Climate change impedes scleractinian corals as primary reef ecosystem engineers
Christian Wild A L , Ove Hoegh-Guldberg B , Malik S. Naumann C , M. Florencia Colombo-Pallotta D , Mebrahtu Ateweberhan E F , William K. Fitt G , Roberto Iglesias-Prieto D , Caroline Palmer H I , John C. Bythell H , Juan-Carlos Ortiz B , Yossi Loya J and Robert van Woesik KA Coral Reef Ecology Group (CORE), Leibniz Center for Tropical Marine Ecology (ZMT) and University of Bremen, Fahrenheitstr. 6, 28359 Bremen, Germany.
B Global Change Institute, University of Queensland, Brisbane, Qld 4072, Australia.
C Centre Scientifique de Monaco, Avenue Saint-Martin, 98000 Monaco, Principality of Monaco.
D Reef Systems Unit, Instituto de Ciencias del Mar y Limnología, UNAM, Puerto Morelos, México.
E Coral Reef Conservation Project, Mombasa, Kenya.
F Department of Biological Sciences, University of Warwick, CV4 7AL, Coventry, United Kingdom.
G Odum School of Ecology, University of Georgia, Athens, GA 30602, USA.
H School of Biology, Newcastle University, Newcastle Upon Tyne, United Kingdom.
I ARC Centre of Excellence for Coral Reef Studies and School of Marine and Tropical Biology, James Cook University, Townsville, Qld 4811, Australia.
J Zoology Department, Tel Aviv University, Tel Aviv, Israel.
K Department of Biological Sciences, Florida Institute of Technology, Melbourne, FL 32901, USA.
L Corresponding author. Email: christian.wild@zmt-bremen.de
Marine and Freshwater Research 62(2) 205-215 https://doi.org/10.1071/MF10254
Submitted: 13 October 2010 Accepted: 14 January 2011 Published: 24 February 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Coral reefs are among the most diverse and productive ecosystems on our planet. Scleractinian corals function as the primary reef ecosystem engineers, constructing the framework that serves as a habitat for all other coral reef-associated organisms. However, the coral’s engineering role is particularly susceptible to global climate change. Ocean warming can cause extensive mass coral bleaching, which triggers dysfunction of major engineering processes. Sub-lethal bleaching results in the reduction of both primary productivity and coral calcification. This may lead to changes in the release of organic and inorganic products, thereby altering critical biogeochemical and recycling processes in reef ecosystems. Thermal stress-induced bleaching and subsequent coral mortality, along with ocean acidification, further lead to long-term shifts in benthic community structure, changes in topographic reef complexity, and the modification of reef functioning. Such shifts may cause negative feedback loops and further modification of coral-derived inorganic and organic products. This review emphasises the critical role of scleractinian corals as reef ecosystem engineers and highlights the control of corals over key reef ecosystem goods and services, including high biodiversity, coastal protection, fishing, and tourism. Thus, climate change by impeding coral ecosystem engineers will impair the ecosystem functioning of entire reefs.
Additional keywords: bleaching, ecosystem goods and services, ocean warming and acidification.
Scleractinian corals as reef ecosystem engineers
Ecosystem engineers are organisms that modulate the availability of resources to other species by causing physical state changes in biotic or abiotic materials (Jones et al. 1994, 1997). Scleractinian corals act as key reef ecosystem engineers in two main ways: first, they are autogenic engineers, because through their calcification and ensuing reef accretion, they change the physical, chemical, and biological environment and thereby provide habitats for associated reef organisms. The generation of complex, hard, and stable substrates by scleractinian corals underpins the high biodiversity characteristic of tropical coral-reef ecosystems (Bellwood and Hughes 2001).
Second, corals also act as allogenic ecosystem engineers because they intensively generate and transform inorganic and organic materials. Coral-derived inorganic calcareous skeletons are transformed into calcareous reef sands by reef-associated bio-eroding organisms (such as molluscs, echinoderms, and sponges) and by other biological, physical, and chemical erosion processes (Glynn 1997; Hallock 1997), so that corals, through the production of inorganic materials, control adjacent sediments. These highly permeable biogenic sediments with generally large grain sizes allow intense advective coupling between the water column and the seafloor (Huettel et al. 2003; Rasheed et al. 2003; Wild et al. 2005a). Calcareous reef sands support abundant associated heterotrophic microbes (Wild et al. 2006). Coral-generated calcareous sediments thereby act as biocatalytic filter systems that facilitate rapid processing and recycling of organic matter (Wild et al. 2004a, 2005a, 2005b, 2008). Corals also continuously release large amounts of dissolved and particulate organic materials, which may function as energy carriers and particle traps (Wild et al. 2004a; Huettel et al. 2006; Naumann et al. 2009). This release of organic material promotes the formation of mucus–particle aggregations in the water column that increase the sedimentation and recycling rates by which essential elements are retained (Wild et al. 2004b, 2005b; Huettel et al. 2006). Consequently, a wide range of biogeochemical processes, important in coral reef functioning, are directly controlled by scleractinian corals acting as the principal ecosystem engineers.
This review sets out to expand our understanding of the impacts of global climate change on coral reef ecosystems owing to the direct impediment of the reef’s primary ecosystem engineer – the scleractinian corals. Rather than focusing on the plight of corals per se, we chose to explore how the impacts of ocean warming and acidification may affect the corals’ ability to act as engineers within this complex ecosystem. We discuss the impact of climate change on scleractinian corals at the organism level and how this translates into responses at the reef ecosystem level.
The impact of global climate change on the coral engineer
As stenothermic and calcifying organisms, corals are particularly sensitive to both ocean acidification and warming. There are several indications that carbon pollution-induced increases in ocean acidity and temperature are impacting the metabolism and growth of reef-building corals (Langdon and Atkinson 2005; De’ath et al. 2009; Tanzil et al. 2009; Manzello 2010).
Ocean acidification and coral calcification
Increasing acidity of oceanic waters represents a direct threat to reef-building scleractinian corals, with various implications for their role as ecosystem engineers. Ocean acidification is the consequence of global oceanic uptake of increasing anthropogenic atmospheric CO2 (e.g. Kleypas et al. 1999). Such uptake increases CO2 partial pressure (pCO2) in the water column, decreases seawater pH, increases concentrations of total dissolved CO2 ([CO2] and [HCO3]), and reduces concentrations of [CO32–] in seawater (Caldeira and Wickett 2003; Feely et al. 2004). Physiological processes (e.g. calcification) in corals may respond to these changes in ocean chemistry (Langdon and Atkinson 2005).
The reduction in [CO32–], at constant seawater calcium concentration [Ca2+], consequently results in the decrease of the saturation state of aragonite (Ωarag), the polymorph of CaCO3 produced by coral calcification. Presently, tropical surface waters, with the exception of the eastern Pacific Ocean, are about 4-fold supersaturated with respect to aragonite (Hoegh-Guldberg et al. 2007). However, Ωarag is expected to significantly decrease to levels of 2.5–3.0 by the year 2100 (Feely et al. 2009). Scleractinian corals generally require seawater that is super-saturated in aragonite for efficient aragonite accretion. In acidified seawater, lowered external Ωarag impedes the essential increase of Ωarag within the internal calcifying fluid, and causes a corresponding decrease in calcification rate (reviewed in Cohen and Holcomb 2009). This decrease in skeletal growth performance, caused by ocean acidification, directly translates to a decline in the engineering capacity of scleractinian corals to construct essential reef habitats.
Various studies have documented the negative effect of ocean acidification and the consequential reduction in seawater Ωarag on coral calcification in both the laboratory (e.g. Anthony et al. 2008; Jokiel et al. 2008) and the field (Bak et al. 2009; De’ath et al. 2009; Tanzil et al. 2009). However, a very recent study (Jury et al., in press) presents significant differences in calcification rates at equal [CO32–] and further suggests [HCO3–] as a potentially more important driver for coral calcification, thereby questioning the reliability of Ωarag or [CO32–] as sole predictors of the effect of ocean acidification on coral calcification. Another recent study argues that deleterious effects caused by elevated [CO2], as a result of ocean acidification, may be ameliorated by inorganic nutrient enrichment (Holcomb et al. 2010). These authors conclude that naturally elevated inorganic nutrient levels may thus support increased primary and secondary production, consequently facilitating coral calcification in environmenst with naturally high concentrations of CO2. However, species-specific differences in sensitivity to ocean acidification and thermal stress may occur (Manzello 2010). This could have tremendous effects on the structure of communities in future coral reefs (Loya et al. 2001).
Ocean acidification can also affect coral reproduction by reducing sperm motility (Morita et al. 2009) or settlement and post-settlement development of planula larvae and coral recruits (Albright et al. 2008; Cohen et al. 2009). At experimentally reduced aragonite saturation states (Ωarag), the early skeleton of coral recruits showed progressive changes in aragonite crystal morphology and a decline in crystal growth rate (Cohen et al. 2009). This implies that ocean acidification may significantly affect recruitment rates and the competitive capacity of coral populations, and may consequently lead to a shift in coral community structure. In addition, a recent study showed that spawning female corals of the temperate species Astrangia poculata are more susceptible to the negative effects of ocean acidification than spawning male corals (Holcomb et al., in press). This gender discrimination may be a result of the energetically expensive egg production process, leaving only limited resources to compensate for the effects of acidification on calcification. On a longer time-scale, this lack of energy and growth may reduce recruitment success for gonochoric-spawning coral species.
