Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea
C. Clarke A B , J. S. E. Lea A C F and R. F. G. Ormond A D EA Danah Divers Marine Research Facility, Beach Palace, P.O. Box 10646, Jeddah, 21443, Saudi Arabia.
B University of London Marine Biological Station, Millport, Isle of Cumbrae, Scotland, KA28 0EG, UK.
C University of Plymouth, Drake Circus, Plymouth, Devon, PL4 8AA, UK.
D Centre for Marine Biodiversity & Biotechnology, Heriot-Watt University, Riccarton, Edinburgh, EH14 4AS, UK.
E Save Our Seas Foundation, 5/6 Lang Rigg, South Queensferry, Edinburgh, EH30 9WN, UK.
F Corresponding author. Email: james.lea@plymouth.ac.uk
Marine and Freshwater Research 62(6) 668-675 https://doi.org/10.1071/MF10171
Submitted: 24 June 2010 Accepted: 11 January 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Many shark populations are experiencing critical declines from overfishing, triggering potentially detrimental cascade effects on marine ecosystems. Silky sharks, Carcharhinus falciformis, have experienced some of the most severe declines, yet little information exists on their behavioural ecology to inform management decisions. In the present study, the movement patterns of a sexually segregated subpopulation of female silky sharks on reefs in the Central Red Sea were investigated using acoustic telemetry to characterise habitat-use and residency patterns. Frequent baiting of sharks at a particular reef-site significantly increased time spent in the vicinity, although no increases in use of other reef areas 5–10 and 50–60 km away were recorded, and regular use of all three reef areas persisted in the absence of bait. Observed residency patterns varied considerably, from being present almost year-round to visiting only intermittently. The sharks spent significantly longer times at study reefs during daylight hours, even within bait-free regions, suggesting the diel bias is normal. This pattern became less distinct nearer the full moon when there is more ambient light. The regular, perennial use of these reefs by mature and near-mature female silky sharks highlights the importance of this habitat in the Red Sea for recruitment into the local shark population.
Additional keywords: acoustic telemetry, Indian Ocean region, provisioning, reef sharks.
Introduction
The silky shark, Carcharhinus falciformis, is a large (up to 3.5 m total length), slender species that until recently was regarded as one of the three most common pelagic shark species (Compagno 1984). However, in recent decades they have been subject to very high mortality, especially as the high demand for shark fins made them a lucrative bycatch species in long-line fisheries (Clarke et al. 2006). Thus, the silky shark population in the Gulf of Mexico has declined by more than 90% since the 1950s (Baum and Myers 2004), and it has been estimated that fishing mortality in the north-west Atlantic would need to be reduced by ~60% to ensure the survival of this sensitive species (Myers and Worm 2005). Although the exact magnitude of these declines may be overestimated (Burgess et al. 2005), the implications of depleted shark stocks remain severe. The removal of predators such as sharks can have profound environmental consequences, triggering cascade effects on marine ecosystems that can result in their collapse (Ferretti et al. 2010). Despite this, silky sharks remain absent from CITES listings and are described as only ‘near threatened’ on the IUCN Red List (Bonfil et al. 2007; CITES 2010).
Despite their past abundance, silky sharks remain largely overlooked in the scientific literature, in part owing to the logistical difficulty of observing them in their pelagic habitat. There is a lack of validated age and growth information, limiting the ability of fisheries managers to make informed decisions (Beerkircher and Shivji 2003; Watson et al. 2009). In addition, there is insufficient knowledge of their movement patterns that may confound management efforts, exemplified in the ongoing collapse of reef shark populations on the Great Barrier Reef, Australia (Robbins et al. 2006), where grey reef sharks, Carcharhinus amblyrhynchos, have been reported to display limited reef fidelity (Heupel et al. 2010).
Although silky sharks are generally regarded as oceanic or epipelagic in nature, they are most common in deep water close to a continental or insular shelf edge (Compagno 1984). In addition, they are occasionally seen on reefs adjacent to deep water (Tricas et al. 1997). Such behaviour appears to be especially characteristic of silky sharks in the central Red Sea, where their regular occurrence on offshore reefs in the Jeddah region provided the opportunity to study their behaviour in greater detail than has hitherto been possible. In the present study, a population of mature and near-mature female silky sharks has been subject to baiting and feeding at two sites since 1995. As a result, close observation of the sharks became possible, allowing individual sharks to be distinguished either as a result of distinctive marks or injuries, or through conventional tagging (C. Clarke et al. unpubl. data). Results of this preliminary study raised questions concerning the marked variation in pattern of visits to feeding stations by different individuals, and the movements and ranges of these sharks at times when they are away from this area.
