ROWLEY REVIEW. Bird migration in the southern hemisphere: a review comparing continents
Hugh DingleA Department of Entomology and Center for Animal Behavior, University of California, Davis, CA 95616, USA.
B Present address: School of Integrative Biology, University of Queensland, St Lucia, Qld 4072, Australia. Email: rdhdingle@ucdavis.edu
Emu 108(4) 341-359 https://doi.org/10.1071/MU08010
Submitted: 4 March 2008 Accepted: 6 November 2008 Published: 9 December 2008
Abstract
To broaden perspectives and stimulate research on migration, I survey the bird species that breed in the northern hemisphere and migrate to the southern hemisphere and species that migrate within the southern hemisphere, comparing routes, seasonal patterns and life histories. Differences in the area and latitudinal extent of land masses on the two sides of the Equator influence patterns of bird migration. In contrast to birds breeding in the northern hemisphere, no land or freshwater birds breeding in the southern hemisphere migrate between continents and only a very few cross the Equator. Furthermore, except for shorebirds, few northern intercontinental migrants reach the southern hemisphere in regions south of the equatorial forest belt, because most encounter, and are filtered out by, suitable habitats en route. Australasia is an extreme case because only 10 land or freshwater migrants from the northern hemisphere regularly occur there (most are uncommon or rare) compared with 42 in Africa and 28 in South America, and no Australasian breeders enter Asia beyond Wallace’s Line. Historical geographical and oceanic barriers may be an additional factor limiting migration to Australasia. There are generally no or only slight differences in frequencies of austral migrants within foraging guilds or families across southern continents. Exceptions are rallids, with more migrants in Africa, and cuckoos and nectarivores, with more Old World than New World migrants. Austral migrations are of shorter distances than most of those of the northern hemisphere, and they appear to vary more with respect to routes and patterns. Breeding and non-breeding ranges frequently overlap. Partial migration is common, but there is no evidence that it differs in frequency from that in northern regions. Because climate is generally milder and drier in the southern hemisphere, rainfall is a more important influence on migration than in the north especially in some nomadic birds, but temperature also predicts migration frequency and pathways for many species. These patterns are similar across southern continents, but each continent has its own characteristics. Southern hemisphere migrants seem to display ecophysiologies and orientation mechanisms similar to those found in northern hemisphere species, but very few southern species have been studied. I argue that the variation present among southern hemisphere migrations provides exceptional opportunities to understand the evolution and ecology of migration systems. In order to take advantage of these systems, we need to focus on variation in movement behaviour, on associated syndromes of traits, and on the particular features of natural selection and ecology setting thresholds that lead to the diverse migration patterns observed.
Introduction
‘… the position of the continents and the habitats they contain serve as the template from which all aspects of migration develop.’ (Faaborg 2005, p. 129)
A glance at a map of our planet immediately reveals a striking difference between the northern and southern hemispheres. There is a massive disparity between the land areas of these two halves of Earth. The southern hemisphere is occupied by all or parts of four continents but of these, two – Africa and South America – extend north of the Equator, predominantly so in the case of Africa, and one, Antarctica, is only sparsely inhabited by living organisms. Excluding Antarctica, the southern hemisphere contains only about one-fifth of the land area of the northern hemisphere (Anon. 2000) and very little of this extends into temperate regions. In this review I ask whether this disparity in land area and its latitudinal extent contribute to patterns in the distribution and biology of migratory birds in the southern hemisphere.
