Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia
Stephen R. Balcombe A E , Fran Sheldon A , Samantha J. Capon A , Nick R. Bond B C , Wade L. Hadwen A , Nick Marsh D and Sofie J. Bernays AA Australian Rivers Institute and eWater Cooperative Research Centre, Griffith University, Nathan, Qld 4111, Australia.
B School of Biological Sciences and eWater Cooperative Research Centre, Monash University, Clayton, Vic. 3800, Australia.
C Present address: Australian Rivers Institute, Griffith University, Nathan, Qld 4111, Australia.
D Yorb Pty Ltd, 906 Sandgate Road, Clayfield, Qld 4011, Australia.
E Corresponding author. Email: s.balcombe@griffith.edu.au
Marine and Freshwater Research 62(9) 1099-1114 https://doi.org/10.1071/MF11059
Submitted: 11 March 2011 Accepted: 28 July 2011 Published: 21 September 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Many aquatic ecosystems have been severely degraded by water-resource development affecting flow regimes and biological connectivity. Freshwater fish have been particularly affected by these changes and climate change will place further stress on them. The Murray–Darling Basin (MDB), Australia, represents a highly affected aquatic system with dramatically modified flow regimes. This has impaired the health of its rivers, and potentially limited the adaptive capacity of its biota to respond to a changing climate. Here, we present our predictions of the potential impacts of climate change on 18 native fish species across their distributional ranges against the back-drop of past and continuing water-resource development (WRD). Because most of these species are found across a wide range of geographical and hydrological settings, we classified the MDB into 10 regions to account for likely variation in climate-change effects, on the basis of latitude, elevation and WRD. Cold water-tolerant species will be under greater stress than are warm water-tolerant species. In some regions, the negative impacts on exotic fish such as trout are likely to improve current conditions for native species. Because the impacts of climate change on any given species are likely to vary from region to region, regional fish assemblages will also be differentially affected. The most affected region is likely to occur in the highly disturbed Lower Murray River region, whereas the dryland rivers that are less affected in the northern MDB are likely to remain largely unchanged. Although climate change is a current and future threat to the MDB fish fauna, the continued over-regulation of water resources will place as much, if not more, stress on the remnant fish species.
Additional keywords: conceptual models, native fish, regionalisation, riparian vegetation, water-resource development.
Introduction
Climate change, with associated changes in land use, atmospheric CO2 concentration, nitrogen deposition and acid rain, as well as introductions of exotic species, is considered one of the most important determinants of current declines in global biodiversity (Sala et al. 2000). The challenge for ecologists is to predict the likely responses of species and communities to climate change over and above the background natural ecological variability (Verschuren et al. 2000) and, in heavily modified areas, above and beyond the effects of human development.
Humans already withdraw ~50% of available freshwater resources globally (Szöllosi-Nagy et al. 1998), with requirements increasing almost 10-fold during the 20th century (Biswas 1998). The effects of this development on aquatic ecosystems have been severe, evidenced by the numerous global and regional assessments that depict accelerating losses of biodiversity and declines in ecosystem function (Vörösmarty et al. 2000). These impacts in freshwater ecosystems stem from changes to the natural flow regime (Poff and Zimmerman 2010), loss of river–floodplain connectivity (Tockner et al. 2008) and channelisation and construction of in-stream barriers, to name a few. The added effects of climate change in already stressed ecosystems will only further exacerbate the decline in freshwater biota (Pittock and Finlayson 2011).
In Australia, most of the larger rivers in the south-east of the country suffer from extensive agricultural and rural development and a consequent decline in river ecosystem health (Mercer and Marden 2006). The most notable are the rivers of the Murray–Darling Basin (MDB) located in the south-east of Australia. The MDB occupies ~14% of the continent's surface area (1.07 million km2), produces ~40% of the country's annual agricultural production (Crabb 1997) and annually contributes ~AU$18 billion worth of produce to the national economy (MDBA 2010). As a focus for intensive agricultural production, there is intense interest in the condition of aquatic ecosystems in the MDB, as well as the possible impacts of climate change (Davies et al. 2010).
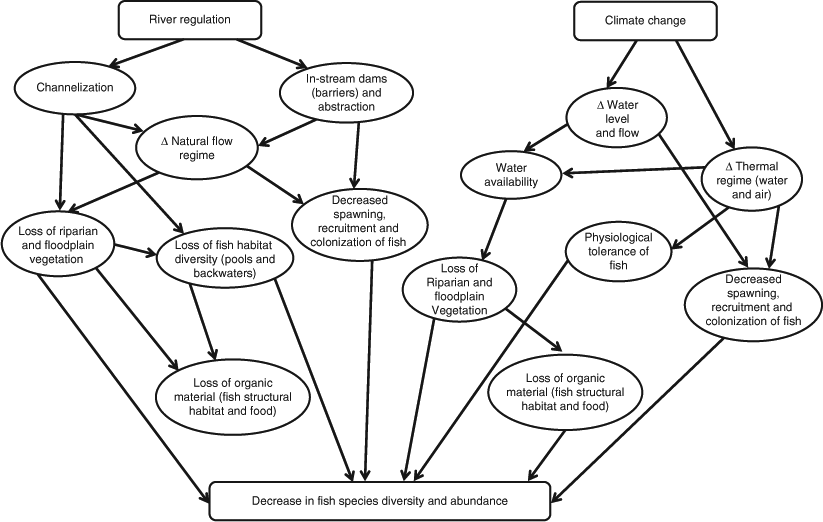
There has been widespread degradation of both riverine and floodplain biota in the MDB, with large tracts of floodplain forest transitioning to terrestrial ecosystems (Pittock and Finlayson 2011). In their assessment of fish species, hydrology and macroinvertebrates, Davies et al. (2010) found 20 of the 24 river basins to be in poor or very poor condition. The impacts of flow modifications, including thermal pollution, have been implicated in the demise of native fish in the MDB-regulated rivers, because of their impacts on physiology, spawning and movement (Gehrke and Harris 2001; Growns 2008). Native fish numbers are now only 10% of pre-European levels (MDBC 2004) because of factors such as changes to the natural flow regime, habitat degradation and barriers to biological connectivity (Pratchett et al. 2011). Assessing how climate change will affect fish species in the MDB must consider these previous and continuing impacts.
Much in-stream fish habitat has been lost as a result of wood removal across the MDB (Koehn et al. 2004). Disruption of riparian–riverine linkages and the loss of riparian and floodplain flora will continue to result in impacts to MDB fish, aside from the direct impacts of river regulation. In the present review, we begin by exploring the effects of water resource and climate change on riparian and in-stream vegetation in the MDB. We then assess how climate change might alter native fish assemblages across the MDB by focussing on 18 individual species, spanning the majority of fish within any local assemblage within the MDB (largely on the basis of distribution maps from Lintermans (2007)). These impacts are presented in a regional context to demonstrate how responses are likely to differ across climate zones and levels of water-resource development.