Underwater acoustic telemetry was used to investigate several key questions important to efforts to secure the sustainable management of silky shark populations in the central Red Sea:
Do these sharks only visit the reefs in response to baiting, or do they make regular use of this habitat even when baiting does not occur?
Are silky sharks present in the area only at those times of year when baiting and observations were possible, or do they visit these reefs year round?
Do silky sharks make use of only a limited area of reefs, or do they make use of a large section of coast?
Materials and methods
Study site
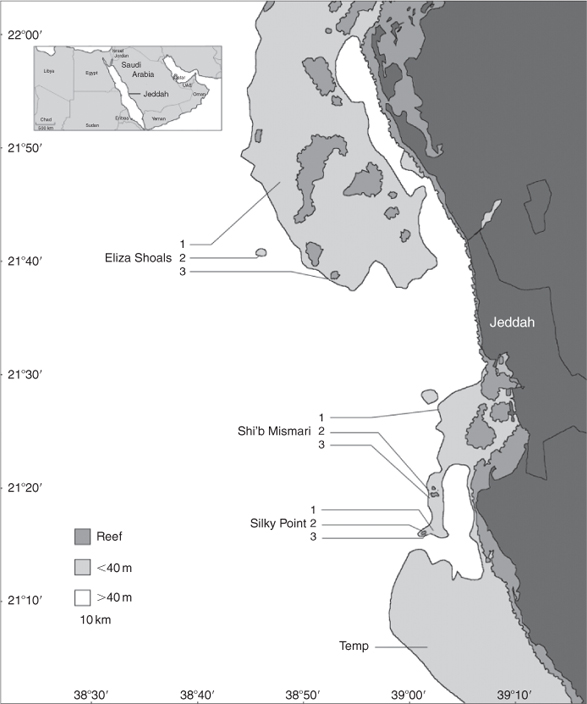
The study was conducted between August 2007 and June 2009 on a series of coral reefs distributed 10–30 km offshore from the region of Jeddah, Saudi Arabia, in the central Red Sea (Fig. 1). Three reef areas were involved: Eliza Shoals (ES), Shi’b Mismari (MM), and Silky Point (SP); within each of these areas three sites (named ES1, ES2, ES3; MM1, MM2; SP1, SP2, etc.) were used. The site SP2 has been a baited dive site since 1995, where sharks are attracted using bonito, ground to facilitate odour dispersal. Shi’b Mismari represents a bait-free area, whereas occasional baiting has occurred at the site ES2. Most individual reefs are small (area 0.5–1 km2) and slope steeply from the surface to ~40 m depth, before rapidly dropping to depths of several hundred metres. Exceptions to this trend were sites ES1, which is an extended coral plateau of 20 m depth, and ES2, which is a seamount that rises to within 40 m of the surface. The geographical range of the study was determined by accessibility from our research base on the coast north of Jeddah.
|
Tagging
Fourteen female silky sharks were tagged externally with coded acoustic transmitters (V16, Vemco Ltd, Canada; 60–120 s delay), which had a battery life of greater than 15 months. Tagging was undertaken at SP2 and ES2 (Fig. 1) and procedures were similar to previous studies that avoided hooking individuals (e.g. Laroche et al. 2007). The transmitters (tags) were attached via a short wire tether to a small plastic umbrella dart (Domeier et al. 2005), placed adjacent to the base of the dorsal fin. This was achieved either by using a modified spear gun in the water (for those tagged at SP2), or by immobilising the individual with a tail-rope in the water and using a purpose-built, small handheld spear to insert the umbrella tag (for those tagged at ES2). Shark total length was estimated against a graduated PVC pole of known length.
Autonomous acoustic receivers
An array of up to 12 separate acoustic receivers (VR2W, Vemco Ltd, Canada) was deployed to cover the three study reefs and the surrounding areas (Fig. 1). Nine made up a core set: three around SP, three around MM, and three along the outer part of ES. The three other receivers were placed at more distant sites on the periphery of the study area to explore the extent of shark movement for future study.
