Rediscovery and redescription of the smoothtooth blacktip shark, Carcharhinus leiodon (Carcharhinidae), from Kuwait, with notes on its possible conservation status
Alec B. M. Moore A B F , William T. White C , Robert D. Ward C , Gavin J. P. Naylor D and Richard Peirce EA RSK Environment Ltd, Spring Lodge, 172 Chester Road, Helsby, Cheshire, WA6 OAR, UK.
B Bangor University, School of Ocean Sciences, Menai Bridge, LL59 5AB, UK.
C CSIRO Marine and Atmospheric Research, Wealth from Oceans Flagship, GPO Box 1538, Hobart, Tas. 7001, Australia.
D Florida State University, School of Computational Science/Department of Biological Science, Tallahassee, FL 32304, USA.
E Shark Conservation Society, Dulverton House, 8 Crooklets, Bude, Cornwall, EX23 8NE, UK.
F Corresponding author. Email: amoore@rsk.co.uk
Marine and Freshwater Research 62(6) 528-539 https://doi.org/10.1071/MF10159
Submitted: 18 June 2010 Accepted: 9 November 2010 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
The smoothtooth blacktip shark, Carcharhinus leiodon, is one of the rarest whaler shark species of the genus Carcharhinus, previously known only from the holotype collected over 100 years ago from the Arabian Sea coast of Yemen. Recent market surveys in the Persian (Arabian) Gulf rediscovered 25 specimens (~2% of individual sharks recorded) in Kuwait, ~3000 km away from the type location. This study combined morphometric and molecular approaches to provide a detailed redescription of this species based on new material, as well as the first information on fresh colouration, size range and maturity. Sequences from two separate regions of the mitochondrial genome (COI and ND2) support the identity of C. leiodon as a distinct species, closely related to C. limbatus, C. amblyrhynchoides and C. tilstoni. Carcharhinus leiodon is superficially similar to, but clearly distinct from, C. melanopterus and C. amblyrhynchoides. The previously uncertain type locality of C. leiodon is considered to be correct, and the narrow range and unusual disjunct distribution, relatively rare for a marine carcharhinid, is discussed. The Kuwait population of C. leiodon, including juveniles, is subject to fisheries by-catch and is in an area of extensive habitat alteration. As a result, C. leiodon is considered vulnerable, requiring urgent conservation action.
Additional keywords. : Chondrichthyes, elasmobranch, Western Indian Ocean.
Introduction
The whaler or requiem sharks (Carcharhiniformes: Carcharhinidae) are the most species-rich and economically important family of sharks occurring in tropical, continental shelf regions of the world (White and Sommerville 2010). Given their commercial importance and varying life histories, accurate identification of species is essential for successful fisheries management. Many whaler shark species are morphologically similar and accurate identifications are often difficult, particularly if good field characters are not available. Furthermore, ontogenetic changes in whaler shark species can add to misidentification issues. Fisheries observers are often faced with the added difficulty of having to identify species from finned carcasses in which many of the key characters are lacking. In these instances, molecular methods can become important and DNA barcoding has proven to be an extremely useful tool for determining the species composition of confiscated dried shark fins (Holmes et al. 2009).
The Carcharhinidae consists of 54 nominal species belonging to 12 genera; Carcharhinus is the most species-rich genus with 30 species (Compagno et al. 2005). A thorough revision of the genus Carcharhinus by Garrick (1982) provided detailed descriptions of all species described before 1982. In 1985, Garrick described a further species, Carcharhinus leiodon, from a single juvenile male specimen that he found in the fish collection of the Naturhistorisches Museum in Vienna and which had been collected in 1902 from southern Arabia. This was the first new species of Carcharhinus described since C. altimus (Springer) and C. tilstoni (Whitley) in 1950, and one of only seven described in the 20th Century. Although his description was based solely on the holotype, Garrick (1985: p. 13) stated: ‘I have no hesitation in describing it as a new species because it very clearly differs from all other species of Carcharhinus including even those that superficially are very similar to it’. This species has been considered the rarest Carcharhinus species, as there have been no published records since its description more than 25 years ago and the collection of the holotype more than a century ago. As a result of its rarity, very restricted distribution and assumed small population size, C. leiodon is currently listed as Vulnerable in the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (Compagno 2005).
This paper provides the first validated records of C. leiodon since the collection of the first specimen in 1902. The new specimens were collected during surveys of fish markets in Kuwait on the Persian (Arabian) Gulf (hereafter referred to as the Gulf), around 3000 km from the type locality. A redescription of C. leiodon is provided based on additional specimens, which includes adult males, and colour is described from both fresh and preserved specimens. Relationships with closely related and morphologically similar Carcharhinus species, including mitochondrial DNA sequence variation, are discussed. Aspects of the range of C. leiodon are also addressed, including the validity of the type locality, the unusual disjunct distribution, and possible habitat. The conservation status of C. leiodon is re-assessed in light of the first information on fisheries interactions of this species and discovery of a previously unknown population.
Materials and methods
The Shark Conservation Society (SCS) conducted the first dedicated surveys of elasmobranchs in the Gulf by investigating the catches at fish markets and landing sites in Kuwait (April 2008), Qatar (April 2009), and Abu Dhabi in the United Arab Emirates (April 2010). Specimens of C. leiodon were recorded from Sharq and Fahaheel fish markets in Kuwait. Four individuals were retained whole and deposited in the Natural History Museum (London) (BMNH; registration numbers: BMNH 2008.7.28.1–3; BMNH 2010.2.4.1) and jaws from two additional specimens were also retained and deposited (BMNH 2010.2.8.1; South African Museum (SAM), unregistered).
Specimens are referred to by the following prefixes for their registration numbers: CSIRO, Australian National Fish Collection (ANFC), Hobart; BMNH, British Natural History Museum, London; NMW, Naturhistorisches Museum, Vienna; SAM, South African Museum, Cape Town. Tissues collected for molecular analyses are deposited at the ANFC (CSIRO) or Florida State University (FSU) and allocated a unique number with the prefix BW or GN, respectively (initials of two of the authors).
Measurement terminology follows Compagno (1984, 1988, 2001), who assigned names and abbreviations to measurements often indicated by descriptive phrases (e.g. snout to upper caudal origin = precaudal length = PCL). Direct measurements were used unless specified otherwise. Some measurements (e.g. head length) were also taken horizontally to account for different measurement protocols followed by other researchers. Dentitional terms generally follow Compagno (1979, 1988, 2001). Vertebral terminology, method of counting and vertebral ratios follow Springer and Garrick (1964) and Compagno (1979, 1988, 2001). The four whole, recently collected specimens of C. leiodon were measured in full, and compared with measurements of the holotype provided in Garrick (1985) (see Accessory Publication on the Marine and Freshwater Research website). In the descriptive section, morphometric and meristic values for the holotype (from Garrick 1985) are given first, followed in parentheses by the range of the four new specimens (BMNH 2008.7.28.1–3; BMNH 2010.2.4.1). Meristics were taken from radiographs of three whole, recently collected specimens of C. leiodon (BMNH 2008.7.28.1–3) and compared with those provided for the holotype by Garrick (1985). Counts were obtained separately for trunk (monospondylous), precaudal (monospondylous + diplospondylous to origin of upper lobe of caudal fin) and caudal (centra of the caudal fin) vertebrae. Tooth row counts were taken from a set of excised jaws (BMNH 2010.2.8.1) and compared with those for the holotype in Garrick (1985). In addition, a further 19 individuals of C. leiodon were recorded in the fish markets, but not retained. For these individuals, total length (TL) and, for males, maturity stage (juvenile: claspers soft, flexible, not extending beyond posterior margin of pelvic fins; adult: clasper hard, not flexible, fully calcified, extending well past free rear tips of pelvic fins) was recorded. Owing to time and logistical constraints, no females were examined for reproductive status.