Finally, and possibly most alarmingly, ocean acidification has been identified as a potential trigger for coral bleaching (Anthony et al. 2008). According to this study, branching and massive coral species experience an increase in bleaching with decreasing seawater pH (8.4–7.6) at low (25–26°C) and high (28–29°C) seasonal temperatures. These findings suggest that ocean acidification may be another primary bleaching-trigger, causing similar and/or other deleterious consequences for scleractinian corals as reef ecosystem engineers.
Ocean warming and coral bleaching
Reef-building corals form a primary habitat for zooxanthellae (endosymbiotic dinoflagellates) of the genus Symbiodinium, which are crucial to the physiological integrity and ecological function of reef-building corals within tropical-reef systems. Coral–zooxanthellae interactions reflect a close evolutionary relationship between host and symbiont, with temporal and spatial variability allowing the coexistence of diverse symbionts within the coral host (Rowan et al. 1997). In the eastern Pacific Ocean, Pocillopora verrucosa colonies inhabiting shallow-water habitats contained different clades of zooxanthellae than Pavona gigantea colonies living in deeper water and more shaded habitats (Iglesias-Prieto et al. 2004). Similarly, Symbiodinium spp., within Acropora tenuis appear to be distributed according to light exposure, with two different types of Symbiodinium spp. found in sunny and shady parts of the colony, respectively (Van Oppen et al. 2001). Such tight interaction leads to the conclusion that the two organisms cannot be considered separately (Iglesias-Prieto et al. 2004).
One of the most dramatic impacts of ocean warming on coral reefs is mass coral bleaching, which is the breakdown of the symbiosis between corals and zooxanthellae. Bleaching is associated with a pronounced loss of colour from affected corals caused by the expulsion of the photopigment-rich zooxanthellae (Fig. 1). Mass coral bleaching is strongly associated with anomalously high sea-surface temperatures (Glynn 1993), but it can also be triggered by a range of other environmental stress factors (e.g. altered salinity, light, sedimentation, and toxins). Since the early 1980s, mass coral bleaching events have increased in extent and frequency in response to steadily warming ocean-water temperatures (Hoegh-Guldberg 1999).
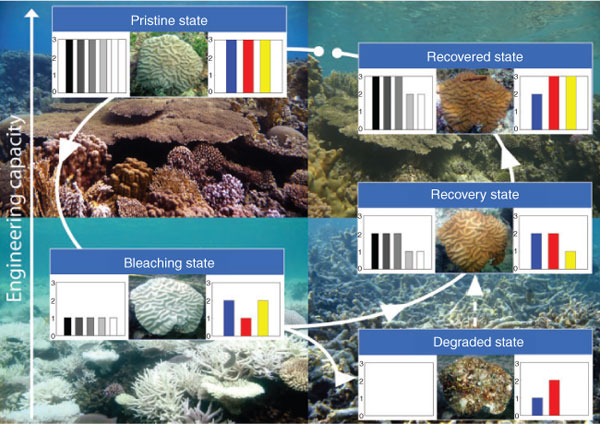
|
Coral bleaching is a reversible process (Fig. 1) if the stressful conditions are relatively mild and short-term, with zooxanthellae populations inside the host tissues returning to pre-bleaching levels after 2–6 months depending on the coral species (Hoegh-Guldberg and Smith 1989; Brown 1997; Hoegh-Guldberg and Jones 1999). Bleached corals, however, are physiologically compromised because of the reduction in the abundance of zooxanthellae and/or their pigments. Under normal conditions, zooxanthellae translocate up to 95% of their photosynthetically fixed carbon to the coral host (Muscatine et al. 1984). They also cover 30% of the host’s nitrogen requirements for growth, reproduction and maintenance (Bythell 1988) from dissolved nutrient uptake (Bythell 1990). During bleaching, the photosynthetic ability of reef-building corals is greatly reduced (Porter et al. 1989; Suzuki and Kawahata 2003; Rodrigues and Grottoli 2006), leading to the depletion of energy stores, which results in ‘food shortage’ and starvation (Porter et al. 1989; Fitt et al. 1993).
As calcification strongly depends on the photosynthetic efficiency of the zooxanthellae within the tissues of corals (Gattuso et al. 1999; Allemand et al. 2004; Colombo-Pallotta et al. 2010), bleached and/or thermally stressed corals have lower growth rates (Jokiel and Coles 1977; Leder et al. 1991) and tissue regeneration capacities (Meesters and Bak 1993). In Montastraea faveolata, one of the major reef-builders of the Caribbean, bleaching reduced photosynthetic efficiency by 4.6-fold and capacity by up to 4.8-fold (Rodríguez-Román et al. 2006). These changes in photosynthetic activity translate directly into a decline in calcification. Calcification rates of bleached M. annularis colonies were only 37% of the mean annual calcification observed before bleaching (Leder et al. 1991). Laboratory experiments revealed that calcification rates in Montastraea faveolata, immediately after bleaching, can be as low as 22% of unbleached control corals (F. Colombo-Pallotta, pers. comm.).
The physiological consequences of bleaching and the time required to return to normal conditions is species-specific. Many branching corals of the genus Acropora die within 10 to 14 days after bleaching, whereas some massive species may show greater resistance and recover after several months (Rodrigues and Grottoli 2006; Rodrigues et al. 2008). Early life stages of corals seem to be particularly vulnerable to climate change stressors (Edmunds 2007), thus affecting the demographics of coral reefs. Bleaching may impact the reproductive output and planula larvae development of corals (Baird and Marshall 2002) for up to several years (Ward et al. 2002). The observed reduction in reproductive output of scleractinian corals that survived bleaching indicates that recovery to former levels is slow (Baird and Marshall 2002), suggesting that sub-lethal bleaching leads to long-term effects on corals and associated ecosystem functioning. Re-establishment of reef corals after major disturbance events with mass coral mortality usually takes years to decades (e.g. Loya 1976, 2004; Done 1992; Loch et al. 2004).
The effect of light
For corals under thermal stress, ambient light conditions can significantly affect the onset and severity of bleaching events. Low or reduced irradiance during times of high water temperature reduces photoinhibition and suppresses coral bleaching (Iglesias-Prieto et al. 1992; Mumby et al. 2001; Brown et al. 2002; Takahashi et al. 2004). By contrast, reductions in cloudiness exacerbate thermal stress. Nonetheless, the response of clouds to climate change remains one of the largest sources of uncertainty in modelling climate at regional and local scales. Some studies show that warming oceans could reduce upper-level cloudiness, particularly in the tropics, because the higher temperatures increase turnover efficiency (Mumby et al. 2001; Lau and Wu 2003; Lau et al. 2005). A cause for concern is the modelled simulations of doubled atmospheric CO2 that predict reductions in total cloudiness, leading to greater absorption of shortwave and long-wave radiation by the oceans (Sud et al. 2008).
Coral immunity and diseases
Several studies have correlated climate change, in general (Harvell et al. 2001), or temperature anomalies, in particular (Willis et al. 2004; Miller et al. 2006; Bruno et al. 2007; Muller et al. 2008), to the increased prevalence of coral disease, and disease incidence has been shown to increase following coral bleaching (Brandt and McManus 2009). Invertebrate immunity can be reduced under starvation conditions (Feder et al. 1997; Moret and Schmid-Hempel 2000; Seppälä et al. 2008) similar to those induced during coral bleaching. Compromised coral immunity during and following a bleaching event (Fig. 1) (Mydlarz et al. 2009; Reed et al. 2010) will therefore limit the activation and efficacy of a response to invading organisms or physical injury (Meszaros and Bigger 1999; Rinkevich 1999; Stedman 2000), leading to an increase in disease prevalence following coral bleaching (Harvell et al. 2001; Miller et al. 2006).
There is also growing consensus that microbes interact strongly with corals, forming an integral part of a symbiosis that includes coral, zooxanthellae, and associated microflora. This has been referred to as the ‘holobiont’ to connote a biological ‘unit’ that operates as one, within both ecological and evolutionary time frames (Rohwer et al. 2002). The concept of the coral holobiont emerged following several research advances using culture-based and culture-independent molecular techniques. Coral-associated bacterial communities show greater similarity between distantly-separated colonies of the same host species than between adjacent colonies of different host species (Rohwer et al. 2002), implying that bacterial communities are host-coral specific. Healthy and diseased corals show distinct differences in microbial community composition (Cooney et al. 2002; Frias-Lopez et al. 2002, 2004; Ritchie 2006), including the apparently healthy tissues remote from the disease lesion of infected corals (Pantos et al. 2003; Pantos and Bythell 2006). This indicates that in a changing climate, there will be pronounced changes in community composition and diversity of microbes associated with corals.
Such microbial associates may perform several different functions, including that of N-fixation (Williams et al. 1987; Shashar et al. 1994; Lesser et al. 2004), and as an antibiotic agent in the case of bacteria associated with coral mucus (Ritchie 2006). The experimental evidence on selective properties of antibacterial substances in the mucus of the coral Acropora palmata (Ritchie 2006) indicates a probiotic role of bacteria in corals (Knowlton and Rohwer 2003; Pantos et al. 2003; Reshef et al. 2006; Rypien et al. 2010), whereby the normal microbial flora inhibits the colonisation and growth of potential pathogens via competition and release of antimicrobial compounds (Ritchie 2006; Rypien et al. 2010).