Data analysis
For visual assessment of any apparent temporal or geographical patterns, raw data from the receivers were plotted over time. General Linear Models (GLMs; Minitab release 15, Minitab Inc., State College, PA, USA) were used to determine which factors influenced observed variation in site visitation frequency and time spent in particular areas. Post-hoc Tukey’s tests were conducted to determine the locations of significant differences. Factors tested in these models (against an H0 of no influence) were: (i) individual shark; (ii) receiver; (iii) time of year (month); (iv) baiting activity at SP; (v) moonlight (% illumination of the moon); and (vi) the interaction between receiver and bait at SP. Response variables modelled against these factors were: (a) daily presence/absence; (b) the proportion of hours present per day (calculated from hourly presence/absence data and angular-transformed); and (c) the proportion of nocturnal hours present per day (also calculated on an hourly presence/absence basis and angular-transformed). The latter model included an interaction term between individual shark and moonlight. The GLM results are interpreted with caution as the data were not normal even after transformations. To test the null hypothesis that there was no diel bias in habitat use, paired t-tests were used to compare the proportion (after angular-transformation) of diurnal and nocturnal hours spent at each site. Three of the 14 sharks tagged were excluded from statistical analyses owing to the limited duration of their tracks providing insufficient data (≤two days, Sharks 6, 8 and 9; Table 1).

|
Results
Spatial patterns
Sharks did not visit different reefs with similar frequency. The majority of detections (35 412, 64.34% of total) were made by the receivers positioned at SP (where Sharks 1–10 were tagged), nearly a third by a receiver at ES2 (16 284, 29.58%; none at either ES1 or 3), and fewer at MM (3345, 6.08%). The relatively high number of detections at ES2 is in part attributable to the four sharks (11–14) tagged there later in the study period (Table 1). At both SP2 and ES2, up to three tagged sharks were detected simultaneously. Two sharks tagged at SP2 were detected at ES2, giving a maximum known displacement of a shark in the present study of 56 km (Table 1). However, most tagged sharks (79%) were only detected at receivers within 10 km of their tagging location (Table 1). Although there was a significant correlation between tracking period and total detections (r = 0.885, d.f. = 13, P < 0.001), this significance was lost with the exclusion of Shark 2 (r = 0.472, d.f. = 12, P = 0.103). Evidently, there was higher use of the study area by some individuals than by others.
Although the GLM for presence/absence data only accounted for 18.56% of the observed variation, much of this was attributable to variation in ‘receiver’ (Table 2a). Specifically, SP1 and SP2 were visited most frequently, with ES1, 2, 3 and MM1 and 2 being visited infrequently by comparison. As indicated by the correlation between tracking period and total detections, variations in individual sharks also significantly influenced the observed variation in site presence (Table 2a), with Sharks 1, 2 and 3, specifically, visiting the study area more frequently than others. Only the interaction term between ‘bait at SP’ and receiver was significant as opposed to ‘bait at SP’ of itself (Table 2a), highlighting that baiting only significantly affected shark visitation frequency at SP.
The second GLM, to investigate visit duration (Table 2b), showed a similar pattern in variation accounted for by the factors, explaining 45.37% of the observed variation. Significantly greater amounts of time were spent at SP2 and ES2. Time spent at SP2 was also positively influenced by bait, with tagged sharks spending ~70% longer there on baited days. Despite its relative proximity to SP (5–10 km), time spent near to receivers at MM was not influenced by baiting activity at SP, and tagged sharks continued to use each of the study reef areas even in the absence of bait. Again, some individual sharks, particularly Sharks 1, 11 and 12, spent significantly more time around receivers than other sharks.
Temporal patterns
Only five (36%) of the tags provided tracking periods in excess of three months (1, 2, 4, 5, 7; Table 1). However, two of the sharks were detected for approaching a year or more (Table 1), including one (Shark 2) that because of a distinctive dorsal fin injury had been individually recognised and seen at SP for approximately three years before being tagged. Although many of the sharks spent a substantial proportion of their tracking periods within the study area, there were also many days where tagged individuals did not visit (Table 1). For instance, two of the sharks were absent from the detection record for periods in excess of a month, but then returned (4, 5; Table 1).