Carcharhinus leiodon specimens were sequenced for both the cytochrome c oxidase I (COI) DNA barcoding fragment (~650 base pairs (bp), see Hebert et al. 2003; Ward et al. 2005; Holmes et al. 2009) and the entire protein-coding region of the mitochondrial gene nicotinamide adenine dinucleotide dehydrogenase subunit 2 (ND2; 1047 bp). The C. leiodon sequences were compared with corresponding sequences for three Carcharhinus species that are closely related to C. leiodon, i.e. C. amblyrhynchoides, C. tilstoni and C. limbatus, and for two other species that are genetically similar to this group (i.e. C. melanopterus and C. sorrah, see Ward et al. 2008). GenBank accession numbers of all COI and ND2 sequences are given in the Accessory Publication. Unfortunately, because this work was carried out in two different laboratories, the same individuals were not used to represent each species. Nonetheless, three of the C. leiodon specimens were common to both studies and the within- versus between-species differences in both the COI and ND2 datasets suggest that any differences due to the particular specimens sequenced do not affect the validity of our conclusions. A subsequent comparison of the C. leiodon barcodes with those from 170 individuals of 17 additional Carcharhinus species was also conducted to determine if any diagnostic bases could be found.
For the COI sequencing (DNA barcoding), DNA extractions, PCR reactions and sequencing followed either (for those sequenced earlier in the study) Stage 1 or (for those sequenced later) Stage 2 protocols given in Holmes et al. (2009). For the ND2 sequencing, total DNA was extracted from the tissue samples using the High Pure PCR Template Preparation Kit (Roche Diagnostics Corporation, Indianapolis, IN). Extracted total DNA was stored at –20°C. Subsets of the extracted template were diluted to 1/10 of original strength and stored for subsequent use in PCR reactions. Samples were PCR amplified using Hot Start Taq (Promega Corporation, Madison, WI) using primers designed to target the complete coding sequence for ND2 (Naylor et al. 2005).
The number of pairwise nucleotide differences among individuals was determined for both the COI and ND2 datasets separately, using PAUP* 4.0 (Swofford 2002). Kimura two-parameter (K2P, Kimura 1980) pairwise genetic distances were also estimated for both the COI and ND2 datasets. These distances were subsequently subjected to neighbour-joining to generate trees for each dataset. Bootstrap support values were estimated from the data (1000 iterations) to assign confidence levels to branches. Software package MEGA4 (Tamura et al. 2007) was used to generate and bootstrap the trees.
Results
Carcharhinus leiodon Garrick, 1985 (Figs 1a, b, 2–5)

|

|
|
|
|
Holotype (not examined). NMW 61465, 750 mm TL, juvenile male, Gulf of Aden, Qishn, 1902.
Material examined
Six specimens: BMNH 2008.7.28.1, female 865 mm TL, 4.15 kg; BMNH 2008.7.28.2, BW-A6069, juvenile male 888 mm TL, 4.25 kg; BMNH 2008.7.28.3, BW-A6070, GN5015, juvenile male 673 mm TL, 1.94 kg; BMNH 2010.2.4.1, BW-A6073, GN6483, adult male 1236 mm TL, 14.9 kg; BMNH 2010.2.8.1 (jaws only), BW-A6072, GN5013, adult male 1230 mm TL; SAM unregistered (jaws only), BW-A6068, juvenile male 701 mm TL; collected from Sharq fish market, Kuwait City, Persian (Arabian) Gulf, 29°23′N, 47°58′E, 22 April 2008.
Comparative material
Carcharhinus amblyrhynchoides: BMNH 2008.7.28.4, BW-A6074, GN6487, female 837 mm TL, 3.61 kg; BMNH 2010.2.8.2 (jaws only), BW-A6071, GN5014, male 888 mm TL; collected from Sharq fish market, Kuwait City, Persian (Arabian) Gulf, 29°23′N, 47°58′E, 22 April 2008. Tissues from related species C. tilstoni, C. limbatus, C. melanopterus and C. sorrah were also sequenced for COI and ND2 (see Figs 4 and 5).
Diagnosis
A Carcharhinus with the following combination of characters: a short and bluntly pointed snout as seen in dorsoventral view; upper and lower teeth narrow, erect (to semi-oblique in uppers), smooth-edged; anteroposterior tooth row counts 16 (2–3) 16/14–15 (3) 14–15; total tooth row counts (including symphysials) 34–35/31–33, or 65–68; interdorsal ridge absent, interdorsal space 18.2–20.2% TL; first dorsal fin relatively small, not falcate, length 14.9–16.8% TL, its origin over mid-length of pectoral-fin inner margin; second dorsal-fin small, height 39–45% of first dorsal-fin height; anal fin height 89–116% of second dorsal-fin height; total vertebral counts 197–198, monospondylous precaudal counts 60–65, diplospondylous precaudal counts 50–54, diplospondylous caudal counts 83–94, precaudal counts 113–115. Dorsal and lateral surfaces uniformly greyish green to greenish yellow (greyish in preservative); broad blackish stripe on dorsal surface of second dorsal–caudal space extending onto dorsal caudal margin as a broad blackish anterior margin; all fins with distinct blackish apical tips.
Description
Body moderately stout; trunk somewhat triangular to subcircular in section at first dorsal-fin base; length of trunk from fifth gill slits to vent 1.06–1.14 (in recently collected specimens) times head length. Predorsal, interdorsal and postdorsal ridges absent from midline of back; lateral ridges absent from body. Caudal peduncle stout, rounded–hexagonal in section at second dorsal-fin insertion; postdorsal and postventral spaces flattened and with a very shallow median groove; lateral surfaces rounded to somewhat angular, no lateral ridges; height of caudal peduncle at second dorsal-fin insertion 0.96–1.23 times its width, 1.51–1.82 times in dorsal–caudal space. Precaudal pits present; upper pit pronounced, deep, arcuate and crescentic; lower pit smaller, narrower and shallower but pronounced. Head length to fifth gill opening 0.91–1.05 times in pectoral–pelvic space. Head moderately short and stout, not flattened, ellipsoidal in shape in cross-section at eyes. Outline of head in lateral view slightly undulated dorsally; medially slightly concave on snout, convex above eye, concave at nape and convex above gills and progressively elevated towards first dorsal fin; convex ventrally along lower jaws and beneath gills. In dorsoventral view, head narrowly parabolic, with gill septa expanded slightly outwards. Snout relatively short, preoral snout length 0.70 in holotype (0.62–0.73 in recently collected specimens) times mouth width; tip bluntly pointed in dorsoventral view and very weakly indented anterior to nostrils; snout narrowly rounded in lateral view, convex above and below.