Climate change impacts in the Murray–Darling Basin
Projected changes to temperature, rainfall and evaporation, particularly in inland Australia, will alter the frequency and magnitude of heavy rain events, floods and droughts, and affect runoff, soil moisture and salinity on a regional basis (Pittock 2003). In the northern and southern MDB, declines of between 8% and 12% of median runoff, with <5% change in the central, eastern and southern upland regions, are predicted (PMSEIC 2007). The frequency of drought is predicted to increase by 20–40% by 2030 (compared with 1975–2000 average) (PMSEIC 2007). Most global climate models indicate that future winter rainfall is likely to be lower across the MDB, particularly in the southern MDB (CSIRO 2008), which is significant because most of the rainfall and runoff occurs in winter in this region.
Although the median 2030 climate projections suggest an overall reduction of 11% in average surface-water availability across the MDB, there are strong regional variations, with reductions of 3% in the Paroo catchment, 12% for the Murray River at Wentworth and 21% in the Wimmera (CSIRO 2008). These changes in runoff- and surface-water availability must be set against a backdrop of historical levels of water use in the basin of ~50%, which have already caused widespread changes in the nature of riverine and floodplain ecosystems.
Predicted impacts on riparian and floodplain vegetation in the Murray–Darling Basin
River red gum (Eucalyptus camaldulensis)
River red gums are the iconic trees of the MDB and contribute significant structural woody habitat and finer organic material (Koehn et al. 2004) that provide shelter from predators, food and nutrients. River red gums form extensive forests within the Murray and Murrumbidgee catchments (e.g. Barmah–Millewa Forest) and fringe most of the main rivers and tributaries throughout the basin (Roberts 2001). The species is found across a large temperature gradient and can utilise a range of water sources, including surface- and groundwater sources (Thorburn et al. 1994). Their distribution, density and condition, however, are closely linked to flooding, particularly flood frequency (Roberts 2001). Consequently, changes in flood frequency are usually associated with changes in river red gum condition. In the Barmah Forest, for example, river red gum trees have historically withstood an absence of flooding for up to 18 months during droughts in 1982 and perhaps also during droughts of a similar duration in 1904, 1915, 1944 and 1967 (Bren et al. 1987). The extreme drought in the lower MDB since the mid-1990s, however, has stressed or killed a large number of river red gums.
Impacts of water-resource development on river red gums have occurred as a result of changes in flood frequency, duration and magnitude (Cunningham et al. 2007). Where parts of the floodplain have been permanently inundated, large stands of river red gum have perished. Individual trees and river red gum forests in other locations are stressed because of a lack of flooding of sufficient duration and frequency. Reductions in flooding associated with river regulation and water abstraction have probably impaired recruitment. Increased groundwater salinity, also associated with river regulation in parts of the MDB, has further contributed to declining river red gum health (Overton et al. 2006).
Climate-change impacts on river red gums are most likely to manifest themselves through changes in the frequency and magnitude of floodplain inundation events. Trees and forests already stressed by water-resource development may exhibit mass mortality as a result of the increased stress associated with further reductions in flooding caused by climate change. Increased duration of dry spells, along with higher temperatures, may reduce survivorship of seedlings. Such impacts to river red gums, leading to less structural woody habitat, will have significant implications for fish, such as Murray cod (Maccullochella peelii) (Koehn 2009) and trout cod (Maccullochella macquariensis) (Nicol et al. 2007). Reduced inputs of organic matter from finer material such bark and leaves will also have an impact on food supply for small-bodied fish and cascade upwards through food webs, whereas the loss of stream-side canopy may increase algal growth and further exacerbate rising water temperatures as a result of reduced shading.
Lignum (Muehlenbeckia florulenta)
Lignum shrubland occurs throughout the MDB, with large stands on the floodplains of the lower Warrego River, Barwon–Darling River and northern Darling tributaries (Roberts 2001). In the eastern MDB, it fringes the more ephemeral or temporary waterbodies. Lignum is very tolerant of drought conditions and can survive as a leafless shrub for several years on low rainfall with little to no flooding (Craig et al. 1991; Capon 2003). After long dry spells, lignum responds quickly to either flooding or rainfall through rapid leaf growth and flowering (Capon et al. 2009). Lignum shrubland is particularly significant in the MDB as breeding habitat for colonial waterbirds, especially where it comprises large, mature shrubs in intermediately flooded areas (Capon et al. 2009).
Impacts of water-resource development on lignum shrubland in the MDB are likely to have been minimal in comparison with the widespread historic clearing of this species. Lignum is intolerant of prolonged flooding (Roberts 2001; Capon 2003), so reductions in flood frequency and duration associated with water-resource development do not appear to have resulted in significant declines in the health of remaining lignum stands. Instead, this may have allowed lignum to increase its range into areas that were previously flooded more frequently or for longer durations, such as in the terminating wetlands of the northern MDB dryland rivers.
Higher air temperatures and reductions in rainfall and flood frequency and duration associated with climate-change scenarios may alter the character and condition of lignum stands throughout the MDB and, therefore, their value as habitat. Lignum recruitment appears to be closely linked to hydrology (Capon et al. 2009) and further alterations to flooding patterns may change the population structure of lignum stands as well as facilitating the further encroachment into channels and open water areas by seedlings. Such impacts are likely to have implications for fish populations in the MDB by altering habitat and food-web structure, via flow-on effects of impacts to piscivorous waterbirds that rely on lignum shrublands for habitat and via changes to inputs of organic material (e.g. lignum leaves).
Aquatic grasses, reeds, rushes and sedges
Emergent macrophytes, including grasses, sedges, reeds and rushes, are widespread across the MDB's floodplains, wetlands and waterway fringes. Prominent and ecologically significant species include grasses such as couch (Cynodon dactylon), Moira grass (Pseudoraphis spinescens), water couch (Paspalum distichum) and common reed (Phragmites australis) as well as bulrushes (Typha spp.) and sedges such as Eleocharis spp. and Cyperus spp. Most species exhibit some tolerance to both inundation and drying, although life-history responses vary widely, reflected by species distributions (Roberts 2001). Some species (e.g. Moira grass and water couch) require frequent, seasonal inundation, whereas others (e.g. common reed) can persist through considerable periods of drought both as mature plants and as persistent rhizomes in the soil. In contrast, bulrushes grow in water to 2 m deep and, although they can tolerate water regimes that vary from permanently wet to seasonally or periodically dry, they do not tolerate permanent flooding over 2 m deep and can only tolerate dry conditions for short periods after the growing season (Roberts 2001).
The impacts of water-resources development are likely to have varied amongst species in this group, depending on their particular environmental tolerances and life histories. Anecdotal evidence, for instance, suggests that common reed was historically more widespread in terminal floodplains of the MDB and the decline in the extent of this species may reflect reductions in flood frequency, as well as grazing pressure, in such areas (Roberts 2001). Moira grass plains on floodplains of the Murray River are also threatened as a result of water-resources development, both as a direct result of changes in flood frequency, duration and timing on the life history of this species, as well as indirectly as a result of their encroachment by both river red gum and giant rush because of altered flooding patterns (Bren 1992; Roberts 2001).
Further reductions in the duration and frequency of flooding, which are likely under many climate-change scenarios, will have a major impact on many plant species in this group, and further reductions in the extent of Moira grass plains and stands of common reeds are of particular concern. Higher air temperatures, combined with lower humidity, have the potential to affect germination and regeneration of these species through reduced soil moisture. Survival of persistent propagules (e.g. rhizomes) may also decline as a result of declining soil moisture and flood frequency. Bulrushes are also likely to be affected by altered flooding patterns associated with water-resources development and climate change.