Detection records for several sharks showed noticeably greater presence on the study reefs during daylight hours than at night (e.g. Shark 2, Fig. 2). To test this, the proportions of diurnal and nocturnal hours during which sharks were present were compared for each of the reef areas (Table 3). Thus, it appears that tagged sharks in the present study spent longer at each of ES, MM and SP during daylight hours than at night. This diel bias in habitat use remains evident at bait-free MM. However, the tagged sharks were not entirely absent from reefs in the study area at night (e.g. Shark 2, Fig. 2). From the GLM, done to model factors that might influence time spent on reefs at night (Table 2c), it is apparent that although baiting activity accounted for most of the explained variation, followed closely by receiver, the interaction between moonlight and individual shark was also significant. Sharks 1, 2, 4 and 7 spent more time on reefs under greater moonlight, whereas Shark 12 appeared to spend less time than would be expected by chance under greater moonlight.
Analysis of longer-term patterns has to be undertaken more tentatively owing to the small number of sharks with substantial track periods (only five individuals with three months or more, Table 1). It is not possible to discern any clear long-term patterns, but from the GLMs (Table 2a, b), time of year accounted for more of the observed variance than would be expected by chance alone. Although silky sharks were to some extent present in the study area year round, January and September experienced higher visitation frequencies compared with other months, whereas March and April displayed troughs in shark presence. However, these patterns are likely influenced by tag deployment dates and brief tracks, as the months during which the most time was spent within the study area were September to November, coinciding with the first months after tags 1–5 were deployed.
Discussion
Spatial patterns
Tagged silky sharks used each of the reef areas ES, MM and SP, although the majority of detections occurred at SP, where 10 of the 14 sharks were tagged. In addition, tagged sharks spent considerably more time (~70% longer) in the vicinity of SP when baiting occurred. However, the data from this study also clearly show that the sharks visited both the SP baiting station and other bait-free reef areas (e.g. MM) in the absence of bait and even during longer periods, albeit less frequently, when no baiting had been taking place. Taken together these observations suggest that, in this Red Sea population, females and sub-adults regularly visit such reefs in the absence of bait, and that such behaviour is probably normal. It seems that silky sharks are drawn to these reef habitats for reasons extraneous to baiting activity, but that they may linger at particular locations (e.g. SP) when bait is applied. Nonetheless, the intermittent nature of the detection records also suggests that individuals typically spend much of their time away from the reef study areas, most probably in deeper water further offshore, as supported by the complete lack of detections during some periods.
The fact that this loose aggregation comprises predominantly female sharks is of particular management significance, owing to the apparent sexual segregation. Overexploitation of any population representing a reproductive stage or sex-biased aggregation could disrupt population dynamics and cause a sudden collapse in numbers. This could happen despite other population units not being exploited directly, and the population as a whole may even reside across political boundaries, highlighting the potential need for collaborative management (Lucifora et al. 2002).
In addition to sexual segregration, there also appears to be individual variation in habitat-use. For instance, Sharks 1, 2 and 3 used the study area significantly more frequently than other tagged individuals. Two extremes are illustrated by Sharks 2 and 5; the former being detected within the study area on 68.3% of its 703 day detection record, with the latter showing both few detections and prolonged absences over its three-month data record. A switch between these two modes of habitat use in the study area was apparent in the detection record of Shark 4, which displayed regular presence for six months before disappearing for five months, after which it was detected again, though only over four days. Assuming that in each case the shark’s tag was operating correctly, the contrasting records indicate considerable differences in residency pattern, ranging from present all year, through intermittent visiting, to present for only part of the year. Such a high degree of individual variation in residency has also been revealed in other large shark species, e.g. tiger shark, Galeocerdo cuvier, and Galapagos shark, Carcharhinus galapagensis (Lowe et al. 2006), grey reef shark (Heupel et al. 2010) and some teleost fish, e.g. Australian snapper, Pagrus auratus (Egli and Babcock 2004).
A disparity in habitat use behaviours among individual silky shark is also consistent with the varying displacement distances recorded as a result of conventional tagging studies (Kato and Carvallo 1967; Stevens 1984; Kohler et al. 1998). Taken together, such studies suggest that although silky sharks may range considerably less than might be expected of a truly vagrant epipelagic species, moderately large displacements over short periods may also occur. Directed movement in response to specific factors may explain the periods of absence in the detection record.