Reef ecosystem-level responses
Habitat provisioning by corals and effects on associated organisms
Climate change has the capacity to change reef landscapes and benthic communities. Coral-generated production of framework structures will substantially decrease with climate change-induced bleaching and subsequent reduction of coral calcification. Such declines will influence associated organisms and significantly alter the reef’s biogeochemical functioning (Fig. 1).
On a larger scale, impeding coral ecosystem engineers may cause phase shifts from scleractinian corals to other invertebrates such as gorgonians, soft corals, ascidians and sponges (Bak et al. 1996; Maliao et al. 2008) or even benthic algae (Hughes 1994; McCook 1999; Diaz-Pulido and McCook 2002; Bellwood et al. 2006) with obvious negative effects on provisioning of three-dimensional surfaces and habitats for associated high biodiversity. Climate change will contribute to such ‘phase shifts’ and potentially to long-term destruction of reef framework and changes in reef functioning (Glynn 1993; Aronson and Precht 1997; Diaz-Pulido and McCook 2002; Aronson et al. 2004). Dead corals are rapidly colonised by a diverse community of algae (Diaz-Pulido and McCook 2002), and living corals are directly overgrown or damaged by macroalgae (Smith et al. 2006; Haas et al. 2010). Algal colonisation of surfaces of dead or impaired corals therefore likely influences the recovery of neighbouring corals from climate change-induced stress such as bleaching of the coral engineer. This may also include dramatic changes of biogeochemical processes such as the fixation of nitrogen via coral-associated cyanobacteria. On a reef-wide scale, within weeks of a major bleaching event and mass coral mortality, the production of new nitrogen can be 30-fold greater than that associated with living corals under undisturbed conditions (Davey et al. 2008). Thermally-induced mortality of the coral engineer therefore has the potential to significantly alter the reef systems’ overall dynamics and integrity. In addition, the loss of live tissue will affect natural recycling of nutrients within the system.
On a smaller scale, this involves changes in the scleractinian coral community. Branching, framework-building scleractinian corals including the genera Acropora, Seriatopora, Pocillopora, and Stylopora are examples of morphologies that are more sensitive to thermal stress than massive and encrusting growth forms (Marshall and Baird 2000; Loya et al. 2001; McClanahan et al. 2002). In addition, large colonies of these genera were more susceptible to thermal stress than small colonies (Loya et al. 2001; Mumby et al. 2001; Nakamura and van Woesik 2001; Bena and van Woesik 2004). Therefore, climate change will reduce the framework-building and habitat-generation capacity of reef-coral communities.
Calcifying activities of reef-building corals ultimately result in a three-dimensional matrix that provides space, shelter, and food for a multitude of organisms (Srinivasan 2003; Sale et al. 2005; Raes et al. 2007). High reef rugosity, for example where large massive Porites coral colonies dominate the seascape, is associated with high abundances of reef fishes (McClanahan and Shafir 1990). Similarly, a high abundance of micro-structures within coral colonies is strongly and positively correlated with high fish and invertebrate diversity (Nanami and Nishihira 2004; Nanami et al. 2005). Reduction in rugosity reduces the availability of habitat space at a variety of scales and leads to a considerable reduction in reef biodiversity (McClanahan and Shafir 1990). In the Caribbean, the architectural complexity of reefs has already declined non-linearly over the last 40 years (Alvarez-Filip et al. 2009) as a combined result of climate-change effects and local disturbances.
The fate of reef corals and their community composition is not only fundamental for future reefs to maintain their ability to accrete and form coastal barriers, but also in determining whether or not they will continue to support the plethora of coral-associated diversity through time. Loss of corals and shifts in species composition reduces the variety of habitats available at macro- and micro-scales, which likely leads to subsequent losses of habitat complexity and associated biodiversity of invertebrates and fish (Reaka-Kudla 1997; Loya et al. 2001; Wilson et al. 2006). This includes obligate associates such as corallivorous fishes in the short term, but also subsequent reductions in overall fish diversity 3–4 years following a thermal stress event (Pratchett 2007; Munday et al. 2008).
Control of biocatalytical sand filters
The commonly observed phase-shifts from the dominance of corals to that of fleshy algae will additionally decrease the production of inorganic material in the long term, but will increase sediment-forming erosion processes in the short term, because of the breakdown of the reef framework (Fig. 1). The extent of this reef degradation is obviously dependent on the functional group of algae involved in phase shift. In areas dominated by crustose coralline algae, fragmentation of the reef framework can be mitigated by cementation and binding of sediments and loose coral fragments (Kennedy et al. 2002; Payri 1988). Some coralline algae species can even induce coral larval settlement, thereby facilitating new recruitment and reef recovery (Fabricius and De’ath 2001; Harrington et al. 2004; Golbuu and Richmond 2007). This beneficial effect may, however, depend on the extent of climate change, as crustose coralline algae are particularly sensitive to ocean warming and acidification (Anthony et al. 2008). Calcareous green and articulated corallines contribute less to sediment binding, although their sediment production, in particular by calcareous green algae (e.g. Halimeda spp.), can be high (Bach 1979; Drew 1983; Payri 1988).
Carbonate production by Halimeda spp. is likely quantitatively lower than that by scleractinian corals (Bosence 1989). In addition, sand and coralline sediments derived from Halimeda spp. are different in density and chemistry from coral-derived sediments (Borowitzka and Larkum 1977; Braga et al. 1996; Bosence and Wilson 2003). Therefore, these algae-generated sediments will very likely have shorter residence times, and therefore will most likely not function as biocatalytical filter systems as described for coral-generated sediments (Wild et al. 2005a, 2009; Werner et al. 2006).
Generation and fate of coral-derived organic material
Under undisturbed conditions, scleractinian corals release particulate organic carbon (POC) in quantities of 0.3–7.1 mg POC m–2 coral surface h–1 (Wild et al. 2004a, 2005b; Naumann et al. 2010). To date, there are no available studies on the effect of acidification on coral-derived organic matter release. However, during thermal-induced bleaching, two different kinds of organic matter are released in increased quantities: (1) zooxanthellae, and (2) coral-derived particulate organic matter (POM) (Niggl et al. 2009). Degradation of these substrates by reef microbes differs considerably, with very low rates observed for symbiotic dinoflagellates (<1% h–1), and high rates for coral-derived POM (>5% h–1) (Wild et al. 2005a). This could indicate that a major fraction of the symbiotic dinoflagellate-composed POM does not remain in the reef system long enough to be recycled, and thus likely involves a considerable loss of essential nutrients. In contrast, the coral-derived POM may function as an energy carrier and particle trap (Wild et al. 2004a; Huettel et al. 2006). This material potentially stays within the system and is recycled, particularly by the benthic community. A short-term pulse of labile organic matter can result, and studies have demonstrated that this organic matter can be recycled by the planktonic and benthic reef community within two weeks (Wild et al. 2004c, 2008; Eyre et al. 2008; Glud et al. 2008).
Histological analyses (Fitt et al. 2009), however, indicate that internal mucus reservoirs in the coral tissue are depleted during bleaching, so that mucus–POM release by corals is most likely only stimulated during the early phase of bleaching, dropping to low levels the longer the bleaching event lasts (Fig. 1). This dynamic flux of POM was confirmed by Piggot et al. (2009), who demonstrated that the number of mucus-producing cells (mucocytes) in the coral tissue increases with increasing sea-surface temperatures, but declines to low levels after the initial bleaching response.
Coral bleaching thereby largely reduces the metabolic exchange between corals and associated organisms, as well as reducing the capacity of corals to trap organic matter. This may lead to further loss of POM from the reef system. Therefore, not only are corals affected by thermal stress, but the entire reef system changes and functions differently during and after a bleaching event, particularly when bleaching results in extensive coral mortality. Corals can recover from bleaching, which allows for the return of the described reef engineering functions through production of organic matter by the coral engineer (Fig. 1). The respective recovery time scales range from weeks to months (Gates 1990; Jokiel and Coles 1990), so that short- to mid-term effects on biogeochemical processes can be expected from a brief impediment of the coral engineer. However, these effects may include long-term changes in the reef’s recycling capacity.
Value of coral engineer-driven reef ecosystem goods and services
Reef-building corals act as ecosystem engineers because they provide the physical structure as well as biotic and biogeochemical services to reefs (Moberg and Folke 1999). Such services are functionally similar to other ecosystem engineers such as rainforest trees, mangroves or seagrasses (Guitierrez et al., in press), but usually much more persistent because of the calcareous material of the coral skeleton. However, corals will most likely be the ecosystem engineers most affected by climate change because they are comparatively slow-growing, stenothermic and calcifying organisms, and therefore particularly susceptible to ocean warming and acidification. Global climate change, especially ocean warming that includes coral bleaching, impedes the engineering capacity of scleractinian corals (Fig. 1). This also includes the loss of reef ecosystem services for coastal protection, tourism, and productivity.
Protection of coastal areas and their high associated biodiversity strongly depend on the production of inorganic material by scleractinian corals. These reef structures can absorb 70–90% of wind-generated wave energy (Wells et al. 2006) and differentiate habitats and physical environments that influence biological diversity at all scales. The attraction of coral reefs for tourism is probably a reflection of such coral-driven biodiversity.