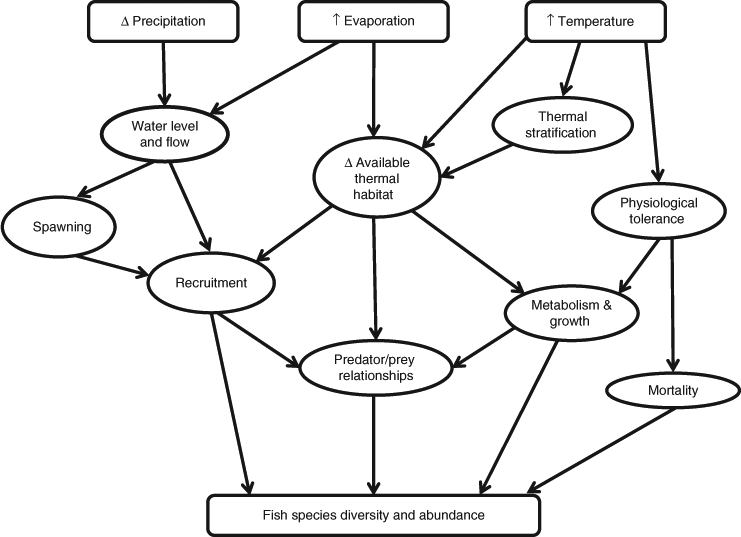
Although Typha stands may well expand in certain regions of the MDB under climate-change scenarios, favoured by warm, nutrient-rich conditions, in other areas where water levels and soil moisture decline and the periods between flooding are extended, Typha stands may disappear. Such loss of grasses and reeds, particularly in terminating and off-channel wetlands, are likely to have a significant impact on smaller-bodied fish species, such as pygmy perch and carp gudgeons, that rely on them for both cover and food. Furthermore, plants such as reeds and grasses that fringe river channels also provide significant organic inputs to fish food webs; losses would reduce fish abundance and diversity (Fig. 1).
|
Conceptual models of predicted climate-change impacts on some iconic MDB fish species
Environmental filters have been used to predict changes in fish species presence on the basis of their physiological and habitat requirements (Poff 1997; Bond et al. 2011). However, patchy information on environmental tolerances of many MDB fish taxa limits predictions of impacts from climate change. Many MDB fish species appear to be ecological generalists (flexible breeding strategies, diet and habitat), given their wide distribution across varying climatic and geographical regions (Lintermans 2007). Therefore, the main climate-change drivers for generalist fish within the MDB will relate to flow (and therefore changes in habitat availability) and to physiological impacts felt through increased water temperatures influencing spawning times and reduced oxygen levels impairing fitness and survival (Fig. 2; Pankhurst and Munday 2011). Given the over-riding impacts of current levels of water-resource development on fish throughout the MDB, our predictions of climate-change impacts acknowledge how individual species have already been affected, and will continue to be so, by water-resource development (MDBC 2004).
The impact of climate change on invasive species will also be relevant to native fish. Invasive species are expected to undergo a mix of responses to climate change, reflecting the diverse range of climatic conditions under which they originally evolved (Rahel and Olden 2008). For example, Bond et al. (2011) predicted a contraction in the distribution of brown (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) in the southern part of the MDB. The reduction of these species, especially in upland regions, would be expected to benefit many native species. Morrongiello et al. (2011) suggested that gambusia may benefit from increased water temperatures, again, especially in the south. Any increase in the distribution and abundance of this species is likely to be negative, especially for small-bodied native fish. Common carp (Cyprinus carpio) has opportunistic recruitment patterns and high rates of population growth that allow it to persist in suboptimal conditions (Koehn 2004). Hence, this fish species is unlikely to be affected by climate change and will probably increase in some places, as a result of increased temperature and thus breeding and growth response when periodic recruitment opportunities (occasional floods) occur.
On the basis of the conceptual model in Fig. 2, we developed five derivative models for different groups of MDB fish species. Fish were grouped according to ecological similarities in diet, breeding strategies (such as spawning flexibility and type of spawning cue), habitat associations (e.g macrophytes, snags, open water) and temperature tolerances (e.g cold water and warm water). For brevity, only two of these models are presented here to demonstrate how we assessed climate-change impacts on species diversity and abundance.
The first model predicts the abundance of Murray cod (Fig. 3). This model demonstrates two pathways of effect, as follows: (1) decreased precipitation leading to reduced water levels and flow, affecting spawning and recruitment, and (2) increased temperature influencing the timing of spawning and physiological tolerance, ultimately reducing Murray cod abundance (Table 1). Overall, Murray cod will decline across the MDB, especially in the northern regions where reduced flows will exacerbate impacts of loss of hydrological connectivity and reduce thermal (summer) refugia because of the loss of deep pools (Table 1). There may be some localised increases in populations where stream temperature rises partially negate impacts of cold-water pollution below bottom-release dams (Table 1). Additionally, there are likely to be longer-term indirect impacts throughout the MDB where riparian and floodplain trees are lost, given they provide significant habitat and food for Murray cod (Table 1).
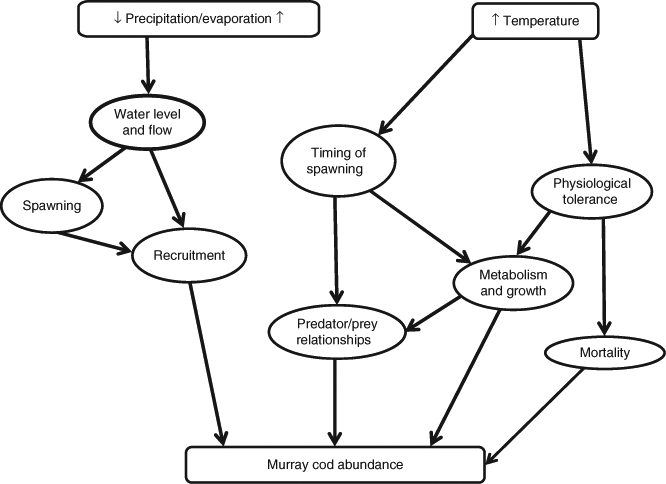
|
Our second example (Fig. 4) demonstrates some of the key drivers influencing five medium- to large-bodied fish species that are either widely distributed across the whole MDB (yellowbelly (Macquaria ambigua), eel-tailed catfish (Tandanus tandanus) and silver perch (Bidyanus bidyanus)) or across the northern MDB (Hyrtl's tandan (Neosilurus hyrtlii) and spangled perch (Leiopotherapon unicolor)) (Table 1). Increased temperature will directly influence predator–prey relationships by affecting lower levels of the food web, including primary productivity, potentially creating more niches for these fish. Changes in precipitation and evaporative loss will reduce the amount of water in the river, impairing connectivity and recruitment of these species. In the northern MDB, increased temperatures will allow species such as yellowbelly, spangled perch and Hyrtl's tandan with high temperature tolerances and opportunistic breeding strategies to take advantage of the high primary productivity (Table 1). Silver perch and eel-tailed catfish are unlikely to gain much benefit from such changes in the northern MDB because the drier climate will have a further impact on hydrology, particularly connectivity (Table 1). In the southern MDB, however, these two species will probably benefit from increases in stream temperature, given how much they have been affected by cold-water pollution (Table 1).