Temporal patterns
Silky shark presence within the study area showed an exceptionally strong diel pattern, with detections of tagged sharks significantly more frequent during daylight hours. This finding is consistent with other aspects of shark behaviour found to exhibit diel periodicity, e.g. diving (Weng and Block 2004; Sims et al. 2005; Rowat et al. 2007) and feeding (Sims et al. 2006). Although the diurnal bias of tagged sharks was apparent across all sites, the most pronounced disparity in time spent between day and night was at SP.
Strong daytime bias shown by the silky sharks in the present study could result from baiting activity at SP occurring only during the day, as cases are known where baiting has influenced a species’ diel behaviour, e.g. stingrays at the popular site in the Cayman Islands, known as ‘Stingray City’ (Nelson 1995). However, such shifts in diel behaviour have not been found in other fish species when baited (e.g. Chateau and Wantiez 2008), and the finding of a statistically significant diel pattern at each site, including the bait-free MM, suggests the observed diel pattern in reef presence may reflect a normal preference. Although diurnal use of reef habitats may be natural behaviour, the use of reefs as either natural daytime foraging habitat or as refuges during less active periods cannot be determined – any response to bait may simply reflect opportunistic exploitation of an available resource, regardless of normal foraging habits.
Despite the diurnal bias, tagged sharks were not entirely absent from the study area at night: four of the tagged sharks displayed a significant increase in nocturnal presence nearer the full moon, whilst a fifth showed the opposite effect. A fuller moon results in greater nocturnal ambient light, and with potentially very high retinal sensitivity (Bres 1993; Lisney and Collin 2008), some silky sharks may opportunistically extend normal daytime behaviours into the night as the extra ambient light permits. This extension of diurnal behaviour is consistent with the diving behaviour of a juvenile white shark off California, which performed only a small proportion of its dives at night, but the majority of these occurred near the full moon (Dewar et al. 2004). The lack of an apparent relationship between lunar phase and the nocturnal presence of other sharks in the study could result from their records spanning insufficient lunar cycles for an effect to be detected.
Regarding longer-term and seasonal residency patterns, although it is difficult to decide the issue unequivocally, owing to tag loss, it is clear that there is a degree of perennial residency and habitat use in these reef areas. Further, although there are indications in the record of peaks in shark presence around January and September, there are no marked seasonal trends. However, the small sample size of sharks with sufficiently long detection spans limits interpretation. Silky sharks are believed to possess a biennial reproductive cycle (Bonfil et al. 1993), although there are differing reports regarding the seasonality of birthing, i.e. seasonal (Branstetter 1987; Bonfil et al. 1993) v. perennial (Bass et al. 1973; Stevens and McLoughlin 1991; Hazin et al. 2007). There may be geographical variation in the degree to which silky shark gestation is seasonal, related to the narrower seasonal amplitude of sea-surface temperatures closer to the equator (Hazin et al. 2007). Thus, Red Sea silky sharks could mate and pup throughout the year, making individuals’ biennial reproductive cycles asynchronous; this could partly explain the lack of a congruent seasonal pattern in the presence and absence of the tagged sharks.
Influence of baiting
The disproportionate amount of time spent at SP compared with other sites, and the significantly increased presence at SP on baited days, indicate that not only are the tagged sharks responding to the easy food availability at the baiting station, but that this availability is modifying the local habitat use, at least of the tagged sharks. The actual effect of baiting at SP may be even greater than that observed here, as the visitation frequency on non-baiting days was likely to have been elevated through feeding prior to the present study, and use of the area thus having been reinforced through past reward. A related concern is if bait-induced modifications to habitat use are not transient, then they may increase susceptibility to local fishing operations.
If Red Sea silky sharks make frequent use of reefs adjacent to deep water, their foraging activities may play a key role in the natural ecology of these reefs, as suggested for similar shark species elsewhere, e.g. Galapagos shark (Okey et al. 2004). Baiting activity may have second-order effects, either by increasing the number of these predators and the time they spend on the reef, or alternatively, if baiting is sufficiently frequent, by reducing the predation pressure on their natural prey. However, there are currently no empirical data to indicate whether impacts may occur through baiting of silky sharks, and data for Caribbean reef sharks, Carcharhinus perezi, show no evidence for shifts in behaviour or ecological impact despite long-term baiting (Maljković and Côté 2010). Further work in this area should include sequential assessment of reef community structure to track any differences or long-term changes.