External eye opening of fleshy orbit without anterior or posterior notches, circular in shape, with height 1.03–1.22 in eye length. Eyes small, length 13.32 (12.39–16.85) times in head length; situated slightly ventrolateral on head, with lower edges crossing horizontal head rim in ventral view; eyes not visible in dorsal view; subocular ridges absent. Nictitating lower eyelids internal, with deep subocular pouches and secondary lower eyelids fused to upper eyelids. Spiracles absent. Gill slits moderately large, first four gill slits about equal in height, third or fourth gill opening largest, fifth smallest; fifth slit ~0.71 (0.67–0.82) of height of third; height of third ~5.62 (5.23–6.32) in head length, 2.37 (2.25–3.22) times eye length. First three gill slits nearly straight to weakly concave, fourth and fifth becoming more oblique and slightly more concave; upper edges of gill slits three and four most elevated; upper end of highest gill opening about level with upper third of eye. Gill filaments not visible from outside in lateral view. Gill-raker papillae absent from gill arches.
Nostrils with large, subcircular incurrent apertures; prominent triangular anterior nasal flaps with short, bluntly pointed tips, posterior nasal flap vestigial, small suboval excurrent apertures; well in front of mouth; nostril width 3.76–4.15 in internarial width, 1.02–1.26 in eye length, 2.54–3.06 in longest gill-opening.
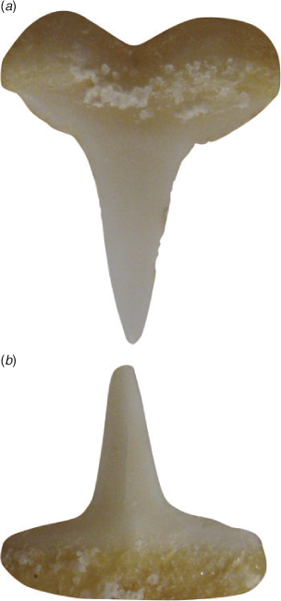
Mouth moderately rounded and relatively large; width 2.64 (2.54–2.74) in head length; mouth length 1.75 (1.74–1.85) in mouth width. Lips fully concealing teeth when mouth is closed. Tongue large, flat and broadly rounded, filling floor of mouth. Labial furrows very short, restricted to mouth corners, lower labial furrow concealed below lip, uppers 0.67 (0.81–1.23) times as long as lowers. Teeth (including symphysials) in 35 (34)/33 (31) rows or 68 (65) total rows (both jaws), two series functional; not arranged in diagonal files, no toothless spaces at symphysis; similar in both upper and lower but differentiated along jaws; tooth formula of upper jaw 16 (16) + 3 (2) + 16 (16), lower jaw 15 (14) + 3 (3) + 15 (14); upper teeth narrow, with erect to slightly oblique, smooth-edged cusps, becoming notched laterally (Fig. 3a); lower teeth narrow, with erect, smooth-edged cusps, not becoming notched laterally (Fig. 3b). Lateral trunk denticles of BMNH 2008.7.28.2 with flat, rhomboidal crowns slightly wider than long. Crown with three prominent longitudinal ridges that extend its entire length onto the cusps; medial cusp short but strong, slightly shorter than the rest of crown; one or two pairs of shorter lateral cusps present. Denticle crowns close together, imbricate, with skin not visible between them. Denticles absent from insertion of the fins and from dorsal surface of claspers.
Pectoral fin moderately large, weakly falcate; anterior margin moderately convex, apices narrowly rounded; posterior margin nearly straight distally, mesial half shallowly and broadly concave; free rear tip moderately rounded, inner margin convex; base broad ~53–62% of fin length; length from origin to rear tip 1.05–1.11 in anterior margin length; much greater in area than first dorsal fin; origin about under fourth gill opening; fin apex about well posterior to inner margin when fin is elevated and adpressed to body. Pelvic fins triangular and not falcate; length of anterior margin 0.42–0.49 of pectoral–fin anterior margins; area larger than that of anal fin; anterior margin nearly straight and concave near base; apices moderately rounded to angular; posterior margin nearly straight to very weakly concave; free rear tip bluntly rounded, inner margin nearly straight. Claspers of adult male long, robust and broad-based, tapering from pelvic fin free rear tip; apex bluntly rounded, extending just posterior to anal-fin origin.
First dorsal fin relatively small, low, not falcate; anterior margin moderately convex, shallowly concave basally; apex bluntly pointed; posterior margin nearly straight distally, broadly concave basally; free rear tip acutely pointed, inner margin nearly straight; origin just anterior to pectoral-fin free rear tip, midpoint of base 0.9–1.4 times closer to pectoral insertions than pelvic origins; free rear tip just anterior to pelvic-fin origins by less than half eye diameter; posterior margin almost vertical distally then appears to be arcing strongly posteroventrally at free rear tip. First dorsal-fin base 1.52–1.96 in interdorsal space, 2.21 (2.05–2.49) in dorsal caudal margin; height 1.07 (1.17–1.32) in base length; inner margin 1.95–2.21 in height, 2.33–2.72 in base length.
Second dorsal fin small, apically moderately broad, triangular, very weakly falcate; height 0.39 (0.39–0.45) times first dorsal-fin height; base 0.46 (0.56–0.59) times first dorsal-fin base; anterior margin concave basally, becoming weakly to moderately convex distally; apex moderately rounded to angular; posterior margin distally very slightly convex and basally concave; free rear tip acutely pointed, inner margin nearly straight; origin well behind pelvic-fin free rear tips and about opposite anal-fin origin; rear tip about opposite anal-fin free rear tip, in front of upper caudal-fin origin by 0.7–1.1 times its inner margin length; posterior margin curving posteroventrally from apex; insertion about level with or just posterior to fin apex. Second dorsal-fin base 0.93–1.38 in dorsal–caudal space; height 1.24 (1.52–1.78) in base; inner margin 0.94–0.99 in height, 1.54–1.82 in base.
Anal fin apically narrow and strongly falcate; height 1.07 (0.89–1.16) times second dorsal-fin height, base length 1.10 (0.96–1.20) times second dorsal-fin base; anterior margin concave basally and distally broadly convex; apex narrowly rounded; posterior margin deeply notched; free rear tip acutely pointed, inner margin nearly straight to shallowly concave; origin about opposite second dorsal-fin origin; insertion about opposite second dorsal-fin insertion, anterior to fin apex; free rear tip in front of lower caudal-fin origin by about two-thirds its inner margin length; posterior margin slanting anterodorsally distally and then abruptly posterodorsally at mid-margin. No preanal ridges obvious. Anal-fin base 0.90–1.02 in anal–caudal space; height 1.27 (1.57–1.92) in base; inner margin 0.74–0.99 in height, 1.94–2.13 in base.
Caudal fin narrow-lobed and asymmetrical, with short-terminal lobe and prominent, long, narrow, non-falcate ventral lobe; dorsal caudal margin proximally and distally convex, and slightly concave just anterior to subterminal notch, with prominent lateral undulations; preventral margin strongly convex, tip of ventral caudal-fin lobe narrowly rounded; lower postventral margin very slightly concave; upper postventral margin nearly straight except for convex section at subterminal notch; subterminal margin very slightly concave, terminal margin concave, lobe formed by these margins rounded, tip of tail narrowly rounded. Length of dorsal caudal margin 3.07 (2.76–2.96) in precaudal length, preventral caudal margin 1.89 (1.93–2.04) in dorsal caudal margin, terminal lobe from caudal tip to subterminal notch ~3.55–3.82 in dorsal caudal margin, subterminal margin length 1.67–2.18 in terminal margin.
Counts of total vertebral centra (TC) 198 (197), precaudal centra (PC) 115 (113–115), monospondylous precaudal (MP) centra (60–65), diplospondylous precaudal (DP) centra (50–54), diplospondylous caudal (DC) centra 83 (83–94); MP centra 30.5–32.5%, DP centra 25.4–26.9%, and DC centra 41.9–42.6% of TC centra. Ratios of DP/MP centra 0.78–0.88, DC/MP centra 1.30–1.40. Last few MP centra before MP–DP transition not enlarged and not forming a ‘stutter zone’ of alternating long and short centra.