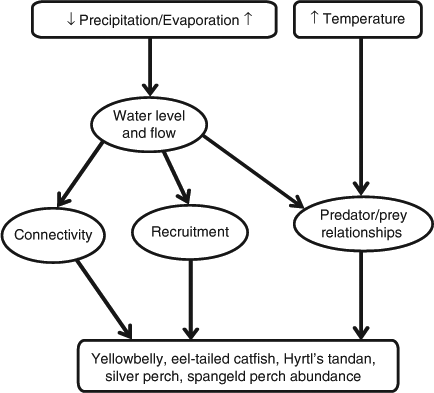
|
Of the remaining species presented in Table 1, we grouped them as follows: Group A, including carp gudgeons and bony bream; Group B, including river blackfish, two-spined blackfish, trout cod, mountain galaxiids and Macquarie perch; and Group C, containing Australian smelt, un-specked hardyhead, southern pygmy perch, flat-headed gudgeon and Murray–Darling rainbowfish. Group A fish tolerate an extremely wide range of environmental conditions and their abundance is often strongly linked to levels of primary productivity (Table 1). The increase in temperature resulting from climate change should, therefore, increase the food base of these fish directly and any change to flows is likely to have no major influence on them (Table 1).
The distributions of Group B fish all extend into the cooler upland streams of the MDB (Lintermans 2007) and these fish could be loosely termed as ‘cold-water tolerant’. Increased stream temperatures are likely to have a direct physiological effect on these fish, particularly where they are located at their high temperature limits (e.g. river blackfish and mountain galaxiids in the northern MDB, Table 1). In the southern MDB, increased temperatures may lead to increased abundances in local populations of fish such as trout cod, two-spined blackfish and Macquarie perch affected by cold-water pollution (Table 1). Such changes in temperature regime could affect larval recruitment success of species such as trout cod and Macquarie perch with short breeding seasons, by affecting the timing of zooplankton emergence, for example (Table 1). It is likely that all of the species within this group have been largely affected by the presence of exotic trout, so the expected decline of trout (Bond et al. 2011) because of increased stream temperatures may lead to more native species in these rivers (Table 1).
Group C comprises smaller-bodied fish, often found in wetlands and off-river channel waterbodies often associated with macrophytes (Table 1). The reduction since European settlement of species such as Murray–Darling rainbowfish, un-specked hardyhead and southern pygmy perch can be largely attributed to a loss of connectivity within riverine ecosystems (Table 1). Distributions of these species will become further fragmented as flow diminishes as a result of climate change. Losses of floodplain vegetation (e.g. rushes and reeds in floodplain wetlands) will also further affect fish such as flat-headed gudgeons and southern pygmy perch associated with macrophytes (Table 1). Increased temperatures will also have an impact on small-bodied fish with short breeding seasons and relatively narrow diet ranges, such as Australian smelt, also found in many floodplain habitats (Table 1). These fish are likely to be affected because any shift in seasonal thermal regime is likely to disrupt the synchrony between the seasonal peak in smelt larvae and specific species and size classes of their zooplankton prey.
Regional impacts on fish assemblages in the MDB
Given that the impacts of climate change are predicted to vary with latitude as well as with the degree of flow modification, we classified the MDB into 10 regions on the basis of their latitudinal position and the degree of flow management (Fig. 5). Ecological communities will tend to vary over an elevation gradient, so we used the largely elevation-driven regionalisation boundaries in the Sustainable Rivers Audit (Davies et al. 2008) to differentiate uplands from lowlands and to distinguish the alpine region. By using this classification and the predicted effects of climate change on individual species (Table 1), we can predict regional changes in fish assemblages throughout the MDB.
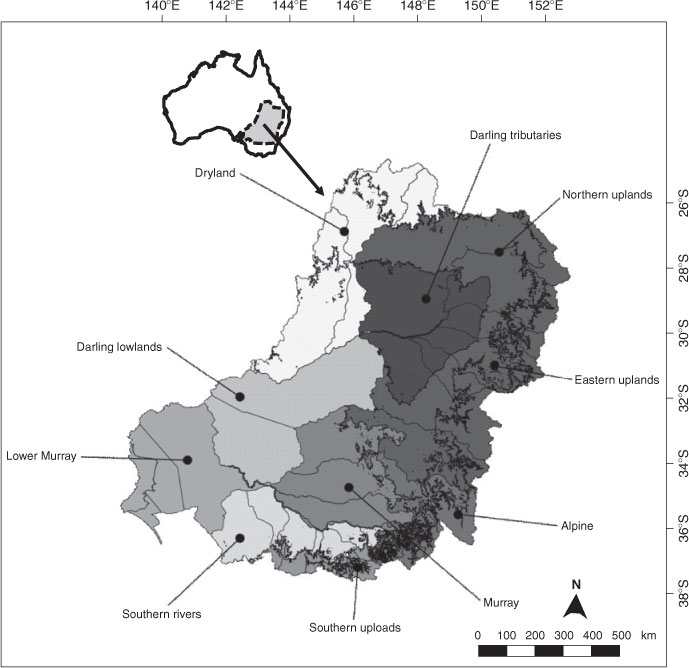
|
Northern and eastern uplands
Both of these regions, located in the northern MDB, with rivers ultimately flowing into the Darling River (Fig. 5), represent highly regulated rivers because of the presence of water storages (Table 2). There are significant effects of cold-water pollution in these regions, owing to bottom-release off-takes in water storages. Therefore, any warming as a result of climate change will confer an advantage to those fish species that have been reduced in their range and abundance from cold-water pollution. It would be expected that both range and abundance of Murray cod and river blackfish could increase slightly in both of these regions because increased water temperatures partially mitigate effects such as reduced spawning and growth from thermal pollution (Table 1). Although increased temperatures could also facilitate the range expansion of generalist fish species such as yellowbelly, eel-tailed catfish, silver perch and bony bream, these highly regulated rivers are likely to have less water overall. As a result, drought refugia will dry out faster, leaving fewer suitable habitats across the landscape and probably cancelling out any gains made though increased water temperature.

|

|
Southern uplands
The rivers of the southern MDB comprise a mixture of regulated and unregulated rivers. Most predicted changes in this region relate to increased water temperatures, although in the regulated rivers, effects will be further exacerbated by reduced flows (Table 2). These increases are likely to affect cold water-tolerant species such as river and two-spined blackfish and trout cod, reducing their breeding activities and thus overall abundance (Table 1). However, balanced against these predicted reductions in native species will be the likely displacement of exotic trout (Bond et al. 2011), which currently has a significant impact on native riverine food webs as a major predator of invertebrates and fish (Lintermans 2007). Such an impact on trout could facilitate increases in vulnerable species such as mountain galaxias and the two blackfish species.
Alpine
The Alpine region contains many headwater streams of the Murray and Murrumbidgee rivers in the eastern MDB (Fig. 5). For the regulated rivers, the impacts of water-resource development would outweigh any small changes to flow regime brought about by climate change. Therefore, the impacts of climate change will be mostly restricted to increases in stream temperatures that alter the distributions of the cold water-tolerant species, especially two-spined blackfish (Table 2). It is likely that temperature increases will reduce river blackfish distributions at lower altitudes and affect the spawning time and food availability for Macquarie perch. As with the southern uplands, we predict that significant impacts on exotic trout will greatly benefit native fish species such as mountain galaxias and the two blackfish species through reduced competition and predation.