Study limitations
The present study suffers from several weaknesses. Foremost is tag loss, which not only dramatically reduced the reliable sample size of substantial detection periods, but also made it difficult to interpret the cause of a tag falling silent, unless it or the shark were detected again upon the subsequent return of the individual. At least three tags were confirmed to have become detached, as indicated by severed tethers. Surgical implantation is being considered to address this in future studies. An associated complication was the likelihood that the results were strongly skewed towards patterns displayed by Shark 2, owing to its disproportionately large detection record.
A deeper issue relates to the unknown extent to which the observed shark population’s behaviour was altered by the long-term baiting regime. Thus, behaviour of these sharks may not be representative of normal silky shark behaviour.
Wider implications
The regular use of reefs in this area of the Red Sea by a reproductively valuable subpopulation of silky sharks is evident, despite the potential influences of a long-term baiting regime. This highlights the importance of this region for recruitment into the local silky shark population, and management strategies to limit existing exploitation of sharks in the area need to be developed. Local exploitation of sharks presently persists unchecked, despite a recent governmental decree prohibiting targeting of any sharks. Given the study area represents only a subset of the effective population, as males were rarely seen, effective management will require consideration of all life stages, and so most likely regulation over a larger marine area. Genetic studies will help determine the scale of management required by revealing how reproductively isolated local silky sharks are from silky sharks in other regions. A wider implication of the comparatively confined habitat use displayed at least by some individuals during the present study is that this population of the epipelagic shark species may be more isolated than originally anticipated. This could have major management implications if, for example, the Red Sea and Indian Ocean populations were found to be reproductively isolated. How shark species interact with bait is also an issue of increasing importance to management, given the expanding tourism industry of baited shark diving, which is increasingly presented as an alternative to fishing and as providing a mortality-free revenue source from sharks.
Acknowledgements
We thank the Founder and Board of the Save Our Seas Foundation for funding and providing all facilities for this work. We also especially thank all the divers, ships’ crews and engineers at the Foundation’s Marine Facility for their assistance during fieldwork and preparation. In addition, we thank the Guest Editor and referees for their comments and advice when preparing this manuscript for publication.
References
Bass, A. J., D’Aubrey, J. D., and Kistnasamy, N. (1973). Sharks of the east coast of southern Africa. I. The genus Carcharhinus (Carcharhinidae). Oceanographic Research Institute, Durban. Investigational Report 33, 29–32.Baum, J. K., and Myers, R. A. (2004). Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecology Letters 7, 135–145.
| Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Beerkircher, L., and Shivji, M. (2003). A Monte Carlo demographic analysis of the silky shark (Carcharhinus falciformis): implications of gear selectivity. Fishery Bulletin 101, 169–174.
Bonfil, R., Mena, R., and de Anda, D. (1993). Biological parameters of commercially exploited silky sharks, Carcharhinus falciformis, from the Campeche Bank, Mexico. NOAA Technical Report NMFS 115, 73–86.
Bonfil, R., Amorim, A., Anderson, C., Arauz, R., Baum, J., et al. (2007). The IUCN Red List of Threatened Species: Carcharhinus falciformis. Available at http://www.iucnredlist.org/apps/redlist/details/39370/0 [Verified 14 March 2011].
Branstetter, S. (1987). Age, growth and reproductive biology of the silky shark, Carcharhinus falciformis, and the scalloped hammerhead, Sphyrna lewini, from the northwestern Gulf of Mexico. Environmental Biology of Fishes 19, 161–173.
| Age, growth and reproductive biology of the silky shark, Carcharhinus falciformis, and the scalloped hammerhead, Sphyrna lewini, from the northwestern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Bres, M. (1993). The behaviour of sharks. Reviews in Fish Biology and Fisheries 3, 133–159.
| The behaviour of sharks.Crossref | GoogleScholarGoogle Scholar |
Burgess, G. H., Beerkircher, L. R., Cailliet, G. M., Carlson, J. K., Cortés, E., et al. (2005). Is the collapse of shark populations in the Northwest Atlantic Ocean and Gulf of Mexico real? Fisheries 30, 19–26.