The total annual economic value of coral reefs via ecosystem services has been estimated at least USD100 000–600 000 km–2 (Wells et al. 2006). The impediment of corals as reef engineers will drastically reduce these economic values, even without consideration of the ecological values (e.g. high biodiversity) if reefs are further degraded under climate change.
Coral engineer-controlled reef biodiversity also has an increasing economic importance for the delivery of new biologically active molecules (e.g. Fung et al. 1997; Fung and Ding 1998) that are being developed as pharmaceuticals. Reef productivity and ensuing fishery biomass largely depend on the production of scleractinian corals, but also on their reef-engineering functions including the initiation of biogeochemical processes, leading to rapid recycling, and conservation of essential elements, such as nitrogen and phosphorus.
Conclusions
This review indicates that anthropogenic climate change has the potential to drive major changes across the entire reef ecosystem and indeed is already doing so (Fig. 1). These fundamental changes have the ability to cause both reversible and irreversible changes to reef ecosystem functioning. As part of these interactions, several related aspects are likely to feed back on the role that corals play as ecosystem engineers. Bleaching-induced death of the coral engineers includes the generation of bare skeletons that are particularly sensitive to physical, chemical and biological erosion processes (Stoddart 1969). In addition, colonisation of these stable surfaces by microbial biofilms, algae or other invertebrates may not only reduce recruitment success (e.g. Webster et al. 2004), but also change the biogeochemical processes such as nitrogen fixation (Davey et al. 2008).
The initiation of carbon and nutrient cycles by coral-derived organic matter will likely be significantly reduced in warmer oceans (Fig. 1), which may alter nutrient dynamics to favour other organisms such as cyanobacteria rather than corals.
Production of calcareous reef sands will probably increase immediately after bleaching-induced mass coral mortality as a result of increased erosion. However, this is a short-term effect lasting only until the carbonate supply diminishes. At large scales, a net loss in carbonate accretion will hypothetically influence the biocatalytic filter systems so that the processing of imported organic matter, for example via eutrophication or river discharge, is reduced (Fig. 1). This may ultimately decrease reef resilience and increase vulnerability of coral-reef ecosystems.
Similar, but not as pronounced, feedback effects on the coral engineer can be expected during and after sub-lethal bleaching (Fig. 1). As the energetic status of corals will be affected, less energy may be invested in the production of inorganic and organic matter, including carbonates, secondary metabolites, and antibiotic substances (Ritchie 2006) that prevent overgrowth by other organisms and promote resistance against pathogens. Such reductions may change the diversity and activity of coral-associated organisms, which consequently may overgrow or infect corals so that recovery from bleaching is suppressed.
The impacts of global climate change on fundamental physiological processes such as scleractinian coral growth, calcification, defence, maintenance and reproduction result in broad-scale consequences for ecosystem functions and services provided by the reef-building coral engineers. Reduced growth and reproduction translate directly to a reduced resilience of coral-dominated reef communities. Similarly, compromised immune systems and reduction in competitive abilities (Mydlarz et al. 2009; Reed et al. 2010) lead to fundamental changes in the community structure of tropical benthic assemblages (Hoegh-Guldberg et al. 2007). The consequences of reduced calcification rates as a result of ocean warming and acidification will manifest as a reduction in skeletal extension (Jokiel et al. 2008), changes in skeletal crystal morphology (Cohen et al. 2009) or, hypothetically, the formation of less dense skeletons that are highly susceptible to rapid physico–chemical and biological erosion.
Such deleterious impacts of climate change will thereby modify the ability of scleractinian corals to maintain their engineering roles in coral-reef ecosystems. Global influences will also interact with more local and direct disturbances to exacerbate the adverse effects of overfishing and declining water quality (Hughes et al. 2007) that additionally impede the functioning of corals as reef ecosystem engineers.
Acknowledgements
We thank the GEF Coral Reef Targeted Research and Capacity Building for Management Project (CRTR) for support. Coordination and development of this review was also supported by German Research Foundation grant Wi 2677/2–1 to C.W. We would also like to explicitly thank editor Prof. Andrew Boulton and three anonymous reviewers for their constructive help in improving this manuscript.
References
Albright, R., Mason, B., and Langdon, C. (2008). Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 27, 485–490.| Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae.Crossref | GoogleScholarGoogle Scholar |
Allemand, D., Ferrier-Pages, C., Furla, P., Houlbreque, F., Puverel, S., et al. (2004). Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. Comptes Rendus Palevol 3, 453–467.
| Biomineralisation in reef-building corals: from molecular mechanisms to environmental control.Crossref | GoogleScholarGoogle Scholar |
Alvarez-Filip, L., Dulvy, N. K., Gill, J. A., Cote, I. M., and Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: region-wide decline in architectural complexity. Proceedings of the Royal Society of London, Series B. Biological Sciences 276, 3019–3025.
| Flattening of Caribbean coral reefs: region-wide decline in architectural complexity.Crossref | GoogleScholarGoogle Scholar |
Anthony, K. R. N., Kline, D. I., Diaz-Pullido, G., Dove, S., and Hoegh-Guldberg, O. (2008). Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences of the United States of America 105, 17 442–17 446.
| Ocean acidification causes bleaching and productivity loss in coral reef builders.Crossref | GoogleScholarGoogle Scholar |
Aronson, R. B., and Precht, W. F. (1997). Stasis, biological disturbance, and community structure of a Holocene coral reef. Paleobiology 23, 326–346.
Aronson, R. B., Macintyre, I. G., Wapnick, C. M., and O’Neill, M. W. (2004). Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 85, 1876–1891.
| Phase shifts, alternative states, and the unprecedented convergence of two reef systems.Crossref | GoogleScholarGoogle Scholar |
Bach, S. D. (1979). Standing crop, growth and production of calcareous Siphonales (Chlorophyta) in a South Florida Lagoon. Bulletin of Marine Science 29, 191–201.
Baird, A. H., and Marshall, P. A. (2002). Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Marine Ecology Progress Series 237, 133–141.
| Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Bak, R. P. M., Lambrechts, D. Y. M., Joenje, M., Nieuwland, G., and Van Veghel, M. L. J. (1996). Long-term changes on coral reefs in booming populations of a competitive colonial ascidian. Marine Ecology Progress Series 133, 303–306.
| Long-term changes on coral reefs in booming populations of a competitive colonial ascidian.Crossref | GoogleScholarGoogle Scholar |
Bak, R. P. M., Nieuwland, G., and Meesters, E. H. (2009). Coral growth rates revisited after 31 years: what is causing lower extension rates in Acropora palmata? Bulletin of Marine Science 84, 287–294.
Bellwood, D. R., and Hughes, T. P. (2001). Regional-scale assembly rules and biodiversity of coral reefs. Science 292, 1532–1535.
| Regional-scale assembly rules and biodiversity of coral reefs.Crossref | GoogleScholarGoogle Scholar | 11375488PubMed |
Bellwood, D. R., Andrew, S. H., Ackerman, J. H., and Depczynski, M. (2006). Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Global Change Biology 12, 1587–1594.
| Coral bleaching, reef fish community phase shifts and the resilience of coral reefs.Crossref | GoogleScholarGoogle Scholar |
Bena, C., and van Woesik, R. (2004). The impact of two bleaching events on the survival of small coral colonies (Okinawa, Japan). Bulletin of Marine Science 75, 115–125.
Borowitzka, M. A., and Larkum, A. W. D. (1977). Calcification in the green alga Halimeda. I. An ultrastructure study of thallus development. Journal of Phycology 13, 6–16.
Bosence, D. (1989). Biogenic carbonate production in Florida Bay. Bulletin of Marine Science 44, 419–433.
Bosence, D., and Wilson, J. (2003). Maerl growth, carbonate production rates and accumulation rates in the NE Atlantic. Aquatic Conservation: Marine and Freshwater Ecosystems 13, S21–S31.
| Maerl growth, carbonate production rates and accumulation rates in the NE Atlantic.Crossref | GoogleScholarGoogle Scholar |
Braga, J. C., Martín, J. M., and Riding, R. (1996). Internal structure of segment reefs: Halimeda algal mounds in the Mediterranean Miocene. Geology 24, 35–38.
| Internal structure of segment reefs: Halimeda algal mounds in the Mediterranean Miocene.Crossref | GoogleScholarGoogle Scholar |
Brandt, M. E., and McManus, J. W. (2009). Disease incidence is related to bleaching extent in reef-building corals. Ecology 90, 2859–2867.
| Disease incidence is related to bleaching extent in reef-building corals.Crossref | GoogleScholarGoogle Scholar | 19886494PubMed |
Brown, B. E. (1997). Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138.
| Coral bleaching: causes and consequences.Crossref | GoogleScholarGoogle Scholar |
Brown, B. E., Dunne, R. P., Goodson, M. S., and Douglas, A. E. (2002). Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21, 119–126.
Bruno, J. F., Selig, E. R., Casey, K. S., Page, C. A., Willis, B. L., et al. (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biology 5, e124.
| Thermal stress and coral cover as drivers of coral disease outbreaks.Crossref | GoogleScholarGoogle Scholar | 17488183PubMed |
Bythell, J. C. (1988). A total nitrogen and carbon budget for the elkhorn coral Acropora palmata (Lamarck). Proceedings of the 6th International Coral Reef Symposium 6, 535–540.