Dryland
The dryland region in the upper western corner of the MDB (Fig. 5) consists largely of the unregulated semiarid rivers. While there are no major diversion weirs or water storages on these rivers, there are many low-level weirs that currently impose the largest threat to fish migration and breeding patterns in the region (Table 2). The fish of these rivers are mostly ecological generalists, adapted to cope with the highly variable flows of these rivers (Gehrke et al. 1995; Balcombe et al. 2006, 2011). It is unlikely that climate change will substantially change these fish assemblages, given the ability of the species to cope with extremes of climate. However, where the impact of climate change interacts with the effects of barriers on fish migration (such as low flows), the fish species that migrate as part of their breeding cycle (e.g. Hyrtl's tandan, Balcombe and Arthington 2009; Kerezsy et al. 2011) could be affected in some rivers. There are likely to be some localised impacts of less flow reaching terminal wetlands (climate change added to water-resource development), affecting floodplain and wetland vegetation, especially encroachment from lignum. This could enhance the local abundance of fish species, including exotic species such as carp and gambusia, because of the presence of added structural habitat and increased organic input when these systems are flooded.
Darling tributaries
Originating in the northern and eastern upland region, the Darling tributaries region refers only to the lowland section of these rivers (Fig. 5). All are affected by water-resource development to varying degrees because of the presence of headwater storages, low-level weirs and significant levels of water abstraction for irrigation (Table 2). Climate change is likely to lead to fewer refuge pools (remaining pools will also be shallower) during dry periods, which will have a flow-on effect for fish that are close to their upper thermal limits, such as Murray cod. Generalist species such as eel-tailed catfish, yellowbelly and bony bream may increase in abundance in response to declines in Murray cod. Balanced against this is the potential expansion of the distributions of exotic fish, particularly of common carp and gambusia, which are established and significant competitors for food resources in these rivers (Gehrke et al. 1995; Balcombe et al. 2011). The combined effects of reduced flows and reduced connectivity from development will also have an impact on species, including carp gudgeons, Murray–Darling rainbowfish and un-specked hardyheads, that use more marginal waterbodies such as anabranches and billabongs (Lintermans 2007).
Darling lowland
The Darling lowland region in the mid-western MDB (Fig. 5) has been significantly affected by water-resource development, with major abstractions and many low-level weirs (Table 2). Because of the high levels of water-resource development and the associated decline of the fish assemblages in the region, climate-change impacts are not likely to cause too many further changes. However, further reductions in flow and increased temperatures may decrease available drought refugia for Murray cod. Furthermore, bony bream may increase its abundance in such refuge habitats in response to fewer predators and because of its ability to capitalise on increased algal resources associated with high water temperatures. These increases in primary productivity in degraded habitats could also favour common carp (Koehn 2004), placing further stress on native fish. Lignum may encroach into drier sections of rivers such as wetlands, which could exacerbate impacts of exotic fish.
Murray
The Murray region in the lower MDB (Fig. 5) is a highly regulated riverine system with many weirs and locks. Flows are managed via release from major water storages. Fish stocks have been largely affected by cold-water pollution for significant lengths of river below water storages. As a result, increased temperatures are likely to ameliorate some of these impacts and thus increase the abundance and range of species such as trout cod, Murray cod, yellowbelly and silver perch (Table 2). Given the lack of connectivity of this highly modified and regulated river with off-channel and floodplain habitats, any further reductions to flow will mean fewer floodplain connections and further degradation of fish such as yellowbelly, silver perch, Australian smelt, flat headed gudgeons, un-specked hardyheads and pygmy perch that use floodplains and associated waterbodies (Table 1). Less floodplain inundation will also have an impact on riparian vegetation, especially river red gum. This will have longer-term effects on the habitat use and food resources of native fish. However, fewer floodplain connections may have an impact on common carp recruitment because inundated floodplains provide a significant source of carp recruits in the MDB (Crook and Gillanders 2006).
Southern rivers
The southern rivers of the MDB that feed into the mid- and lower Murray (Fig. 5) are mostly regulated with storages on all but the Ovens River (Table 2). The likely reduced flows and reductions in flood frequency will have an impact on in-channel and floodplain habitats. Although weirs are likely to maintain refuge pools in regulated river reaches, overall flow reductions may exacerbate the loss of longitudinal and lateral connectivity. With less flow, there is also likely to be less macrophyte habitat, leading to fewer small-bodied species such as pygmy perch, carp gudgeons and flat headed gudgeons as they become more vulnerable to predation (Table 2). In headwater streams, increased temperatures will drive an upstream contraction of cold water-tolerant species such as river and two-spined blackfish and trout cod. Again, barrier effects may impede such movements in more developed catchments.
Lower Murray
The lower Murray region represents the termination of the Murray–Darling system (Fig. 5) and is highly regulated via locks and weirs (Table 2). Consequently, the assemblages of riverine fish are already highly degraded and subtle changes to either temperature or flow will probably not result in much impact (Table 2). Nonetheless, increased water temperatures combined with less flow, and thus less lateral connectivity, are likely to further affect the already degraded habitats for wetland fish species such as pygmy perch, flat headed gudgeons, un-specked hardyheads and Murray–Darling rainbowfish because of loss of macrophytes and increased salinity (Table 1). Given the high temperature tolerance of gambusia, it is likely to flourish in these wetland habitats and further affect small-bodied native fish species. Further declines in river red gum woodlands will also have flow-on effects to the riverine fish assemblages because of reduced structural woody habitat, carbon inputs and food resources. Given the raft of impacts already known for this region, the future looks bleak for several species that are restricted in distribution and abundance, including river blackfish, purple-spotted gudgeon (Mogurnda adspersa) and Murray hardyhead (Craterocephalus fluviatilis).
Conclusions
Most of the regions of the MDB contain highly altered river systems. The Lower Murray region is characterised by weirs and locks that constrain longitudinal connectivity throughout the river and lateral connectivity with associated floodplains. In the northern uplands, reaches show significant effects of thermal pollution and flow regulation from water storages, again leading to riverine systems that have been largely altered. As such, the fish assemblages of the MDB are highly degraded and unlikely to improve significantly under current management regimes. Climate change will further stress these already ‘degraded’ fish assemblages. However, in some regions the change will be limited given the already degraded environment, whereas in others there could be an improvement for native fish, given that temperature increase is likely to at least partially ameliorate some of the effects of thermal pollution and increase stress on resident exotic taxa.
The effects of climate change on fish assemblages should not be considered in isolation from existing water-resource development in any river catchment, especially in heavily altered systems such as the MDB, where historical levels of water-resource use exceed the likely impacts of climate change. In this respect, future management plans that aim to improve the condition of rivers in the MDB must consider both the added pressure of climate change as well as the current stress associated with agricultural and human water use.
Acknowledgements
We thank Andrew Boulton and John Koehn for their helpful comments and feedback on this manuscript and also two anonymous referees who provided insightful comments on an earlier draft. Tori Grice assisted with the regionalisation of the MDB and subsequent graphics. This paper was completed as part of the eWater CRC Project C1 ‘Modelling hydroclimatic variability and impacts on water resources and aquatic ecosystems’ and a Murray–Darling Basin Authority project on climate-change impacts on aquatic ecology in the MDB.