| Is the collapse of shark populations in the Northwest Atlantic Ocean and Gulf of Mexico real?Crossref | GoogleScholarGoogle Scholar |
Chateau, O., and Wantiez, L. (2008). Human impacts on residency behaviour of spangled emperor, Lethrinus nebulosus, in a marine protected area, as determined by acoustic telemetry. Journal of the Marine Biological Association of the United Kingdom 88, 825–829.
| Human impacts on residency behaviour of spangled emperor, Lethrinus nebulosus, in a marine protected area, as determined by acoustic telemetry.Crossref | GoogleScholarGoogle Scholar |
Clarke, S. C., McAllister, M. K., Milner-Gulland, E. J., Kirkwood, G. P., Michielsens, C. G. J., et al. (2006). Global estimates of shark catches using trade records from commercial markets. Ecology Letters 9, 1115–1126.
| Global estimates of shark catches using trade records from commercial markets.Crossref | GoogleScholarGoogle Scholar | 16972875PubMed |
Compagno, L. J. V. (1984). FAO species catalogue. Vol. 4. Sharks of the World. An annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. FAO Fisheries Synopsis 4, 470–472.
Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Checklist (2010). Available at http://www.cites.org/eng/resources/pub/checklist08/Checklist.pdf [Verified 14 March 2011].
Dewar, H., Domeier, M., and Nasby-Lucas, N. (2004). Insights into young of the year white shark, Carcharodon carcharias, behavior in the Southern California Bight. Environmental Biology of Fishes 70, 133–143.
Domeier, M. L., Kiefer, D., Nasby-Lucas, N., Wagschal, A., and O’Brien, F. (2005). Tracking Pacific blue fin tuna (Thunnus thynnus orientalis) in the northeastern Pacific with an automated algorithm that estimates latitude by matching sea-surface-temperature data from satellites with temperature data from tags on fish. Fishery Bulletin 103, 292–306.
Egli, D. P., and Babcock, R. C. (2004). Ultrasonic tracking reveals multiple behavioural modes of snapper (Pagrus auratus) in a temperate no-take marine reserve. ICES Journal of Marine Science 61, 1137–1143.
| Ultrasonic tracking reveals multiple behavioural modes of snapper (Pagrus auratus) in a temperate no-take marine reserve.Crossref | GoogleScholarGoogle Scholar |
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., and Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecology Letters 13, 1055–1071.
| 20528897PubMed |
Hazin, F. H., Oliveira, P. G. V., and Macena, B. C. L. (2007). Aspects of the reproductive biology of the silky shark, Carcharhinus falciformis (Nardo, 1827), in the vicinity of Archipelago of Saint Peter and Saint Paul, in the equatorial Atlantic Ocean. Collective Volume of Scientific Papers: ICCAT 60, 648–651.
Heupel, M. R., Simpfendorfer, C. A., and Fitzpatrick, R. (2010). Large-scale movement and reef fidelity of grey reef sharks. Public Library of Science ONE 5, e9650..
| 20224793PubMed |
Kato, S., and Carvallo, A. H. (1967). Shark tagging in the eastern Pacific Ocean, 1962–1965. In ‘Sharks, Skates, and Rays’. (Eds P. W. Gilbert, R. F. Matheson and D. P. Rall.) pp. 93–109. (Johns Hopkins Press: Baltimore.)
Kohler, N. E., Casey, J. G., and Turner, P. A. (1998). NMFS cooperative shark tagging program, 1962–93: an atlas of shark tag and recapture data. Marine Fisheries Review 60, 1–87.
Laroche, R. K., Kock, A. A., Dill, L. M., and Oosthuizen, W. H. (2007). Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias. Marine Ecology Progress Series 338, 199–209.
| Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias.Crossref | GoogleScholarGoogle Scholar |
Lisney, T. J., and Collin, S. P. (2008). Retinal ganglion cell distribution and spatial resolving power in elasmobranchs. Brain, Behavior and Evolution 72, 59–77.
| Retinal ganglion cell distribution and spatial resolving power in elasmobranchs.Crossref | GoogleScholarGoogle Scholar | 18679025PubMed |
Lowe, C. G., Wetherbee, B. M., and Meyer, C. G. (2006). Using acoustic telemetry monitoring techniques to quantify movement patterns and site fidelity of sharks and giant trevally around French Frigate Shoals and Midway Atoll. Atoll Research Bulletin 543, 281–303.
