogleScholarGoogle Scholar |
Hueter, R. E., Tyminski, J. P., and delaParra, R. (2008).The geographical movements of whale sharks tagged with pop-up archival satellite tags off Quintana Roo, Mexico. In ‘Proceedings of the 2nd International Whale Shark Conference, Holbox, Quintana Roo, Mexico, 15–20 July 2008’. Available at http://www.domino.conanp.gob.mx/doc_conf/Bob.pdf [Accessed October 2010].
Klimley, A. P., and Brown, S. T. (1983). Stereophotography for the field biologist: measurement of lengths and three-dimensional positions of free-swimming sharks. Marine Biology 74, 175–185.
| Stereophotography for the field biologist: measurement of lengths and three-dimensional positions of free-swimming sharks.Crossref | GoogleScholarGoogle Scholar |
Meekan, M. G. (2008). Satellite tagging and photo identification of whale sharks; reconciling conflicting data. In ‘Proceedings of the 2nd International Whale Shark Conference, Holbox, Quintana Roo, Mexico, 15–20 July 2008’. Available at http://www.domino.conanp.gob.mx/doc_conf/Mark.pdf [Accessed October 2010].
Meekan, M. G., Bradshaw, C. J. A., Press, M., McLean, C., Richards, A., et al. (2006). Population size and structure of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia. Marine Ecology Progress Series 319, 275–285.
| Population size and structure of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pierce, S., Brunnschweiler, J., Tilley, A., Dudgeon, C., and Marshall, A. (2008). Whale shark population ecology and movement patterns in southern Mozambique. In ‘Proceedings of the 2nd International Whale Shark Conference, Holbox, Quintana Roo, Mexico, 15–20 July 2008’. Available at http://www.domino.conanp.gob.mx/doc_conf/Alex.pdf [Accessed October 2010].
Quiros, A. (2008). Overview of whale sharks and whale shark tourism and whale shark tourism in the Philippines. In ‘Proceedings of the 2nd International Whale Shark Conference, Holbox, Quintana Roo, Mexico, 15–20 July 2008’. Available at http://www.domino.conanp.gob.mx/doc conf/Angela.pdf [Accessed October 2010].
Riley, M. J., Hale, M. S., Harman, A., and Rees, R. G. (2010). Analysis of whale shark Rhincodon typus aggregations near South Ari Atoll, Maldives Archipelago. Aquatic Biology 8, 145–150.
| Analysis of whale shark Rhincodon typus aggregations near South Ari Atoll, Maldives Archipelago.Crossref | GoogleScholarGoogle Scholar |
Rohner, C. A., Richardson, A. J., Marshall, A. D., Weeks, S. J., and Pierce, S. J. (2011). How large is the world’s largest fish? Measuring whale sharks Rhincodon typus with laser photogrammetry. Journal of Fish Biology 78, 378–385.
| How large is the world’s largest fish? Measuring whale sharks Rhincodon typus with laser photogrammetry.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7hslyqsw%3D%3D&md5=29fe6b4c922627d71135cd53adfd9e11CAS | 21235570PubMed |
Rowat, D. (1997). Seychelles whale shark tagging project – pilot project report. Phelsuma. Nature Protection Trust of Seychelles 5, 77–80.
Rowat, D., and Gore, M. (2007). Regional scale horizontal and local scale vertical movements of whale sharks in the Indian Ocean off Seychelles. Fisheries Research 84, 32–40.
| Regional scale horizontal and local scale vertical movements of whale sharks in the Indian Ocean off Seychelles.Crossref | GoogleScholarGoogle Scholar |
Rowat, D., Meekan, M. G., Engelhardt, U., Pardigon, B., and Vely, M. (2007). Aggregation of juvenile whale shark (Rhincodon typus) in the Gulf of Tadjoura, off Djibouti. Environmental Biology of Fishes 80, 465–472.
| Aggregation of juvenile whale shark (Rhincodon typus) in the Gulf of Tadjoura, off Djibouti.Crossref | GoogleScholarGoogle Scholar |
Rowat, D., Gore, M. A., Baloch, B. B., Islam, Z., Ahmad, E., et al. (2008). New records of neonatal and juvenile whale sharks (Rhincodon typus) from the Indian Ocean. Environmental Biology of Fishes 82, 215–219.
| New records of neonatal and juvenile whale sharks (Rhincodon typus) from the Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Rowat, D., Speed, C. W., Meekan, M. G., Gore, M., and Bradshaw, C. J. A. (2009). Population abundance and apparent survival estimates of the Seychelles whale shark aggregation. Oryx 43, 591–598.
| Population abundance and apparent survival estimates of the Seychelles whale shark aggregation.Crossref | GoogleScholarGoogle Scholar |
Speed, C. W., Meekan, M. G., and Bradshaw, C. J. A. (2007). Spot the match – wildlife photo-identification using information theory. Frontiers in Zoology 4, 2.
| Spot the match – wildlife photo-identification using information theory.Crossref | GoogleScholarGoogle Scholar | 17227581PubMed |
Spitz, S. S., Herman, L. M., and Pack, A. A. (2000). Measuring sizes of humpback whales (Megaptera novaeangliae) by underwater videogrammetry. Marine Mammal Science 16, 664–676.
| Measuring sizes of humpback whales (Megaptera novaeangliae) by underwater videogrammetry.Crossref | GoogleScholarGoogle Scholar |
Uchida, S., Toda, M., Kamei, Y., and Teruya, H. (2000). The husbandry of 16 whale sharks Rhincodon typus, from 1980 to 1998 at the Okinawa expo aquarium. In ‘13th Meeting of the American Elasmobranch Society (Pennsylvania, USA)’. Available at http://elasmo.org/abst1997.php [Accessed October 2010]. [Abstract]
Van Tienhoven, A. M., Den Hartog, J. E., Reijns, R., and Peddemors, V. M. (2007). A computer-aided program for pattern-matching of natural marks of the spotted ragged-tooth shark Carcharias taurus (Rafinesque, 1810). Journal of Applied Ecology 44, 273–280.
| A computer-aided program for pattern-matching of natural marks of the spotted ragged-tooth shark Carcharias taurus (Rafinesque, 1810).Crossref | GoogleScholarGoogle Scholar |
Wintner, S. P. (2000). Preliminary study of vertebral growth rings in the whale shark, Rhincodon typus, from the east coast of South Africa. Environmental Biology of Fishes 59, 441–451.
| Preliminary study of vertebral growth rings in the whale shark, Rhincodon typus, from the east coast of South Africa.Crossref | GoogleScholarGoogle Scholar |
Wolfson, F. H. (1983). Records of seven juveniles of the whale shark Rhiniodon typus. Journal of Fish Biology 22, 647–655.
| Records of seven juveniles of the whale shark Rhiniodon typus.Crossref | GoogleScholarGoogle Scholar |