References
Balcombe, S. R., and Arthington, A. H. (2009). Temporal changes in fish abundance in response to hydrological variability in a dryland floodplain river. Marine and Freshwater Research 60, 146–159.| Temporal changes in fish abundance in response to hydrological variability in a dryland floodplain river.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., and Closs, G. P. (2004). Spatial relationships and temporal variability in a littoral macrophyte fish assemblage. Marine and Freshwater Research 55, 609–617.
| Spatial relationships and temporal variability in a littoral macrophyte fish assemblage.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., and Humphries, P. (2006). Diet of the western carp gudgeon (Hypsleotris klunzingeri Ogilby) in an Australian floodplain lake: the role of water level stability. Journal of Fish Biology 68, 1484–1493.
| Diet of the western carp gudgeon (Hypsleotris klunzingeri Ogilby) in an Australian floodplain lake: the role of water level stability.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Arthington, A. H., Foster, N. D., Thoms, M. C., Wilson, G. G., and Bunn, S. E. (2006). Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin. Marine and Freshwater Research 57, 619–633.
| Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Bunn, S. E., Arthington, A. H., Fawcett, J. H., McKenzie-Smith, F. J., and Wright, A. (2007). Fish larvae, growth and biomass relationships in an Australian arid zone river: links between floodplains and waterholes. Freshwater Biology 52, 2385–2398.
| Fish larvae, growth and biomass relationships in an Australian arid zone river: links between floodplains and waterholes.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Arthington, A. H., Thoms, M. C., and Wilson, G. G. (2011). Fish assemblage patterns across a gradient of flow regulation in an Australian dryland river system. River Research and Applications 27, 168–183.
| Fish assemblage patterns across a gradient of flow regulation in an Australian dryland river system.Crossref | GoogleScholarGoogle Scholar |
Biswas, A. K. (1998). Deafness to global water crisis: causes and risks. Ambio 27, 492–493.
Bond, N. R., and Lake, P. S. (2003). Characterizing fish-habitat associations in streams as the first step in ecological restoration. Austral Ecology 28, 611–621.
| Characterizing fish-habitat associations in streams as the first step in ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Bond, N., Thomson, J., Reich, P., and Stein, J. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Boulton, A. J., and Brock, M. A. (1999). ‘Australian Freshwater Ecology – Processes and Management.’ (Gleneagles Publishing: Adelaide.)
Bren, L. J., O'Neill, I. C., and Gibbs, N. L. (1987). Flooding in the Barmah Forest and its relation to flow in the Murray–Edward River system. Australian Forest Research 17, 127–144.
Bren, L. J. (1992). Tree invasion of an intermittent wetland in relation to changes in the flooding frequency of the River Murray, Australia. Australian Journal of Ecology 17, 395–408.
| Tree invasion of an intermittent wetland in relation to changes in the flooding frequency of the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Brown, A., Nicol, S., and Koehn, J. (1998). ‘National recovery plan for the trout cod Maccullochella macquariensis 1998–2005.’ (Department of Natural Resources and Environment: Melbourne.)
Capon, S. J. (2003). Plant community responses to wetting and drying in a large arid floodplain. River Research and Applications 19, 509–520.
| Plant community responses to wetting and drying in a large arid floodplain.Crossref | GoogleScholarGoogle Scholar |
Capon, S. J., James, C. S., Williams, L., and Quinn, G. P. (2009). Responses to flooding and drying in seedlings of a common Australian desert floodplain shrub: Muehlenbeckia florulenta Meis. (tangled lignum). Environmental and Experimental Botany 66, 178–185.
| Responses to flooding and drying in seedlings of a common Australian desert floodplain shrub: Muehlenbeckia florulenta Meis. (tangled lignum).Crossref | GoogleScholarGoogle Scholar |
Cook, B. D., Bunn, S. E., and Hughes, J. M. (2007). Molecular genetic and stable isotope signatures reveal complementary patterns of population connectivity in the regionally vulnerable southern pygmy perch (Nannoperca australis). Biological Conservation 138, 60–72.
| Molecular genetic and stable isotope signatures reveal complementary patterns of population connectivity in the regionally vulnerable southern pygmy perch (Nannoperca australis).Crossref | GoogleScholarGoogle Scholar |
Crabb, P. (1997). ‘Murray–Darling Basin Resources.’ (The Murray–Darling Basin Commission: Canberra.)
Craig, A. E., Walker, K. F., and Boulton, A. J. (1991). Effects of edaphic factors and flood frequency on the abundance of lignum (Muehlenbeckia florulenta Meissner) (Polygonaceae) on the River Murray floodplain, South Australia. Australian Journal of Botany 39, 431–443.
| Effects of edaphic factors and flood frequency on the abundance of lignum (Muehlenbeckia florulenta Meissner) (Polygonaceae) on the River Murray floodplain, South Australia.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., and Gillanders, B. M. (2006). Use of otolith chemical signatures to estimate carp recruitment sources in the mid-Murray river, Australia. River Research and Applications 22, 871–879.
| Use of otolith chemical signatures to estimate carp recruitment sources in the mid-Murray river, Australia.Crossref | GoogleScholarGoogle Scholar |
CSIRO (2008). Water availability in the Murray–Darling Basin. A report to the Australian Government from the CSIRO Murray–Darling Basin Sustainable Yields Project. CSIRO, Canberra.
Cunningham, S. C., Read, J., Baker, P. J., and MacNally, R. (2007). Quantitative assessment of stand condition and its relationship to physiological stress in stands of Eucalyptus camaldulensis (Myrtaceae). Australian Journal of Botany 55, 692–699.
| Quantitative assessment of stand condition and its relationship to physiological stress in stands of Eucalyptus camaldulensis (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Davies, P. E., Harris, J. H., Hillman, T. J., and Walker, K. F. (2008). SRA Report 1: a report on the ecological health of rivers in the Murray–Darling Basin, 2004–2007. Murray–Darling Basin Commission, Canberra.
Davies, P. E., Harris, J. H., Hillman, T. J., and Walker, K. F. (2010). The sustainable rivers audit: assessing river ecosystem health in the Murray–Darling basin, Australia. Marine and Freshwater Research 61, 764–777.
| The sustainable rivers audit: assessing river ecosystem health in the Murray–Darling basin, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFGrs7Y%3D&md5=4f28a4e5622032c57e03c810de587d26CAS |
Ebner, B. C., Scholz, O., and Gawne, B. (2009a). Golden perch, Macquaria ambigua, are flexible spawners in the Darling river, Australia. New Zealand Journal of Marine and Freshwater Research 43, 571–578.
| Golden perch, Macquaria ambigua, are flexible spawners in the Darling river, Australia.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., McAllister, R. R. J., and Suter, P. S. (2009b). Effects of sample size on numerical estimates of diel prey consumption in a fish population. New Zealand Journal of Marine and Freshwater Research 43, 579–590.
| Effects of sample size on numerical estimates of diel prey consumption in a fish population.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010a). Clarifying an ambiguous evolutionary history: range-wide phylogeography of an Australian freshwater fish, the golden perch (Macquaria ambigua). Journal of Biogeography 37, 1329–1340.
| Clarifying an ambiguous evolutionary history: range-wide phylogeography of an Australian freshwater fish, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010b). Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica. Conservation Genetics 11, 921–934.
| Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica. Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2001). Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia. Regulated Rivers: Research and Management 17, 369–391.
| Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., Brown, P., Schiller, C. B., Moffatt, D. B., and Bruce, A. M. (1995). River regulation and fish communities in the Murray–Darling River system, Australia. Regulated Rivers: Research and Management 10, 15–38.