Lucifora, L. O., Menni, R. C., and Escalante, A. H. (2002). Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. ICES Journal of Marine Science 59, 553–561.
| Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic.Crossref | GoogleScholarGoogle Scholar |
Maljković, A., and Côté, I. M. (2010). Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark. Biological Conservation , .
| Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark.Crossref | GoogleScholarGoogle Scholar | 21113436PubMed |
Myers, R. A., and Worm, B. (2005). Extinction, survival and recovery of large predatory fishes. Philosophical Transactions of the Royal Society of London, Series B. Biological Sciences 360, 13–20.
| Extinction, survival and recovery of large predatory fishes.Crossref | GoogleScholarGoogle Scholar |
Nelson, M. (1995). ‘Swim with the Rays: a Guide to Stingray City, Grand Cayman.’ (Blueline Press: Boulder, CO, USA.)
Okey, T. A., Banks, S., Born, A. F., Bustamante, R. H., Calvopiña, M., et al. (2004). A trophic model of Galápagos subtidal rocky reef for evaluating fisheries and conservation strategies. Ecological Modelling 172, 383–401.
| A trophic model of Galápagos subtidal rocky reef for evaluating fisheries and conservation strategies.Crossref | GoogleScholarGoogle Scholar |
Robbins, W. D., Hisano, M., Connolly, S. R., and Choat, J. H. (2006). Ongoing collapse of coral-reef shark populations. Current Biology 16, 2314–2319.
| Ongoing collapse of coral-reef shark populations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1OhtL3J&md5=ea6e4e5c0751a17505dd47dbe6447cd9CAS | 17141612PubMed |
Rowat, D., Meekan, M. G., Engelhardt, U., Pardigon, B., and Vely, M. (2007). Aggregations of juvenile whale sharks (Rhincodon typus) in the Gulf of Tadjoura, Djibouti. Environmental Biology of Fishes 80, 465–472.
| Aggregations of juvenile whale sharks (Rhincodon typus) in the Gulf of Tadjoura, Djibouti.Crossref | GoogleScholarGoogle Scholar |
Sims, D. W., Southall, E. J., Tarling, G. A., and Metcalfe, J. D. (2005). Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. Journal of Animal Ecology 74, 755–761.
| Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark.Crossref | GoogleScholarGoogle Scholar |
Sims, D. W., Wearmouth, V. J., Southall, E. J., Hill, J. M., Moore, P., et al. (2006). Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. Journal of Animal Ecology 75, 176–190.
| Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark.Crossref | GoogleScholarGoogle Scholar | 16903055PubMed |
Stevens, J. D. (1984). Life-history and ecology of sharks at Aldabra Atoll, Indian Ocean. Proceedings of the Royal Society of London. Series B. Biological Sciences 222, 79–106.
| Life-history and ecology of sharks at Aldabra Atoll, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Stevens, J. D., and McLoughlin, K. J. (1991). Distribution, size and sex composition, reproductive biology and diet of sharks from northern Australia. Australian Journal of Marine and Freshwater Research 42, 151–199.
| Distribution, size and sex composition, reproductive biology and diet of sharks from northern Australia.Crossref | GoogleScholarGoogle Scholar |
Tricas, T. C., Deacon, K., Last, P., McCosker, J. E., Walker, T. I., et al. (1997). In ‘Sharks & Rays’. p. 172. (Harper Collins Publishers: London.)
Watson, J. T., Essington, T. E., Lennert-Cody, C. E., and Hall, M. A. (2009). Trade-offs in the design of fishery closures: management of silky shark bycatch in the eastern Pacific Ocean tuna fishery. Conservation Biology 23, 626–635.
| Trade-offs in the design of fishery closures: management of silky shark bycatch in the eastern Pacific Ocean tuna fishery.Crossref | GoogleScholarGoogle Scholar |
Weng, K. C., and Block, B. A. (2004). Diel vertical migration of the bigeye thresher shark (Alopias superciliosus), a species possessing orbital retia mirabilia. Fishery Bulletin 102, 221–229.