Colouration
In preservative. Dorsal surface of head, trunk and tail slate-grey, graduating variably to white ventrally on midlateral surface. Light and dark surfaces (waterline) of head moderately well demarcated, extending along lateral angle of the snout anteriorly to level of nostrils, directed ventrally besides nostrils, then obliquely directed to the mid-upper eye; continuing post-orbitally along head from upper eye to mid-upper gill slits, merging with first gill slit in mid-upper region, becoming somewhat diffuse; indistinct over gills. Pale region of belly extending above pectoral-fin base anteriorly almost to fifth gill slit and contrasting strongly with greyish base of pectoral fin. Waterline extending along abdomen through the lower third of its depth; interrupted by a paler mid-lateral stripe extending from the tail; waterline indistinct on tail, light and dark tones merging irregularly. Sharply defined, broad blackish stripe extending from insertion of second dorsal fin to origin of upper lobe of caudal fin, its width about equal to width of precaudal pit; contrasting strongly with posterior portion of second dorsal fin viewed from above (not visible in lateral view), continuing along dorsal–caudal margin. Ventral surface largely white with some irregular dusky markings (some specimens stained irregularly brownish from haemorrhaging). First dorsal fin mostly paler than dorsal surface of body, base greyish; apex narrowly black tipped; posterior margin narrowly blackish or dusky. Second dorsal fin basal half paler than dorsal surface of body; anterior margin narrowly black edged; apex broadly black tipped, sharply demarcated from area below; free rear tip pale. Caudal fin slightly paler than body; broad anterior blackish margin, broadest near fin origin (almost equivalent to eye diameter); fin tip with a diffuse blackish marking; posterior margin of terminal lobe narrowly blackish; postventral margin mostly plain, either with an extremely narrow, almost indiscernible blackish edge, or dusky; ventral lobe with a prominent black marking (almost length of prenasal snout), its inner margin diffuse, its anterior margin extending along preventral margin. Anal fin mostly pale, prominent sharp-edged black apical blotch, more prominent than dark marking on second dorsal fin. Pectoral fin greyish dorsally without pale marking at its origin, distinctly paler ventrally, almost white; blackish apical blotch present on both surfaces, slightly larger ventrally. Pelvic fin mostly pale dorsally and ventrally; apex with small, elongate, sharply demarcated black blotch on both surfaces, similar in size on both surfaces. Claspers white (adult males with some dusky areas on dorsal surface of claspers). Eyes silvery yellow with a black pupil.
When fresh. Uniformly greyish green to greenish yellow on dorsal surfaces of head, trunk and tail (becoming greyish in preservative); some specimens with faint scattering of small, ~1 mm, darkish spots on dorsal surface (not shown); fin markings more conspicuous.
Size
Specimens examined range in size from 673 to 1236 mm TL. Size at birth unknown; no individuals with umbilical scars were recorded. Juvenile males ranged from 673 to 888 mm TL and adult males (i.e. with claspers fully calcified) ranged from 1230 to 1236 mm TL, suggesting maturity is attained between 888 and 1230 mm TL.
Distribution and abundance
In the present study, C. leiodon was recorded from both Sharq (29°23′N, 47°58′E) and Fahaheel (29°04′N, 48°08′E) fish markets in Kuwait. All specimens were likely caught by gill-nets operated from small (~7–8 m length) open speedboats fishing in nearshore waters close to these landing sites (pers. obs.), although commercial fishing is not permitted within 3 nautical miles (~5.5 km) of the coast or within Kuwait Bay (J. Bishop, Kuwait Institute for Scientific Research, pers. comm.). At present, confirmed records of this species are disjunct, known only from eastern Yemen (type locality) and Kuwait, ~3000 km apart. It has not been recorded from other waters surrounding the Arabian Peninsula (Randall 1986; Bonfil and Abdallah 2004; Henderson et al. 2007; Golani and Bogorodsky 2010), or in recent surveys of fish markets in Qatar and Abu Dhabi (unpubl. data), ~500 km and 800 km away from Kuwait respectively. Although C. leiodon was not common in the surveys of fish markets in Kuwait (only ~2% of all 1200 sharks recorded), they were as abundant as more widespread species of Indo-Pacific whaler sharks such as C. amboinensis, C. brevipinna, C. leucas and C. limbatus (unpubl. data).
Habitat and ecology
The habitat of the C. leiodon specimens recorded from Kuwait in this study is not known. Based on the likely range of speedboats from their home ports in Kuwait, fishers could be operating in a variety of habitats, including turbid euryhaline waters of the Tigris–Euphrates–Karun system and the limited coral reefs of Kuwait. The main habitat type in the area is soft sediment with maximum depths of 30–40 m. Water temperature in April, when specimens were observed, ranged from 19 to 23°C and salinity from <30 (in estuarine areas) to 38 (Al-Yamani et al. 2004). Stomachs of two fresh specimens (SAM unreg.; BMNH 2010.2.8.1) each contained the body of a single catfish (Ariidae), and the radiograph of a fixed specimen (BMNH 2008.7.28.1) also showed a catfish.