Bythell, J. C. (1990). Nutrient uptake in the reef-building coral Acropora palmata at natural environmental conditions. Marine Ecology Progress Series 68, 65–69.
| Nutrient uptake in the reef-building coral Acropora palmata at natural environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Caldeira, K., and Wickett, M. E. (2003). Anthropogenic carbon and ocean pH. Nature 425, 365.
| Anthropogenic carbon and ocean pH.Crossref | GoogleScholarGoogle Scholar | 14508477PubMed |
Cohen, A. L., and Holcomb, M. C. (2009). Why corals care about ocean acidification: uncovering the mechanism. Oceanography (Washington, D.C.) 22, 118–127.
Cohen, A. L., McCorkle, D. C., de Putron, S., Gaetani, G. A., and Rose, K. A. (2009). Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochemistry Geophysics Geosystems 10, Q07005.
| Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification.Crossref | GoogleScholarGoogle Scholar |
Colombo-Pallotta, M. F., Rodriguez-Roman, A., and Iglesias-Prieto, R. (2010). Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs , .
| Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol.Crossref | GoogleScholarGoogle Scholar |
Cooney, R. P., Pantos, O., Le Tissier, M. D., Barer, M. R., O’Donnell, A. G., et al. (2002). Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environmental Microbiology 4, 401–413.
| Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques.Crossref | GoogleScholarGoogle Scholar | 12123476PubMed |
Davey, M., Holmes, G., and Johnstone, R. (2008). High rates of nitrogen fixation (acetylene reduction) on coral skeletons following bleaching mortality. Coral Reefs 27, 227–236.
| High rates of nitrogen fixation (acetylene reduction) on coral skeletons following bleaching mortality.Crossref | GoogleScholarGoogle Scholar |
De’ath, G., Lough, J. M., and Fabricius, K. E. (2009). Declining coral calcification on the Great Barrier Reef. Science 323, 116–119.
| Declining coral calcification on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar | 19119230PubMed |
Diaz-Pulido, G., and McCook, L. J. (2002). The fate of bleached corals: patterns and dynamics of algal recruitment. Marine Ecology Progress Series 232, 115–128.
| The fate of bleached corals: patterns and dynamics of algal recruitment.Crossref | GoogleScholarGoogle Scholar |
Done, T. J. (1992). Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247, 121–132.
| Phase shifts in coral reef communities and their ecological significance.Crossref | GoogleScholarGoogle Scholar |
Drew, E. A. (1983). Halimeda biomass, growth rates and sediment generation on reefs in the Central Great Barrier Reef province. Coral Reefs 2, 101–110.
| Halimeda biomass, growth rates and sediment generation on reefs in the Central Great Barrier Reef province.Crossref | GoogleScholarGoogle Scholar |
Edmunds, P. J. (2007). Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Marine Ecology Progress Series 341, 1–13.
| Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals.Crossref | GoogleScholarGoogle Scholar |
Eyre, B., Glud, R. N., and Patten, N. (2008). Mass coral spawning: a natural large-scale nutrient addition experiment. Limnology and Oceanography 53, 997–1013.
| Mass coral spawning: a natural large-scale nutrient addition experiment.Crossref | GoogleScholarGoogle Scholar |
Fabricius, K. E., and De’ath, G. (2001). Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 19, 303–309.
| Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Feder, D., Mello, C., Garcia, E., and Azambuja, P. (1997). Immune responses in Rhodnius prolixus: influence of nutrition and ecdysone. Journal of Insect Physiology 43, 513–519.
| Immune responses in Rhodnius prolixus: influence of nutrition and ecdysone.Crossref | GoogleScholarGoogle Scholar | 12770413PubMed |
Feely, R. A., Sabine, C. L., Lee, K., Berelson, W., Kleypas, J., et al. (2004). The impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366.
| The impact of anthropogenic CO2 on the CaCO3 system in the oceans.Crossref | GoogleScholarGoogle Scholar | 15256664PubMed |
Feely, R. A., Doney, S. C., and Cooley, S. R. (2009). Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22, 36–47.
Fitt, W. K., Spero, H. J., Halas, J. C., White, M. W., and Porter, J. W. (1993). Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean ‘bleaching event’. Coral Reefs 12, 57–64.
| Recovery of the coral Montastrea annularis in the Florida Keys after the 1987 Caribbean ‘bleaching event’.Crossref | GoogleScholarGoogle Scholar |
Fitt, W. K., Gates, R. D., Hoegh-Guldberg, O., Bythell, J. C., Jatkar, A., et al. (2009). Response of two species of Indo–Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: the host does matter in determining the tolerance of corals to bleaching. Journal of Experimental Marine Biology and Ecology 373, 102–110.
| Response of two species of Indo–Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: the host does matter in determining the tolerance of corals to bleaching.Crossref | GoogleScholarGoogle Scholar |
Frias-Lopez, J., Zerkle, A. L., Bonheyo, G. T., and Fouke, B. W. (2002). Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Applied and Environmental Microbiology 68, 2214–2228.
| Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces.Crossref | GoogleScholarGoogle Scholar | 11976091PubMed |
Frias-Lopez, J., Klaus, J. S., Bonheyo, G. T., and Fouke, B. W. (2004). Bacterial community associated with black band disease in corals. Applied and Environmental Microbiology 70, 5955–5962.
| Bacterial community associated with black band disease in corals.Crossref | GoogleScholarGoogle Scholar | 15466538PubMed |
Fung, F. M. Y., and Ding, J. L. (1998). A novel antitumour compound from the mucus of a coral, Galaxea fascicularis, inhibits topoisomerase I and II. Toxicon 36, 1053–1058.
| A novel antitumour compound from the mucus of a coral, Galaxea fascicularis, inhibits topoisomerase I and II.Crossref | GoogleScholarGoogle Scholar | 9690797PubMed |
Fung, F. M. Y., Tachibana, S., Chou, L. M., and Ding, J. L. (1997). Cytotoxic and anticancer agents in mucus of Galaxea fascicularis: purification and characterization. Journal of Marine Biotechnology 5, 50–57.
Gates, R. D. (1990). Seawater temperature and sublethal coral bleaching in Jamaica. Coral Reefs 8, 193–197.
| Seawater temperature and sublethal coral bleaching in Jamaica.Crossref | GoogleScholarGoogle Scholar |
Gattuso, J. P., Frankignoulle, M., and Smith, S. V. (1999). Measurement of community metabolism and significance in the coral reef CO2 source–sink debate. Proceedings of the National Academy of Sciences of the United States of America 96, 13 017–13 022.
| Measurement of community metabolism and significance in the coral reef CO2 source–sink debate.Crossref | GoogleScholarGoogle Scholar |
Glud, R. N., Eyre, B., and Patten, N. (2008). Biogeochemical responses on mass coral spawning at the Great Barrier Reef: effects on respiration and primary production. Limnology and Oceanography 53, 1014–1024.
| Biogeochemical responses on mass coral spawning at the Great Barrier Reef: effects on respiration and primary production.Crossref | GoogleScholarGoogle Scholar |
Glynn, P. W. (1993). Coral reef bleaching – ecological perspectives. Coral Reefs 8, 181–191.
| Coral reef bleaching – ecological perspectives.Crossref | GoogleScholarGoogle Scholar |
Glynn, P. W. (1997). Bioerosion and coral reef growth: a dynamic balance. In ‘Life and Death of Coral Reefs’. (Ed. C. Birkeland.) pp. 68–95. (Springer: Berlin.)
Golbuu, Y., and Richmond, R. H. (2007). Substratum preferences in planula larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata. Marine Biology 152, 639–644.
| Substratum preferences in planula larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata.Crossref | GoogleScholarGoogle Scholar |
Guitierrez, J. L., Jones, C. G., Byers, J. E., Arkema, K. K., Berkenbusch, K., et al. (in press). Physical ecosystem engineers and the functioning of estuaries and coasts. In ‘Functioning of Estuaries and Coastal Ecosystems’. (Eds C. H. R. Heip, C. J. M. Philippart and J. J. Middelburg.) (Elsevier: Amsterdam.)
Haas, A. F., al-Zibdah, M., and Wild, C. (2010). Seasonal in-situ monitoring of coral–algae interaction stability in fringing reefs of the Northern Red Sea. Coral Reefs 29, 93–103.
| Seasonal in-situ monitoring of coral–algae interaction stability in fringing reefs of the Northern Red Sea.Crossref | GoogleScholarGoogle Scholar |
Hallock, P. (1997). Reefs and reef limestones in Earth history. In ‘Life and Death of Coral Reefs’. (Ed. C. Birkeland.) pp. 13–42. (Springer: Berlin.)