Growns, I. (2008). The influence of changes to river hydrology on freshwater fish in regulated rivers of the Murray–Darling Basin. Hydrobiologia 596, 203–211.
| The influence of changes to river hydrology on freshwater fish in regulated rivers of the Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., Serafini, L., and King, A. J. (2002). River regulation and fish larvae: variations through space and time. Freshwater Biology 47, 1307–1331.
| River regulation and fish larvae: variations through space and time.Crossref | GoogleScholarGoogle Scholar |
Jackson, P. (1978). Spawning and early development of the River Blackfish, Gadopsis marmoratus (Gadopsiformes: Gadopsidae), in the Makenzie River, Victoria. Australian Journal of Marine and Freshwater Research 29, 293–298.
| Spawning and early development of the River Blackfish, Gadopsis marmoratus (Gadopsiformes: Gadopsidae), in the Makenzie River, Victoria.Crossref | GoogleScholarGoogle Scholar |
Kerezsy, A., Balcombe, S. R., Arthington, A. H., and Bunn, S. E. (2011). Continuous recruitment underpins fish persistence in the arid rivers of far-western Queensland, Australia. Marine and Freshwater Research 62, in press.
| Continuous recruitment underpins fish persistence in the arid rivers of far-western Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Khan, M. T., Khan, T. A., and Wilson, M. E. (2004). Habitat use and movement of river blackfish (Gadopsis marmoratus R.) in a highly modified Victorian stream, Australia. Ecology Freshwater Fish 13, 285–293.
| Habitat use and movement of river blackfish (Gadopsis marmoratus R.) in a highly modified Victorian stream, Australia.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Humphries, P., and Lake, P. S. (2003). Fish recruitment on floodplains: the roles of patterns of flooding and life history characteristics. Canadian Journal of Fisheries and Aquatic Sciences 60, 773–786.
| Fish recruitment on floodplains: the roles of patterns of flooding and life history characteristics.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Mahoney, J. (2009). Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia. River Research and Applications 25, 1205–1218.
| Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2001). Ecological impacts of cold water releases on fish and ecosystem processes. In ‘Thermal Pollution of the Murray–Darling Waterways: Workshop Held at Lake Hume 18–19 June 2001: Statement and Recommendations Plus Supporting Papers’. (Ed. B. Phillips.) pp. 7–11. (Inland Rivers Network and World Wide Fund for Nature: Sydney.)
Koehn, J. D. (2004). Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshwater Biology 49, 882–894.
| Carp (Cyprinus carpio) as a powerful invader in Australian waterways.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. (2009). Multi-scale habitat selection by Murray cod Maccullochella peeli peeli in two lowland rivers. Journal of Fish Biology 75, 113–129.
| Multi-scale habitat selection by Murray cod Maccullochella peeli peeli in two lowland rivers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjnsVOrtg%3D%3D&md5=ce1ca90f25f0cf6eaa9bf57db624af36CAS |
Koehn, J. D., and Harrington, D. J. (2006). Environmental conditions and timing for the spawning of Murray cod (Maccullochella peelii peelii) and the endangered trout cod (M. macquariensis) in southeastern Australian rivers. River Research and Applications 22, 327–342.
| Environmental conditions and timing for the spawning of Murray cod (Maccullochella peelii peelii) and the endangered trout cod (M. macquariensis) in southeastern Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and O'Connor, W. G. (1990). ‘Biological information for management of native freshwater fish in Victoria.’ (Department of Conservation and Environment – Freshwater Fish Management Branch: Melbourne.)
Koehn, J. D., Nicol, S. J., and Fairbrother, P. S. (2004). Spatial arrangement and physical characteristics of structural woody habitat in a lowland river in south-eastern Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 14, 457–464.
| Spatial arrangement and physical characteristics of structural woody habitat in a lowland river in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koster, W., and Crook, D. (2008). Diurnal and nocturnal movements of river blackfish (Gadopsis marmoratus) in a south-eastern Australian upland stream. Ecology Freshwater Fish 17, 146–154.
| Diurnal and nocturnal movements of river blackfish (Gadopsis marmoratus) in a south-eastern Australian upland stream.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: an Introductory Guide.’ (Murray–Darling Basin Commission: Canberra.)
Maddock, I., Toms, M., Jonson, K., Dyer, F., and Lintermans, M. (2004). Identifying the influence of channel morphology on physical habitat availability for native fish: application to the two-spined blackfish (Gadopsis bispinosis) in the Cotter River, Australia. Marine and Freshwater Research 55, 173–184.
| Identifying the influence of channel morphology on physical habitat availability for native fish: application to the two-spined blackfish (Gadopsis bispinosis) in the Cotter River, Australia.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Stuart, I. G. (2003). Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Research and Applications 19, 697–719.
| Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system.Crossref | GoogleScholarGoogle Scholar |
MDBA (2010). ‘Guide to the Proposed Basin Plan: Overview.’ (Murray–Darling Basin Authority: Canberra.)
MDBC (2004). ‘Native Fish Strategy For the Murray Darling Basin 2003–2013.’ (Murray–Darling Basin Commission: Canberra.)
Mercer, D., and Marden, P. (2006). Ecological sustainable development in a ‘quarry’ economy: one step forward, two steps back. Geographical Research 44, 183–203.
| Ecological sustainable development in a ‘quarry’ economy: one step forward, two steps back.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Beatty, S. J., Bennett, J. C., Crook, D. A., Ikedife, D. N. E. N., Kennard, M. J., Kerezsy, A., Lintermans, M., McNeil, D. G., Pusey, B. J., and Rayner, T. (2011). Climate change and its implications for Australia's freshwater fish. Marine and Freshwater Research 62, 1082–1098.
| Climate change and its implications for Australia's freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. J., Barker, R. J., Koehn, J. D., and Burgman, M. A. (2007). Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis Cuvier. Biological Conservation 138, 30–37.
| Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis Cuvier.Crossref | GoogleScholarGoogle Scholar |
O'Connor, J. P., and Zampatti, B. P. (2006). Spawning season and site location of Gadopsis bispinosus Sanger (Pisces: Gadopsidae) in a montane stream of southeastern Australia. Transactions of the Royal Society of South Australia 130, 227–232.
Overton, I. C., Jolly, I. D., Slavich, P. G., Lewis, M. M., and Walker, G. R. (2006). Modelling vegetation health from the interaction of saline groundwater and flooding on the Chowilla floodplain, South Australia. Australian Journal of Botany 54, 207–220.
| Modelling vegetation health from the interaction of saline groundwater and flooding on the Chowilla floodplain, South Australia.Crossref | GoogleScholarGoogle Scholar |
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Marine and Freshwater Research 62, 1015–1026.
| Effects of climate change on fish reproduction and early life history stages.Crossref | GoogleScholarGoogle Scholar |
Pittock, B. (2003). ‘Climate Change: an Australian Guide to the Science and Potential Impacts.’ (Australian Greenhouse Office: Canberra.)