Molecular results
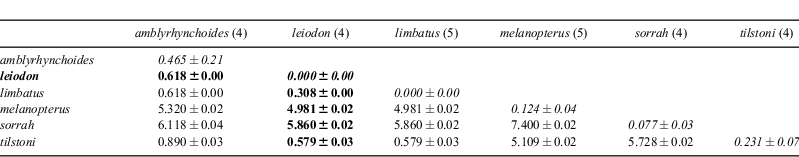
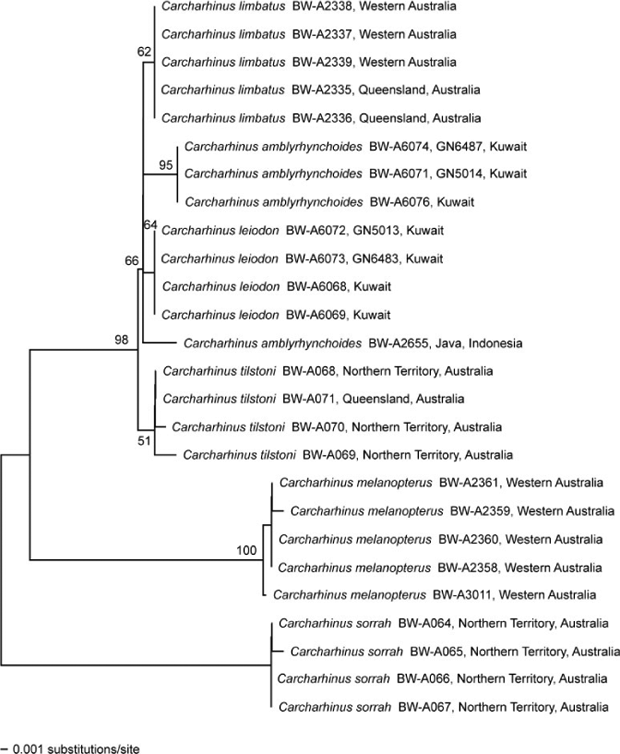
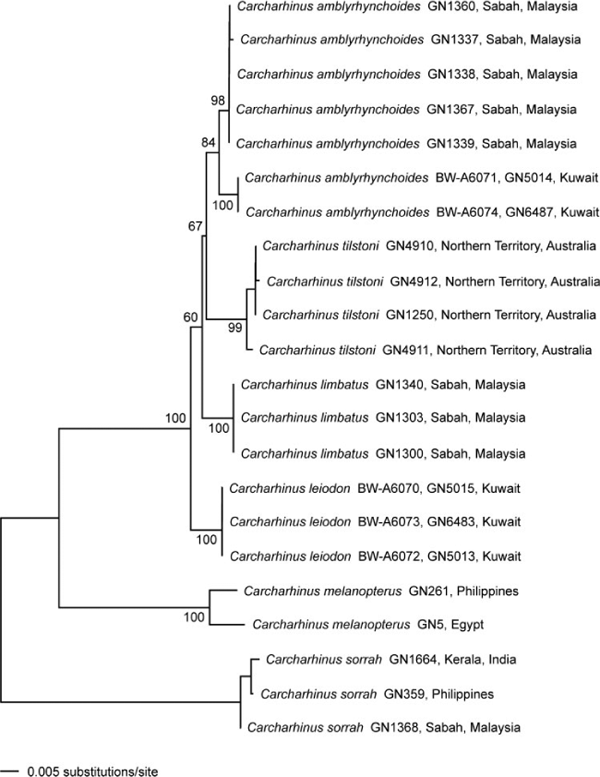
For COI, genetic distances between C. leiodon and the closely related triumvirate of C. limbatus, C. amblyrhynchoides and C. tilstoni were all very low, being less than 1% (Table 1, Fig. 4). Genetic distances to the two other Carcharhinus species examined here were substantially higher, in the range of 5–7%. The four specimens of C. leiodon, fully sequenced for the barcode region, showed an identical haplotype. There was a single base that differentiated these four specimens of C. leiodon from all five other Carcharhinus species assessed, and this base was also found in the fifth specimen of C. leiodon, which was only partially sequenced (BW-A6070). This base was a G (guanine) at position 360 in the barcode sequence; all other specimens and species showed an A (adenosine) here. It was towards the centre of an otherwise conserved sequence within the COI barcode region of these six Carcharhinus species, running from base 343 to base 371: TTAGCTAGCAACTTAGCG/ACATGCTGGACC. A subsequent comparison of the five C. leiodon barcodes with those from 170 individuals of 17 additional Carcharhinus species (not presented here) showed that this base G remained diagnostic for C. leiodon; all other specimens and species had an A at this position. COI data revealed two distinct groupings of C. amblyrhynchoides, with the three Kuwait samples identical in sequence to each other but exhibiting six nucleotide differences from the single Indonesian sample. Thus within-species differentiation among geographically distinct populations of C. amblyrhynchoides (six nucleotides) is larger for the COI data than the among-species differences (four nucleotides) between C. amblyrhynchoides and C. leiodon in Kuwait.
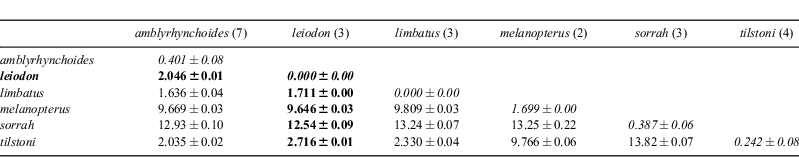
The ND2 sequence data exhibit more variation, both within and among species, than the COI data (Table 2, Fig. 5). All three of the C. leiodon samples are identical to each other and exhibit either 20 or 21 nucleotide differences from the seven C. amblyrhynchoides samples. The ND2 sequence data, like the COI data, show differences among the C. amblyrhynchoides samples, with the five Malaysian samples being either identical or differing by a single nucleotide, whereas the two specimens from Kuwait are eight or nine nucleotides different from the Malaysian samples. However, in contrast to the COI data, the ND2 data suggest that the within-species variation within C. amblyrhynchoides estimated from Malaysian and Kuwaiti specimens is less than the between-species differences between the specimens of C. amblyrhynchoides and C. leiodon in Kuwait. The neighbour-joining trees resulting from the Kimura two-parameter distance data are broadly consistent, but not identical, between the two genes. Both datasets show C. sorrah at the base of the tree with C. melanopterus branching next; C. limbatus, C. tilstoni, C. amblyrhynchoides, and C. leiodon are all closely related. However, the ND2 neighbour-joining tree reveals each of the species to be monophyletic, while the COI data fails to group all of the C. amblyrhynchoides into the same cluster. These inferences are consistent with the patterns of nucleotide differences described above.
Discussion
Comparison with closely related species
The present study provides important new information on field characters of C. leiodon to distinguish it from similar species, most notably its fresh colouration, which was not previously known. C. leiodon is one of the rarest Carcharhinus species and also has one of the most restricted ranges. C. leiodon is very closely related to C. amblyrhynchoides, C. limbatus and C. tilstoni but differs in morphology, fin colouration and teeth morphology. C. leiodon can be clearly distinguished from these three species in dentition (with the upper teeth having narrow and erect cusps with smooth edges v. narrow and erect cusps with serrated edges) and more precaudal vertebrae (precaudal centra 113–115 v. 78–102). It is also distinguished from these species in having the first dorsal-fin origin over mid-length of the pectoral-fin inner margin v. over pectoral-fin insertion. Carcharhinus leiodon can be further distinguished from C. limbatus and C. tilstoni in having a short, bluntly pointed snout v. a moderately long, narrowly pointed snout.
Fresh specimens of C. leiodon closely resemble those of C. amblyrhynchoides but they can be distinguished based on the following aspects of their colouration: anal fin with a prominent, blackish, sharp-edged apical blotch (v. anal-fin apex pale or slightly dusky in C. amblyrhynchoides); dorsal surface of caudal peduncle blackish (v. similar in colouration to upper lateral surfaces); caudal fin with a broad, sharply-demarcated blackish dorsal margin (v. a narrower, more diffuse blackish dorsal margin); demarcation between darker dorsal and paler ventral surfaces diffuse along body (v. more strongly demarcated); pelvic fins with small, elongate, sharp-edged, blackish apical blotches on both dorsal and ventral surfaces (v. pelvic fins with diffuse-edged, dusky tips); whitish marking on body above pectoral-fin base, just posterior to fifth gill slit (v. greyish, more similar to dorsal and upper lateral surfaces); and second dorsal-fin anterior margin blackish (v. anterior margin not distinctly blackish).
The narrow, smooth-edged cusps of C. leiodon teeth are relatively unique within the Carcharhinidae and clearly separate this species from its closest congeners, C. amblyrhynchoides, C. limbatus and C. tilstoni, all of which have serrated cusps. Only C. isodon and juvenile C. brevipinna share this characteristic with C. leiodon (Garrick 1985). The adult males examined in this study also possessed smooth-edged cusps, indicating this feature is not subject to ontogenetic change. C. leiodon clearly differs from C. isodon in having black fin tips, a much higher second dorsal fin (height 3.7–4.1 v. 2.5–2.9% TL) and more precaudal vertebrae (113–115 v. 78–80) (Garrick 1985). It also clearly differs from C. brevipinna in the slightly more posterior position of the first dorsal fin, fin colouration and number of vertebrae (precaudal vertebrae 113–115 v. 76–91, Garrick 1982). When fresh, C. leiodon is superficially similar in appearance to, and probably often misidentified as, C. melanopterus but differs in the following characters: dorsal fin with a small blackish apical tip (v. a very large apical black tip), upper teeth narrow, erect and smooth-edged (v. triangular, more oblique and serrated), and more anteriorly positioned first dorsal fin (origin over mid-length of pectoral-fin inner margin v. over pectoral-fin free rear tips).