Harrington, L., Fabricius, K., De’ath, G., and Negri, A. (2004). Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85, 3428–3437.
| Recognition and selection of settlement substrata determine post-settlement survival in corals.Crossref | GoogleScholarGoogle Scholar |
Harvell, C. D., Kim, K., Quirolo, C., Weir, J., and Smith, G. (2001). Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). Hydrobiologia 460, 97–104.
| Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea).Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50, 839–866.
| Climate change, coral bleaching and the future of the world’s coral reefs.Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O., and Jones, R. J. (1999). Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Marine Ecology Progress Series 183, 73–86.
| Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals.Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O., and Smith, G. J. (1989). The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata (Esper) and Seriatopora hystrix (Dana). Journal of Experimental Marine Biology and Ecology 129, 279–303.
| The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata (Esper) and Seriatopora hystrix (Dana).Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742.
| Coral reefs under rapid climate change and ocean acidification.Crossref | GoogleScholarGoogle Scholar | 18079392PubMed |
Holcomb, M. C., McCorkle, D. C., and Cohen, A. L. (2010). Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). Journal of Experimental Marine Biology and Ecology 386, 27–33.
| Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786).Crossref | GoogleScholarGoogle Scholar |
Holcomb, M. C., Cohen, A. L., and McCorkle, D. C. (in press). Gender bias in the coral response to ocean acidification. Ocean Sciences Meeting Portland, Oregon, USA; 22–26 February 2010 (American Geophysical Union, Washington, DC.)
Huettel, M., Røy, H., Precht, E., and Ehrenhauss, S. (2003). Hydrodynamical impact on biogeochemical processes in aquatic sediments. Hydrobiologia 494, 231–236.
| Hydrodynamical impact on biogeochemical processes in aquatic sediments.Crossref | GoogleScholarGoogle Scholar |
Huettel, M., Wild, C., and Gonelli, S. (2006). The mucus trap in coral reefs: formation and temporal evolution of aggregates caused by coral mucus. Marine Ecology Progress Series 307, 69–84.
| The mucus trap in coral reefs: formation and temporal evolution of aggregates caused by coral mucus.Crossref | GoogleScholarGoogle Scholar |
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551.
| Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar | 17801530PubMed |
Hughes, T., Rodrigues, M., Bellwood, D. R., Ceccarelli, D., Hoegh-Guldberg, O., et al. (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology 17, 360–365.
| Phase shifts, herbivory, and the resilience of coral reefs to climate change.Crossref | GoogleScholarGoogle Scholar | 17291763PubMed |
Iglesias-Prieto, R., Matta, J. L., Robins, W. A., and Trench, R. K. (1992). Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proceedings of the National Academy of Sciences of the United States of America 89, 302–305.
Iglesias-Prieto, R., Beltran, V. H., LaJeunesse, T. C., Reyes-Bonilla, H., and Thome, P. E. (2004). Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proceedings of the Royal Society of London, Series B. Biological Sciences 271, 1757–1763.
| Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific.Crossref | GoogleScholarGoogle Scholar |
Jokiel, P. L., and Coles, S. L. (1977). Effects of temperature on the mortality and growth of Hawaiian reef corals. Marine Biology 43, 201–208.
| Effects of temperature on the mortality and growth of Hawaiian reef corals.Crossref | GoogleScholarGoogle Scholar |
Jokiel, P. L., and Coles, S. L. (1990). Response of Hawaiian and other Indo–Pacific reef corals to elevated temperature. Coral Reefs 8, 155–162.
| Response of Hawaiian and other Indo–Pacific reef corals to elevated temperature.Crossref | GoogleScholarGoogle Scholar |
Jokiel, P. L., Rodgers, K. S., Kuffner, I. B., Andersson, A. J., Cox, E. F., et al. (2008). Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483.
| Ocean acidification and calcifying reef organisms: a mesocosm investigation.Crossref | GoogleScholarGoogle Scholar |
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). Organsims as ecosystem engineers. Oikos 69, 373–386.
| Organsims as ecosystem engineers.Crossref | GoogleScholarGoogle Scholar |
Jones, C. G., Lawton, J. H., and Shachak, M. (1997). Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957.
| Positive and negative effects of organisms as physical ecosystem engineers.Crossref | GoogleScholarGoogle Scholar |
Jury, C. P., Whitehead, R. F., and Szmant, A. M. (). Effects of variations in carbonate chemistry on the calcification rates of Madracis mirabilis (Duchassaing 1861): bicarbonate concentrations best predict calcification rates. Global Change Biology , .
| Effects of variations in carbonate chemistry on the calcification rates of Madracis mirabilis (Duchassaing 1861): bicarbonate concentrations best predict calcification rates.Crossref | GoogleScholarGoogle Scholar | 20431716PubMed |
Kennedy, D. M., Woodroffe, C. D., Jones, B. G., Dickson, M. E., and Phipps, C. V. G. (2002). Carbonate sedimentation on subtropical shelves around Lord Howe Island and Balls Pyramid, Southwest Pacific. Marine Geology 188, 333–349.
| Carbonate sedimentation on subtropical shelves around Lord Howe Island and Balls Pyramid, Southwest Pacific.Crossref | GoogleScholarGoogle Scholar |
Kleypas, J. A., Buddemeier, R. W., Archer, D., Gattuso, J. P., Langdon, C., et al. (1999). Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120.
| Geochemical consequences of increased atmospheric carbon dioxide on coral reefs.Crossref | GoogleScholarGoogle Scholar | 10102806PubMed |
Knowlton, N., and Rohwer, F. (2003). Multispecies microbial mutualisms on coral reefs: the host as a habitat. American Naturalist 162, S51–S62.
| Multispecies microbial mutualisms on coral reefs: the host as a habitat.Crossref | GoogleScholarGoogle Scholar | 14583857PubMed |
Langdon, C., and Atkinson, M. J. (2005). Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. Journal of Geophysical Research 110, C09S07.
| Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment.Crossref | GoogleScholarGoogle Scholar |
Lau, K. M., and Wu, H. T. (2003). Warm rain processes over tropical oceans and climate implications. Geophysical Research Letters 30, 2290.
| Warm rain processes over tropical oceans and climate implications.Crossref | GoogleScholarGoogle Scholar |
Lau, K. M., Wu, H. T., Sud, Y. C., and Walker, G. K. (2005). Effects of cloud microphysics on tropical atmospheric hydrologic processes and intraseasonal variability. Journal of Climatology 18, 4731–4751.
| Effects of cloud microphysics on tropical atmospheric hydrologic processes and intraseasonal variability.Crossref | GoogleScholarGoogle Scholar |
Leder, J. J., Szmant, A. M., and Swart, P. K. (1991). The effect of prolonged ‘bleaching’ on skeletal banding and stable isotopic composition in Montastrea annularis. Coral Reefs 10, 19–27.
| The effect of prolonged ‘bleaching’ on skeletal banding and stable isotopic composition in Montastrea annularis.Crossref | GoogleScholarGoogle Scholar |
Lesser, M. P., Mazel, C. H., Gorbunov, Y., and Falkowski, P. G. (2004). Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305, 997–1000.
| Discovery of symbiotic nitrogen-fixing cyanobacteria in corals.Crossref | GoogleScholarGoogle Scholar | 15310901PubMed |
Loch, K., Loch, W., Schumacher, H., and See, W. R. (2004). Coral recruitment and regeneration on a Maldivian Reef four years after the coral bleaching event of 1998. Part 2: 2001–2002. Marine Ecology 25, 145–154.
| Coral recruitment and regeneration on a Maldivian Reef four years after the coral bleaching event of 1998. Part 2: 2001–2002.Crossref | GoogleScholarGoogle Scholar |
Loya, Y. (1976). Recolonization of Red Sea corals affected by natural catastrophes and man-made perturbations. Ecology 57, 278–289.
| Recolonization of Red Sea corals affected by natural catastrophes and man-made perturbations.Crossref | GoogleScholarGoogle Scholar |
Loya, Y. (2004). The coral reefs of Eilat – past, present and future: three decades of coral community structure studies. In ‘Coral Health and Disease’. (Eds E. Rosenberg and Y. Loya.) pp. 1–34. (Springer: New York.)
Loya, Y., Sakai, K., Yamazato, K., Nakano, Y., Sambali, H., et al. (2001). Coral bleaching: the winners and the losers. Ecology Letters 4, 122–131.
| Coral bleaching: the winners and the losers.Crossref | GoogleScholarGoogle Scholar |
Maliao, R. J., Turingan, R. G., and Lin, J. (2008). Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Marine Biology 154, 841–853.
| Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA.Crossref | GoogleScholarGoogle Scholar |
Manzello, D. P. (2010). Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs 29, 749–758.
| Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific.Crossref | GoogleScholarGoogle Scholar |
Manzello, D. P., Brandt, M., Smith, T. B., Lirman, D., Hendee, J. C., et al. (2007). Hurricanes benefit bleached corals. Proceedings of the National Academy of Sciences of the United States of America 104, 12 035–12 039.
| Hurricanes benefit bleached corals.Crossref | GoogleScholarGoogle Scholar |
Marshall, P. A., and Baird, A. H. (2000). Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19, 155–163.
| Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa.Crossref | GoogleScholarGoogle Scholar |
McClanahan, T. R., and Shafir, S. H. (1990). Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 83, 362–370.