Pittock, J., and Finlayson, C. M. (2011). Australia's Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change. Marine and Freshwater Research 62, 232–243.
| Australia's Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKksbo%3D&md5=c87b710ca2ef52d8bbf6ae5aad474251CAS |
Poff, N. L. (1997). Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16, 391–409.
| Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., and Zimmerman, J. K. H. (2010). Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55, 194–205.
| Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Bay, L. K., Gehrke, P. C., Koehn, J. D., Osborne, K., Pressey, R. L., Sweatman, H. P. A., and Wachenfeld, D. (2011). Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1062–1081.
| Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Prime Minister's Science, Engineering and Innovation Council (PMSEIC) (2007). Climate change in Australia: regional impacts and adaptation – managing the risk for Australia. Report prepared for the Prime Minister's Science, Engineering and Innovation Council, Canberra.
Pusey, B. J., and Arthington, A. H. (2003). Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54, 1–16.
| Importance of the riparian zone to the conservation and management of freshwater fish: a review.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Kennard, M. J., and Arthington, A. H. (2004). ‘Freshwater Fishes of North-eastern Australia.’ (CSIRO Publishing: Melbourne.)
Rahel, F. J., and Olden, J. D. (2008). Assessing the effects of climate change on aquatic invasive species. Conservation Biology 22, 521–533.
| Assessing the effects of climate change on aquatic invasive species.Crossref | GoogleScholarGoogle Scholar |
Rayner, T. S., Pusey, B. J., and Pearson, R. G. (2008). Seasonal flooding, instream habitat structure and fish assemblages in the Mulgrave River, north-east Queensland: towards a new conceptual framework for understanding fish-habitat dynamics in small tropical rivers. Marine and Freshwater Research 59, 97–116.
| Seasonal flooding, instream habitat structure and fish assemblages in the Mulgrave River, north-east Queensland: towards a new conceptual framework for understanding fish-habitat dynamics in small tropical rivers.Crossref | GoogleScholarGoogle Scholar |
Roberts, J. (2001). Large plants. In ‘Rivers as Ecological Systems: the Murray–Darling Basin’. (Ed. W. J. Young.) pp. 187–222. (Murray–Darling Basin Commission: Canberra.)
Rourke, M. L. (2007) Population genetic structure of Murray cod (Maccullochella peelii peelii) and impacts of stocking in the Murray–Darling Basin. Ph.D. Thesis, Monash University, Melbourne.
Rourke, M. L., Teske, P. R., Attard, C. R. M., Gilligan, D. M., and Beheregaray, L. B. (2010). Isolation and characterisation of microsatellite loci in the Australian freshwater catfish (Tandanus tandanus). Conservation Genetics Resources 2, 245–248.
| Isolation and characterisation of microsatellite loci in the Australian freshwater catfish (Tandanus tandanus).Crossref | GoogleScholarGoogle Scholar |
Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., Leemans, R., Lodge, D. M., Mooney, H. A., Oesterheld, M., Poff, N. L., Sykes, M. T., Walker, B. H., and Wall, D. H. (2000). Global biodiversity scenarios for the year 2100. Science 287, 1770–1774.
| Global biodiversity scenarios for the year 2100.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhvVWltLk%3D&md5=b3fd41174f1baf7e859267a12743a59eCAS |
Sherman, B., Todd, C. R., Koehn, J. D., and Ryan, T. (2007). Modelling the impact and potential mitigation of cold water pollution on Murray cod populations downstream of Hume Dam, Australia. River Research and Applications 23, 377–389.
| Modelling the impact and potential mitigation of cold water pollution on Murray cod populations downstream of Hume Dam, Australia.Crossref | GoogleScholarGoogle Scholar |
Sternberg, D., Balcombe, S. R., Marshall, J. C., and Lobegeiger, J. (2008). Food resource variability in an Australian dryland river: evidence from the diet of two generalist native fish species. Marine and Freshwater Research 59, 137–144.
| Food resource variability in an Australian dryland river: evidence from the diet of two generalist native fish species.Crossref | GoogleScholarGoogle Scholar |
Szöllosi-Nagy, A., Najlis, P., and Björklund, G. (1998). Assessing the world's freshwater resources. Nature and Resources 16, 8–18.
Thorburn, P. J., Mensforth, L. J., and Walker, G. R. (1994). Reliance of creek-side river red gums on creek water. Marine and Freshwater Research 45, 1439–1443.
| Reliance of creek-side river red gums on creek water.Crossref | GoogleScholarGoogle Scholar |
Tockner, K., Bunn, S., Gordon, C., Naiman, R. J., Quinn, G. P., and Stanford, J. A. (2008). Flood plains: critically threatened ecosystems. In ‘Aquatic Ecosystems’. (Ed. N. V. C. Polunin.) pp. 45–61. (Cambridge University Press: Cambridge, UK.)
Tonkin, Z., King, A. J., and Mahoney, J. (2008). Effects of flooding on recruitment and dispersal of the southern pygmy perch (Nannoperca australis) at a Murray River floodplain wetland. Ecological Management & Restoration 9, 196–201.
| Effects of flooding on recruitment and dispersal of the southern pygmy perch (Nannoperca australis) at a Murray River floodplain wetland.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Lyon, J., and Pickworth, A. (2010). Spawning behaviour of the endangered Macquarie perch Macquaria australasica in an upland Australian river. Ecological Management & Restoration 11, 223–226.
| Spawning behaviour of the endangered Macquarie perch Macquaria australasica in an upland Australian river.Crossref | GoogleScholarGoogle Scholar |
Verschuren, D., Tibby, J., Sabbe, K., and Roberts, N. (2000). Effects of depth, salinity, and substrate on the invertebrate community of a fluctuating tropical lake. Ecology 81, 164–182.
| Effects of depth, salinity, and substrate on the invertebrate community of a fluctuating tropical lake.Crossref | GoogleScholarGoogle Scholar |
Vörösmarty, C. J., Green, P., Salisbury, J., and Lammers, R. B. (2000). Global water resources: vulnerability from climate change and population growth. Science 289, 284–288.
| Global water resources: vulnerability from climate change and population growth.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Walker, K. F., and Zampatti, B. P. (2007). Habitat separation of Craterocephalus (Atherinidae) species and populations in off-channel areas of the lower River Murray, Australia. Ecology Freshwater Fish 16, 442–449.
| Habitat separation of Craterocephalus (Atherinidae) species and populations in off-channel areas of the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, M. D., and Williams, W. D. (1991). Salinity tolerances of four fish species from the Murray–Darling River system. Hydrobiologia 210, 145–150.
| Salinity tolerances of four fish species from the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Woods, R. J., Macdonald, J. I., Crook, D. A., Schmidt, D. J., and Hughes, J. M. (2010). Contemporary and historical patterns of connectivity among populations of an inland river fish species inferred from genetics and otolith chemistry. Canadian Journal of Fisheries and Aquatic Sciences 67, 1098–1115.
| Contemporary and historical patterns of connectivity among populations of an inland river fish species inferred from genetics and otolith chemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsleit7w%3D&md5=bae795a7812f90ac9d675828835f0400CAS |