Several proportional dimensions reported for the holotype are outside the range of values of the four new specimens (see Accessory Publication). This is likely to be a result of the small sample size (i.e. a single specimen) used by Garrick (1985) to describe the species, as well as possible age and/or preservation artefacts.
Given the similarity of this species to other black-tip whaler sharks, it is likely that it has been commonly misidentified within its range. For example, the Kuwait Institute for Scientific Research (2005) included C. melanopterus but the image is clearly of a specimen of C. leiodon. It is possible that C. leiodon has been present, but not recognised, in the wider Gulf; for example, Hussain et al. (1988) reported ‘C. melanopteras’ (sic) from the Khor Al-Zubair in Iraq, and Basson et al. (1977) noted ‘several species of black-tips rather difficult to distinguish without close examination’, in reference to the Gulf coast of Saudi Arabia.
Molecular analyses
The COI barcode data show that C. leiodon is genetically very closely related to C. limbatus, C. tilstoni and C. amblyrhynchoides, with genetic distances to these species of only 0.25 to 0.60%. Such low intrageneric distances are rare, but have been seen in some other fish species (e.g. tunas of the genus Thunnus, Ward et al. 2005). Genetic distances to the other three Carcharhinus species examined here, of the range of 3 to 7%, are more typical of fish intrageneric comparisons. However, the COI barcode data are consistent with C. leiodon being a true, reproductively isolated species, as it has a single base in the barcode region enabling its discrimination from the 23 other species of Carcharhinus that have been barcoded to date (20 in Ward et al. 2008, plus three unpublished). The ND2 dataset is also consistent with C. leiodon being a distinct species, but this is to be expected as the two markers are linked on the mitochondrial genome and are non-independent. The observation that there are greater differences in the COI barcode sequence among populations of the same nominal species (C. amblyrhynchoides) than there are between individuals of different species (C. leiodon and C. amblyrhynchoides) could give pause to those who would base species description on sequence data without a thorough sampling of all of the geographic variation circumscribed by a given species. Our data are consistent with the close relationship of C. amblyrhynchoides, C. limbatus and C. tilstoni suggested by Ward et al. (2008) and recently confirmed by Ovenden et al. (2010), who in addition to COI, used sequences from the control region and ND4 regions of the mitochondrial genome. However, the exact inferred relationships differ among studies.
Distribution and ecology
Garrick (1985) stated that the accurate capture location of the holotype was not known, with only ‘Gischin’ given. Garrick considered this could be a different spelling of Qishn (15°25′N, 51°41′E) in southern Arabia (currently eastern Yemen, north-east coast of the Gulf of Aden at its border with the Arabian Sea) based on advice from the museum curator that this was where the collector, Wilhelm Hein, obtained specimens. Given this uncertainty and collection of specimens in Kuwait during the present study, ‘Gischin’ may refer to the Iranian island of Qeshm, located in the easternmost Gulf (~26°50′N, 56°0′E). However, on further investigation, Hein, an Austrian anthropologist (1861–1903), is known to have travelled to Yemen in December 1901; he was based in Qishn (Al-Mahra, the easternmost governate of Yemen) where he also collected numerous biological specimens (Speake 2003), and referred to Qishn as Gischin (J. C. Watson, Professor of Arabic Linguistics, University of Salford, pers. comm.). Based on this, we consider the eastern Yemen location for the holotype as likely to be correct.
The two locations in the Western Indian Ocean from which C. leiodon have been recorded are notably different. In recent times, the fully marine coast of eastern Yemen has a narrow continental shelf without permanent watercourses (similar to much of the Arabian Peninsula), adjacent to much deeper waters of more than 2500 m. In contrast, the Gulf is a river valley flooded during the Holocene transgression around 17 000 years ago, and is shallow over its entirety with a maximum depth of ~100 m at the Straits of Hormuz. The north-western Gulf (including Kuwait) is less than 40 m in depth and influenced by the discharge of the major Tigris–Euphrates–Karun river system, a freshwater input of regional significance. However, environmental change in southern Arabia has been significant in recent geological (and evolutionary) history, with periods of wet climate and river activity in the Quaternary (Parker and Goudie 2008). In contrast to the present day, estuaries open to the sea receiving significant inputs of fresh water were present as recently as 420 AD near the type locality of C. leiodon (Hoorn and Cremaschi 2004). Further work on habitat requirements, including possible occurrence in estuaries, is required.
It is likely that in its range, C. leiodon partitions itself from sympatric and morphologically similar species (e.g. C. limbatus and C. amblyrhynchoides) by utilising different habitats or feeding on different prey, as observed for carcharhinids in shallow subtropical environments elsewhere (e.g. White and Potter 2004; White et al. 2004); further research is required. As mentioned previously, fresh specimens of C. leiodon are similar in appearance to C. melanopterus. The latter species is widely distributed throughout the Indo-West Pacific and is one of the commonest inshore sharks in Oman (A. Henderson, Sultan Qaboos University, pers. comm.), and its presence in the eastern Gulf area has been confirmed by photographic evidence from Abu Dhabi (R. Jabado, University of Al Ain, unpubl. data). C. melanopterus was not recorded during market surveys of Kuwait and Qatar (unpubl. data). It is possible that, at least in the waters of Kuwait, C. leiodon replaces C. melanopterus in its shallow-water niche.
As the present study is based on sampling in April only, there are no data available on possible seasonal changes in abundance of C. leiodon. However, closely related species at similar latitudes are known to undertake seasonal north–south migrations, such as C. limbatus off the south-eastern USA (Castro 1996). Off Kuwait, trawl catches of unidentified sharks have been reported as highest in warmer seasons (Goubanov and Shleib 1980), with a similar pattern for trawled carcharhinids in central Gulf waters (FAO 1981). Anecdotal sources also report summer abundances of larger carcharhinids in Kuwait’s waters (Clayton and Pilcher 1983). With water temperature off Kuwait varying from <19°C to >30°C throughout the year (Al-Yamani et al. 2004), it is possible that the distribution and abundance of C. leiodon changes seasonally.
Conservation
While C. leiodon did not appear to be part of a targeted or high-value fishery in Kuwait (pers. obs.), it is clearly subject to by-catch mortality. The Gulf is intensively fished with a range of methods including gill-netting, shrimp trawling and intertidal barrier traps (Bishop 2002; Morgan 2006). As most individuals of all shark species landed in Kuwait in April 2008 were small (50–90 cm TL; unpubl. data) and C. leiodon males appear to become mature at a size around or above the upper part of this range, there is clearly the potential for a fisheries-induced reduction in recruitment, which is of particular concern given its apparently restricted distribution. Status of C. leiodon at the type locality is likely to be even less favourable because Yemen and neighbouring nations, such as Oman and Somalia, are known to operate major targeted shark fisheries (Bonfil and Abdallah 2004; Henderson et al. 2007).