McClanahan, T., Maina, J., and Pet-Soede, L. (2002). Effects of the 1998 coral morality event on Kenyan coral reefs and fisheries. Ambio 31, 543–550.
| 12572820PubMed |
McCook, L. J. (1999). Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 18, 357–367.
| Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Meesters, E. H., and Bak, R. P. M. (1993). Effects of coral bleaching on tissue regeneration potential and colony survival. Marine Ecology Progress Series 96, 189–198.
| Effects of coral bleaching on tissue regeneration potential and colony survival.Crossref | GoogleScholarGoogle Scholar |
Meszaros, A., and Bigger, C. (1999). Qualitative and quantitative study of wound healing processes in the coelenterate Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. Journal of Invertebrate Pathology 73, 321–331.
| Qualitative and quantitative study of wound healing processes in the coelenterate Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences.Crossref | GoogleScholarGoogle Scholar | 10222188PubMed |
Miller, J., Waara, R., Muller, E., and Rogers, C. (2006). Coral bleaching and disease combine to cause extensive mortality on reefs of the US Virgin Islands. Coral Reefs 25, 418.
| Coral bleaching and disease combine to cause extensive mortality on reefs of the US Virgin Islands.Crossref | GoogleScholarGoogle Scholar |
Moberg, F., and Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecological Economics 29, 215–233.
| Ecological goods and services of coral reef ecosystems.Crossref | GoogleScholarGoogle Scholar |
Moret, Y., and Schmid-Hempel, P. (2000). Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168.
| Survival for immunity: the price of immune system activation for bumblebee workers.Crossref | GoogleScholarGoogle Scholar | 11073456PubMed |
Morita, M., Suwa, R., Iguchia, A., Nakamura, M., Shimada, K., et al. (2009). Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18, 103–107.
| Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates.Crossref | GoogleScholarGoogle Scholar |
Muller, E. M., Rogers, C. S., Spitzack, A. S., and van Woesik, R. (2008). Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs 27, 191–195.
| Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands.Crossref | GoogleScholarGoogle Scholar |
Mumby, P. J., Chisholm, J. R. M., Edwards, A. J., Clark, C. D., Roark, E. B., et al. (2001). Unprecedented bleaching-induced mortality in Porites spp. at Rangiroa Atoll, French Polynesia. Marine Biology 139, 183–189.
| Unprecedented bleaching-induced mortality in Porites spp. at Rangiroa Atoll, French Polynesia.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Jones, G. P., Pratchett, M. S., and Williams, A. J. (2008). Climate change and the future for coral reef fishes. Fish and Fisheries 9, 261–285.
| Climate change and the future for coral reef fishes.Crossref | GoogleScholarGoogle Scholar |
Muscatine, L., Falkowski, P. G., and Porter, J. (1984). Fate of photosynthetic fixed carbon in light and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proceedings of the Royal Society of London, Series B. Biological Sciences 222, 181–202.
| Fate of photosynthetic fixed carbon in light and shade-adapted colonies of the symbiotic coral Stylophora pistillata.Crossref | GoogleScholarGoogle Scholar |
Mydlarz, L. D., Couch, C. S., Weil, E., Smith, G., and Harvell, C. D. (2009). Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Diseases of Aquatic Organisms 87, 67–78.
| Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event.Crossref | GoogleScholarGoogle Scholar | 20095242PubMed |
Nakamura, T., and van Woesik, R. (2001). Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series 212, 301–304.
| Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event.Crossref | GoogleScholarGoogle Scholar |
Nanami, A., and Nishihira, M. (2004). Microhabitat association and temporal stability in reef fish assemblages on massive Porites microatolls. Ichthyological Research 51, 165–171.
| Microhabitat association and temporal stability in reef fish assemblages on massive Porites microatolls.Crossref | GoogleScholarGoogle Scholar |
Nanami, A., Nishihira, M., Suzuki, T., and Yokochi, H. (2005). Species-specific habitat distribution of coral reef fish assemblages in relation to habitat characteristics in an Okinawan coral reef. Environmental Biology of Fishes 72, 55–65.
| Species-specific habitat distribution of coral reef fish assemblages in relation to habitat characteristics in an Okinawan coral reef.Crossref | GoogleScholarGoogle Scholar |
Naumann, M., Richter, C., el-Zibdah, M., and Wild, C. (2009). Coral mucus as an efficient trap for picoplanktonic cyanobacteria – implications for pelagic–benthic coupling in the reef ecosystem. Marine Ecology Progress Series 385, 65–76.
| Coral mucus as an efficient trap for picoplanktonic cyanobacteria – implications for pelagic–benthic coupling in the reef ecosystem.Crossref | GoogleScholarGoogle Scholar |
Naumann, M. S., Haas, A., Struck, U., Mayr, C., el-Zibdah, M., et al. (2010). Organic matter release by the dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29, 649–659.
| Organic matter release by the dominant hermatypic corals of the Northern Red Sea.Crossref | GoogleScholarGoogle Scholar |
Niggl, W., Glas, M., Laforsch, C., Mayr, C., and Wild, C. (2009). First evidence of coral bleaching stimulating organic matter release by reef corals. In ‘Proceedings of the 11th International Coral Reef Symposium’. (Ed. B. Riegl.) pp. 905–910. (International Society for Reef Studies: Ft. Lauderdale, FL, USA.)
Pantos, O., and Bythell, J. C. (2006). Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16S rRNA techniques. Diseases of Aquatic Organisms 69, 79–88.
| Bacterial community structure associated with white band disease in the elkhorn coral Acropora palmata determined using culture-independent 16S rRNA techniques.Crossref | GoogleScholarGoogle Scholar | 16703769PubMed |
Pantos, O., Cooney, R. P., Le Tissier, M. D. A., Barer, M. R., O’Donnell, A. G., et al. (2003). The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis. Environmental Microbiology 5, 370–382.
| The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis.Crossref | GoogleScholarGoogle Scholar |
Payri, C. E. (1988). Halimeda contribution to organic and inorganic production in a Tahitian reef system. Coral Reefs 6, 251–262.
| Halimeda contribution to organic and inorganic production in a Tahitian reef system.Crossref | GoogleScholarGoogle Scholar |
Piggot, A. M., Fouke, B. W., Sivaguru, M., Sanford, R. A., and Gaskins, H. R. (2009). Change in zooxanthellae and mucocycte tissue density as an adaptive response to environmental stress by the coral, Montastrea annularis. Marine Biology 156, 2379–2389.
| Change in zooxanthellae and mucocycte tissue density as an adaptive response to environmental stress by the coral, Montastrea annularis.Crossref | GoogleScholarGoogle Scholar |
Porter, J. W., Fitt, W. K., Spero, H. J., Rogers, C. S., and White, M. N. (1989). Bleaching in reef corals: physiological and stable isotopic responses. Proceedings of the National Academy of Sciences of the United States of America 86, 9342–9346.
| Bleaching in reef corals: physiological and stable isotopic responses.Crossref | GoogleScholarGoogle Scholar | 16594090PubMed |
Pratchett, M. S. (2007). Dietary selection by coral-feeding butterfly fishes (Chaetodontidae) on the Great Barrier Reef, Australia. The Raffles Bulletin of Zoology 14, 171–176.
Raes, M., De Troch, M., Ndaro, S. G. M., Muthumbi, A., Guilini, K., et al. (2007). The structuring role of microhabitat type in coral degradation zones: a case study with marine nematodes from Kenya and Zanzibar. Coral Reefs 26, 113–126.
| The structuring role of microhabitat type in coral degradation zones: a case study with marine nematodes from Kenya and Zanzibar.Crossref | GoogleScholarGoogle Scholar |
Rasheed, M., Badran, M., and Huettel, M. (2003). Particulate matter filtration and seasonal nutrient dynamics in permeable carbonate and silicate sands of the Gulf of Aqaba, Red Sea. Coral Reefs 22, 167–177.
| Particulate matter filtration and seasonal nutrient dynamics in permeable carbonate and silicate sands of the Gulf of Aqaba, Red Sea.Crossref | GoogleScholarGoogle Scholar |
Reaka-Kudla, M. L. (1997). The global biodiversity of coral reefs: a comparison with rain forests. In ‘Biodiversity II: Understanding and Protecting our Biological Resources’. (Eds M. L. Reaka-Kudla, D. E. Wilson and E. O. Wilson.) pp. 83–108. (Joseph Henry Press: Washington, DC.)
Reed, K. C., Muller, E. M., and van Woesik, R. (2010). Coral immunology and resistance to disease. Diseases of Aquatic Organisms 90, 85–92.
| Coral immunology and resistance to disease.Crossref | GoogleScholarGoogle Scholar | 20662364PubMed |
Reshef, L., Koren, O., Loya, Y., Zilber-Rosenberg, I., and Rosenberg, E. (2006). The coral probiotic hypothesis. Environmental Microbiology 8, 2068–2073.
| The coral probiotic hypothesis.Crossref | GoogleScholarGoogle Scholar | 17107548PubMed |
Rinkevich, B. (1999). Invertebrates versus vertebrates innate immunity: in the light of evolution. Scandinavian Journal of Immunology 50, 456–460.
| Invertebrates versus vertebrates innate immunity: in the light of evolution.Crossref | GoogleScholarGoogle Scholar | 10564546PubMed |
Ritchie, K. B. (2006). Regulation of microbial populations by coral surface mucus-associated bacteria. Marine Ecology Progress Series 322, 1–14.
| Regulation of microbial populations by coral surface mucus-associated bacteria.Crossref | GoogleScholarGoogle Scholar |
Rodrigues, L. J., and Grottoli, A. G. (2006). Calcification rate and stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochimica et Cosmochimica Acta 70, 2781–2789.