Red List status of C. leiodon
The current IUCN listing of C. leiodon as Vulnerable requires re-assessment as a result of new information presented in this study, notably on range extension and a previously unknown population. However, considerable assumptions are required, especially given the relative lack of reported collection effort in the region. Using Red List geographic range B criteria (IUCN 2001) and assuming C. leiodon is restricted to shallow waters of <40 m depth of an area totalling <20 000 km2 in its two known disjunct ranges off Kuwait and easternmost Yemen, and taking into account likely continuing declines in habitat (at least off Kuwait, e.g. Al-Yamani et al. 2007; Sheppard et al. 2010) and number of adults (through fisheries), the Vulnerable listing would remain unchanged. However, this assumes C. leiodon occurs in all types of waters in its two known ranges. If further work demonstrates that C. leiodon is limited to specific habitats, this could elevate the species to the Endangered category. The status of C. leiodon at its type locality in Yemen requires urgent attention considering the last known occurrence was over a century ago, and it was not recorded in recent elasmobranch surveys in the both the Gulf of Aden/Red Sea area and nearby south-western Oman (Bonfil and Abdallah 2004; Henderson et al. 2007). Demonstrated absence of C. leiodon around the type locality could therefore elevate the Kuwait subpopulation to a Critically Endangered category. Conversely, records from areas neighbouring its currently known distribution (e.g. that of Iraq, Iran and the wider Gulf of Aden) may yet reveal C. leiodon to be more widely distributed, although the elasmobranch fauna in these areas is generally poorly documented.
Although marine carcharhinids are generally widespread, C. leiodon is one of a few species with a very narrow known range. Two other species of small Indo-Pacific Carcharhinus, C. hemiodon and C. sp. A, are known from only a small number of specimens (Compagno et al. 2005) and it is considered that they are highly threatened within their range with little information available for either species. In fact, C. hemiodon is currently listed by the IUCN Red List of Threatened Species as Critically Endangered as it has not been recorded since 1979 (with most of the few known specimens captured before 1900), and is in an area of expanding unregulated fisheries (Compagno et al. 2003).
While the current account provides important new information on C. leiodon, further research on life history and habitat requirements in its known range is required to allow for suitable management practices to be implemented. Additional records of C. leiodon in this poorly-known region would also be beneficial to ascertain the true distribution of this species, and essential to such efforts is its accurate identification.
Acknowledgements
We thank Lt. Gen. Yousef Al-Khorafi, Gen. Sulayman Al-Fahad and Brig. Jassem Failkawi (Kuwait Ministry of Interior), the Public Authority for Agriculture and Fisheries, the Kuwait Scientific Centre (KSC), Gulf Telecom and the Kuwait Institute for Scientific Research (KISR) for support of the Kuwait SCS expedition; Dr James Bishop (KISR) for heroic efforts in transportation of specimens; Tony McEwan (Kuwait Scientific Centre) for temporary storage; Patrick Campbell and Oliver Crimmen (Natural History Museum) for registering, X-raying and accessing specimens; Dr Leonard Compagno (formerly Shark Research Centre, Cape Town) and Dr Aaron Henderson (Sultan Qaboos University, Oman) for initial identification assistance; Bronwyn Holmes, Melody Puckridge, John Pogonoski and Alastair Graham (CSIRO, Australia) for processing DNA barcoding samples; staff at the Biodiversity Institute of Ontario (University of Guelph) for much of the COI sequencing, Kerri Matthes (Florida State University) for PCR amplification and sequencing of the ND2 component; Dr Nick Dulvy (Simon Fraser University) for advice on Red List assessment; Professor Janet C. Watson (University of Salford) for historical help on the location of Hein’s collection; SCS volunteers Dareen Almojil, Tony Bennett, Shane Benzie, Mark Boothman, Emma-Louise Nicholls, Stuart Nicholls, Jacqui Peirce, Al Reeve, Mike Sharland, Andy Sweeney and Mike Webb for their assistance and good humour in market collections, and particularly Simon Collins for assistance with photographing fresh specimens. We are grateful for the comments of referees and Guest Editors that greatly improved the manuscript. Funding for a portion of the molecular component was provided by NSF (DEB 1036500) to G.N. A.B.M.M. thanks Francesca for her support and encouragement throughout.
References
Al-Yamani, F. Y., Bishop, J., Ramadhan, E., Al-Husaini, M., and Al-Ghadban, A. N. (2004). ‘Oceanographic Atlas of Kuwait’s Waters.’ (Kuwait Institute for Scientific Research, Mariculture and Fisheries Department: Safat, Kuwait.)Al-Yamani, F. Y., Bishop, J. M., Al-Rafaie, K., and Ismail, W. (2007). The effects of the river diversion, Mesopotamian marsh drainage and restoration, and river damming on the marine environment of the northwestern Arabian Gulf. Aquatic Ecosystem Health & Management 10, 277–289.
| The effects of the river diversion, Mesopotamian marsh drainage and restoration, and river damming on the marine environment of the northwestern Arabian Gulf.Crossref | GoogleScholarGoogle Scholar |
Basson, P. W., Burchard, J. E., Jr, Hardy, J. T., and Price, A. R. G. (1977). ‘Biotopes of the Western Arabian Gulf: Marine Life and Environments of Saudi Arabia.’ (Aramco Department of Loss Prevention and Environmental Affairs: Dhahran, Saudi Arabia.)
Bishop, J. M. (2002). Fishing and mariculture. In ‘The Gulf Ecosystem: Health and Sustainability’. (Eds N. Y. Khan, M. Munawar and A. R. G. Price.) pp. 253–277. (Backhuys: Leiden.)
Bonfil, R., and Abdallah, M. (2004). ‘Field Identification Guide to the Sharks and Rays of the Red Sea and Gulf of Aden.’ (Food and Agriculture Organization (FAO): Rome.)
Castro, J. I. (1996). Biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bulletin of Marine Science 59, 508–522.
Clayton, D., and Pilcher, C. (1983). ‘Kuwait’s Natural History: An Introduction.’ (Kuwait Petroleum Corporation and Kuwait Oil Company: Ahmadi, Kuwait.)
Compagno, L. J. V. (1979). Carcharhinoid sharks: morphology, systematics and phylogeny. Ph.D. Thesis, Stanford University, Stanford, CA.
Compagno, L. J. V. (1984). FAO Species Catalogue. Vol. 4, Sharks of the World. An annotated and illustrated catalogue of shark species known to date. FAO Fisheries Synopsis No. 125. vol. 4, pt. 2 (Carcharhiniformes), pp. 251–655. FAO, Rome.
Compagno, L. J. V. (1988). ‘Sharks of the Order Carcharhiniformes.’ (Blackburn Press: Caldwell, NJ.)
Compagno, L. J. V. (2001). ‘Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date. Volume 2. Bullhead, Mackerel and Carpet Sharks (Heterdontiformes, Lamniformes and Orectolobiformes).’ (FAO: Rome.)
Compagno, L. J. V. (2005). Carcharhinus leiodon. In ‘IUCN Red List of Threatened Species’. Version 2010.1. Available at www.iucnredlist.org [Accessed 3 June 2010].
Compagno, L., Dando, M., and Fowler, S. (2005). ‘A Field Guide to the Sharks of the World.’ (Harper Collins: London.)
Compagno, L. J. V., White, W., and Fowler, S. (2003). Carcharhinus hemiodon. In ‘IUCN Red List of Threatened Species’. Version 2010.1. Available at www.iucnredlist.org [Accessed 1 June 2010].
FAO (1981). A report on the demersal resources of the Gulf and Gulf of Oman. Regional Fishery Survey (Bahrain, Iran, Iraq, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates) and Development Project FI DP/RAB/71/278/10. FAO, Rome.
Garrick, J. A. F. (1982). Sharks of the genus Carcharhinus. NOAA (National Oceanic and Atmospheric Administration) Technical Report NMFS (National Marine Fisheries Service) Circular 445.