| Calcification rate and stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals.Crossref | GoogleScholarGoogle Scholar |
Rodrigues, L. J., Grottoli, A. G., and Pease, T. K. (2008). Lipid class composition of bleached and recovering Porites compressa (Dana, 1846) and Montipora capitata (Dana, 1846) corals from Hawaii. Journal of Experimental Marine Biology and Ecology 358, 136–143.
| Lipid class composition of bleached and recovering Porites compressa (Dana, 1846) and Montipora capitata (Dana, 1846) corals from Hawaii.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Román, A., Hernández-Pech, X., Thomé, P., Enríquez, S., and Iglesias-Prieto, R. (2006). Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event. Limnology and Oceanography 51, 2702–2710.
| Photosynthesis and light utilization in the Caribbean coral Montastraea faveolata recovering from a bleaching event.Crossref | GoogleScholarGoogle Scholar |
Rohwer, F., Seguritan, V., Azam, F., and Knowlton, N. (2002). Diversity and distribution of coral-associated bacteria. Marine Ecology Progress Series 243, 1–10.
| Diversity and distribution of coral-associated bacteria.Crossref | GoogleScholarGoogle Scholar |
Rowan, R., Knowlton, N., Baker, A., and Jara, J. (1997). Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269.
| Landscape ecology of algal symbionts creates variation in episodes of coral bleaching.Crossref | GoogleScholarGoogle Scholar | 9230434PubMed |
Rypien, K. L., Ward, J. R., and Azam, F. (2010). Antagonistic interactions among coral-associated bacteria. Environmental Microbiology 12, 28–39.
| Antagonistic interactions among coral-associated bacteria.Crossref | GoogleScholarGoogle Scholar | 19691500PubMed |
Sale, P. F., Danilowicz, B. S., Doherty, P. J., and Williams, D. M. (2005). The relation of microhabitat to variation in recruitment of young-of-year coral reef fishes. Bulletin of Marine Science 76, 123–142.
Seppälä, O., Liljeroos, K., Karvonen, A., and Jukka, J. (2008). Host condition as a constraint for parasite reproduction. Oikos 117, 749–753.
| Host condition as a constraint for parasite reproduction.Crossref | GoogleScholarGoogle Scholar |
Shashar, N., Cohen, Y., Loya, Y., and Sar, N. (1994). Nitrogen fixation (acetylene reduction) in stony corals: Evidence for coral-bacteria interactions. Marine Ecology Progress Series 111, 259–264.
| Nitrogen fixation (acetylene reduction) in stony corals: Evidence for coral-bacteria interactions.Crossref | GoogleScholarGoogle Scholar |
Smith, J. E., Shaw, M., Edwards, R. A., Obura, A., Pantos, O., et al. (2006). Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecology Letters 9, 835–845.
| Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality.Crossref | GoogleScholarGoogle Scholar | 16796574PubMed |
Srinivasan, M. (2003). Depth distributions of coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 137, 76–84.
| Depth distributions of coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes.Crossref | GoogleScholarGoogle Scholar | 12856204PubMed |
Stedman, T. L. (2000). ‘Stedman’s Medical Dictionary.’ (Lippincott, Williams and Wilkins: Baltimore.)
Stoddart, D. R. (1969). Ecology and morphology of recent coral reefs. Biological Reviews of the Cambridge Philosophical Society 44, 433–498.
| Ecology and morphology of recent coral reefs.Crossref | GoogleScholarGoogle Scholar |
Sud, Y. C., Walker, G. K., Zhou, Y. P., Schmidt, G. A., and Lau, K. M. (2008). Effects of doubled CO2 on tropical sea surface temperatures (SSTs) for onset of deep convection and maximum SST: Simulations based inferences. Geophysical Research Letters 35, L12707.
| Effects of doubled CO2 on tropical sea surface temperatures (SSTs) for onset of deep convection and maximum SST: Simulations based inferences.Crossref | GoogleScholarGoogle Scholar |
Suzuki, A., and Kawahata, H. (2003). Carbon budget of coral reef systems: an overview of observations in fringing reefs, barrier reefs and atolls of the Indo–Pacific regions. Tellus 55B, 428–444.
Takahashi, S., Nakamura, T., Sakamizu, M., van Woesik, R., and Yamasaki, H. (2004). Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant & Cell Physiology 45, 251–255.
| Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals.Crossref | GoogleScholarGoogle Scholar | 14988497PubMed |
Tanzil, J. T. I., Brown, B. E., Tudhope, A. W., and Dunne, R. P. (2009). Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand, between 1984 and 2005. Coral Reefs 28, 519–528.
| Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand, between 1984 and 2005.Crossref | GoogleScholarGoogle Scholar |
Van Oppen, M. J. H., Palstra, F. P., Piquet, A. M., and Miller, D. J. (2001). Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proceedings of the Royal Society of London, Series B. Biological Sciences 268, 1759–1767.
| Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity.Crossref | GoogleScholarGoogle Scholar |
Ward, S., Harrison, P., and Hoegh-Guldberg, O. (2002). Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In ‘Proceedings of the 9th International Coral Reef Symposium’. pp. 1123–1128. (International Society for Reef Studies: Bali, Indonesia.)
Webster, N. S., Smith, L. D., Heyward, A. J., Watts, J. E. M., Webb, R. I., et al. (2004). Metamorphosis of a scleractinian coral in response to microbial biofilms. Applied and Environmental Microbiology 70, 1213–1221.
| Metamorphosis of a scleractinian coral in response to microbial biofilms.Crossref | GoogleScholarGoogle Scholar | 14766608PubMed |
Wells, S., Ravilious, C., and Corcoran, E. (2006). ‘In the Front Line – Shoreline Protection and Other Ecosystem Services from Mangroves and Coral Reefs.’ (UNEP-WCMC: Cambridge.)
Werner, U., Bird, P., Wild, C., Ferdelman, T., Polerecky, L., et al. (2006). Spatial variability of aerobic and anaerobic mineralization in coral reef sediments (Heron Island, Australia). Marine Ecology Progress Series 309, 93–105.
| Spatial variability of aerobic and anaerobic mineralization in coral reef sediments (Heron Island, Australia).Crossref | GoogleScholarGoogle Scholar |
Wild, C., Huettel, M., Klueter, A., Kremb, S. G., Rasheed, M., et al. (2004). Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428, 66–70.
| Coral mucus functions as an energy carrier and particle trap in the reef ecosystem.Crossref | GoogleScholarGoogle Scholar | 14999280PubMed |
Wild, C., Rasheed, M., Werner, U., Franke, U., Johnstone, R., et al. (2004). Degradation and mineralization of coral mucus in reef environments. Marine Ecology Progress Series 267, 159–171.
| Degradation and mineralization of coral mucus in reef environments.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Tollrian, R., and Huettel, M. (2004). Rapid recycling of coral mass spawning products in permeable reef sediments. Marine Ecology Progress Series 271, 159–166.
| Rapid recycling of coral mass spawning products in permeable reef sediments.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Rasheed, M., Jantzen, C., Cook, P., Struck, U., et al. (2005). Benthic metabolism and degradation of natural particulate organic matter in silicate and carbonate sands of the Northern Red Sea. Marine Ecology Progress Series 298, 69–78.
| Benthic metabolism and degradation of natural particulate organic matter in silicate and carbonate sands of the Northern Red Sea.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Woyt, H., and Huettel, M. (2005). Influence of coral mucus release on nutrient fluxes in carbonate sands. Marine Ecology Progress Series 287, 87–98.
| Influence of coral mucus release on nutrient fluxes in carbonate sands.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Laforsch, C., and Huettel, M. (2006). Detection and enumeration of microbial cells in highly porous carbonate reef sands. Marine and Freshwater Research 57, 415–420.
| Detection and enumeration of microbial cells in highly porous carbonate reef sands.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Jantzen, C., Struck, U., Hoegh-Guldberg, O., and Huettel, M. (2008). Biogeochemical responses on coral mass spawning at the Great Barrier Reef: pelagic–benthic coupling. Coral Reefs 27, 123–132.
| Biogeochemical responses on coral mass spawning at the Great Barrier Reef: pelagic–benthic coupling.Crossref | GoogleScholarGoogle Scholar |
Wild, C., Naumann, M. S., Haas, A., Struck, U., Mayer, F. W., et al. (2009). Coral sand O2 uptake and pelagic–benthic coupling in a subtropical fringing reef, Aqaba, Red Sea. Aquatic Biology 6, 133–142.
| Coral sand O2 uptake and pelagic–benthic coupling in a subtropical fringing reef, Aqaba, Red Sea.Crossref | GoogleScholarGoogle Scholar |
Williams, W. M., Viner, A. B., and Broughton, W. J. (1987). Nitrogen fixation (acetylene reduction) associated with the living coral Acropora variabilis. Marine Biology 94, 531–535.
| Nitrogen fixation (acetylene reduction) associated with the living coral Acropora variabilis.Crossref | GoogleScholarGoogle Scholar |
Willis, B. L., Page, C. A., and Dinsdale, E. A. (2004). Coral disease on the Great Barrier Reef. In ‘Coral Health and Disease’. (Eds E. Rosenberg and Y. Loya.) pp. 69–104. (Springer: New York.)
Wilson, S. K., Graham, N. A. J., Pratchett, M. S., Jones, J. P., and Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biology 12, 2220–2234.
| Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient?Crossref | GoogleScholarGoogle Scholar |