Garrick, J. A. F. (1985). Additions to revision of the shark genus Carcharhinus: synonymy of Aprionodon and Hypoprion, and description of a new species of Carcharhinus (Carcharhinidae). NOAA Technical Report NMFS 34. Available at http://spo.nwr.noaa.gov/tr34.pdf [Accessed May 2011].
Golani, D., and Bogorodsky, S. V. (2010). The fishes of the Red Sea – reappraisal and updated checklist. Zootaxa 2463, 1–135.
Goubanov, E. P., and Shleib, N. A. (1980). ‘Sharks of the Arabian Gulf.’ (Ministry of Public Works, Agricultural Department, Fisheries Division: Kuwait.)
Hebert, P. D. N., Cywinska, A., Ball, S. L., and de Waard, J. R. (2003). Biological identifications through DNA barcodes. Proceedings. Biological Sciences 270, 313–321.
| Biological identifications through DNA barcodes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktVWiu7g%3D&md5=8baaaa53058d9c2058e0c7a9cfff536eCAS |
Henderson, A. C., McIlwain, J. L., Al-Oufi, H. S., and Al-Sheili, S. (2007). The Sultanate of Oman shark fishery: Species composition, seasonality and diversity. Fisheries Research 86, 159–168.
| The Sultanate of Oman shark fishery: Species composition, seasonality and diversity.Crossref | GoogleScholarGoogle Scholar |
Holmes, B. H., Steinke, D., and Ward, R. D. (2009). Identification of shark and ray fins using DNA barcoding. Fisheries Research 95, 280–288.
| Identification of shark and ray fins using DNA barcoding.Crossref | GoogleScholarGoogle Scholar |
Hoorn, C., and Cremaschi, M. (2004). Late Holocene palaeoenvironmental history of Khawr Rawri and Khawr Al Balid (Dhofar, Sultanate of Oman). Palaeogeography, Palaeoclimatology, Palaeoecology 213, 1–36.
| Late Holocene palaeoenvironmental history of Khawr Rawri and Khawr Al Balid (Dhofar, Sultanate of Oman).Crossref | GoogleScholarGoogle Scholar |
Hussain, N. A., Naiama, A. K., and Al-Hassan, L. A. J. (1988). Annotated check list of the fish fauna of Khor Al-Zubair, North West of the Arabian Gulf, Iraq. Acta Ichthyologica et Piscatoria 1, 17–24.
IUCN (2001). IUCN Red List Categories and Criteria: Version 3.1. Available at http://www.iucnredlist.org/technical-documents/categories-and-criteria [Accessed 2 September 2010].
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16, 111–120.
| A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXmtFSktg%3D%3D&md5=b624e05c6d4a1dff9a13279cf8cb9101CAS | 7463489PubMed |
Kuwait Institute for Scientific Research (2005). Elasmobranchs of Kuwait waters. Poster. (Kuwait Institute for Scientific Research, Mariculture and Fisheries Department: Safat, Kuwait.)
Morgan, G. (2006). Country reviews: Iran, Iraq, Kuwait, Bahrain, Qatar, Saudi Arabia, UAE. In ‘Review of the State of World Marine Capture Fisheries Management: Indian Ocean’. (Ed. C. De Young.) FAO Fisheries Technical Paper No. 488, FAO, Rome. Available at http://www.fao.org/docrep/009/a0477e/a0477e00.htm [Accessed 12 December 2009].
Naylor, G. J. P., Ryburn, J. A., Fedrigo, O., and López, J. A. (2005). Phylogenetic relationships among the major lineages of modern elasmobranchs. In ‘Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras, Vol. 3’. (Eds W. C. Hamlett and B. G. M. Jamieson.) pp. 1–25. (Science Publishers: Enfield, NH.)
Ovenden, J. R., Morgan, J. A. T., Kashiwagi, T., Broderick, D., and Salini, J. (2010). Towards better management of Australia's shark fishery: genetic analyses reveal unexpected ratios of cryptic blacktip species Carcharhinus tilstoni and C. limbatus. Marine and Freshwater Research 61, 253–262.
| Towards better management of Australia's shark fishery: genetic analyses reveal unexpected ratios of cryptic blacktip species Carcharhinus tilstoni and C. limbatus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisVahu70%3D&md5=b0a087c800f9dd27ad994b457c0fd847CAS |
Parker, A. G., and Goudie, A. S. (2008). Geomorphological and palaeoenvironmental investigations in the southeastern Arabian Gulf region and the implication for the archaeology of the region. Geomorphology 101, 458–470.
| Geomorphological and palaeoenvironmental investigations in the southeastern Arabian Gulf region and the implication for the archaeology of the region.Crossref | GoogleScholarGoogle Scholar |
Randall, J. E. (1986). ‘Sharks of Arabia.’ (Immel Publishing: London.)
Sheppard, C, Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., Benzoni, F., Dutrieux, E., Dulvy, N. K., Durvasula, S. R. V., Jones, D. A., Loughland, R., Medio, D., Nithyanandan, M., Pilling, G. M., Polikarpov, I., Price, A. R. G., Purkis, S., Riegl, B., Saburova, M., Samimi Namin, K., Taylor, O., Wilson, S., and Zainal, K. (2010). The Gulf: a young sea in decline. Marine Pollution Bulletin 60, 13–38.
| The Gulf: a young sea in decline.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktlSqug%3D%3D&md5=25fa4b3ba17a7659d40173ba24e6de73CAS | 20005533PubMed |
Speake, J. (Ed.) (2003). ‘Literature of Travel and Exploration: An Encyclopaedia. Volume 3: R to Z.’ (Fitzroy Dearborn: London.)
Springer, V. G., and Garrick, J. A. F. (1964). A survey of vertebral numbers in sharks. Proceedings of the United States National Museum 116, 73–96.
Swofford, D. L. (2002). ‘PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.’ (Sinauer Associates: Sunderland, MA.)
Tamura, K., Dudley, J., Nei, M., and Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVGrsL8%3D&md5=927227bc5d8533f730ac83ff20a64694CAS | 17488738PubMed |
Ward, R. D., Zemlak, T. S., Innes, B. H., Last, P. R., and Hebert, P. D. N. (2005). DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society of London, Series B 360, 1847–1857.
| DNA barcoding Australia’s fish species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlSjsrjK&md5=e1f0704a3c9093bcd61999b5e90e1aa2CAS |
Ward, R. D., Holmes, B. H., White, W. T., and Last, P. R. (2008). DNA barcoding Australasian chondrichthyans: results and potential uses in conservation. Marine and Freshwater Research 59, 57–71.
| DNA barcoding Australasian chondrichthyans: results and potential uses in conservation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFKnt7c%3D&md5=cb1feae646109716ca6448cf570cc33cCAS |
White, W. T., and Potter, I. C. (2004). Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia. Marine Biology 145, 1023–1032.
| Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia.Crossref | GoogleScholarGoogle Scholar |
White, W. T., Platell, M. E., and Potter, I. C. (2004). Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: implications for resource partitioning. Marine Biology 144, 439–448.
| Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: implications for resource partitioning.Crossref | GoogleScholarGoogle Scholar |
White, W. T., and Sommerville, E. (2010). Elasmobranchs of tropical marine ecosystems. In ‘Sharks and Their Relatives II: Biodiversity, Adaptive Physiology, and Conservation’. (Eds J. C. Carrier, M. R. Heithaus and J. A. Musick.) pp. 159–240. (CRC Press: Boca Raton, FL.)