My aims in this paper are, firstly, to assess the bird species that breed in the northern hemisphere and migrate into the southern hemisphere and, secondly, to compare the three southern continents with respect to migrations within them. There is still a paucity of information on southern hemisphere bird migrants (Fullagar et al. 1988; Jahn et al. 2004) when compared with the northern hemisphere. Nevertheless, several aspects of southern hemisphere land areas make the analysis of migration from the north and within southern continents potentially interesting (Dingle 2004). In contrast to the northern hemisphere very little of the southern hemisphere is subject to severe winters (outside Antarctica), and this occurs mostly in high mountain regions of limited area. Much of the biogeography and ecology of the southern continents is a function of aridity. Most deserts are a consequence of descending air masses in the vicinity of latitudes 25–30° that warm as a result of compression and absorb moisture (Alerstam 1990; Dingle 1996); these latitudes comprise much of the southern hemisphere land masses. Cold ocean currents along the west coasts of continents are a further contributing factor to desertification, especially in Africa and South America. Because avian breeding in arid climates is frequently tied to erratic rainfall, migration routes can be complex and variable, although often with an underlying north to south pattern (Nix 1976; Jones et al. 2000; Griffioen and Clarke 2002; Cheke and Tratalos 2007). Restricted land area and mild but dry climates can potentially lead to migratory behaviour and ecology that is distinct for the region and among the continents that lie within the region.
In addition to climate and area, the relative position of land masses may further influence patterns of migration. Southern South America lies to the east of North America and the two continents are separated by the narrow Isthmus of Panama and the Caribbean Sea. The southern hemisphere portion of South America is bounded on the north by the rainforests of the Amazon Basin. These forests effectively separate the southerly areas of temperate climate from areas north of the Equator. Africa lies due south of the western Palaearctic, but it is separated from the latter by the Mediterranean. A further separation of southern Africa results from the Sahara Desert and the West African–Congo forest belt, which extends eastward to the Lake Victoria Basin and the Rift Valley lakes to the south. Australasia (New Guinea, Australia and New Zealand) lies on the Australo-Papuan tectonic plate, the only plate lying almost entirely within the southern hemisphere. It lies considerably to the east of mainland Eurasia from which it is separated by large water gaps and the scattered islands of Wallacea and the Sunda Shelf. It is the driest of the southern regions, with ~70% of Australia receiving <500 mm of rainfall per year (Kingsford and Norman 2002). These ‘position effects’ and climate may interact with land area and distance to influence avian migration.
In reviewing the phenomenon of migratory behaviour in the southern hemisphere, I confine myself to land birds and to those birds dependent on fresh water or continental margins like shorebirds, because these are the species most influenced by the characteristics peculiar to the southern hemisphere. The very different ecology of oceanic birds, driven by worldwide geographical features, deserves its own treatment and is beyond the scope of this review. I further confine myself to birds that migrate to or breed in the regions south of the Amazon forests in South America and south of a line formed by the Kunene and Zambezi Rivers in southern Africa or approximately south of 15°S on both continents (Table 1; Joseph 1996; Sinclair et al. 2002). Areas to the north of these boundaries reflect conditions that occur on both sides of the Equator rather than reflecting those primarily prevalent in the southern hemisphere. I shall first consider the migrants that spend northern winters on southern continents and ask what factors may limit their distributions in the south. I shall then consider migrations confined to the southern hemisphere and ask what differences or similarities in pattern and life histories may exist when comparing across hemispheres and within the southern hemisphere. Finally, I shall examine what conclusions can be drawn and how we should approach future analyses and the implications for solving problems in the biology of avian movements. The different patterns of movement likely to be present in the southern hemisphere mean that, first, attention should be given to defining what is meant by ‘migration’.
|
Defining migration
Traditional views of bird migration are strongly influenced by the seasonal round-trip journeys of northern hemisphere migrants occurring at both intra- and inter-continental scales. Definitions of migration by ornithologists usually incorporate such seasonal round-trip patterns as the chief criterion (e.g. Newton 2003, 2008; Allaby 2004; Salewski and Bruderer 2007). There are, however, long-distance movements in southern hemisphere birds (and some in northern hemisphere birds; see Newton 2006) that are complex and variable and not the classic single-year round trips typically used to define bird migration. For example, such movements may not involve return trips in the same year (e.g. African Black Oystercatchers (Haemotopus moquini); Hockey et al. 2003, and below) or to the place of last breeding (Red-billed Quelea (Quelea quelea); Cheke and Tratalos 2007). Nevertheless the birds that undertake them display behavioural and physiological characteristics otherwise typical of migrants (Cooke and Munro 2000; Griffioen and Clarke 2002; Hockey et al. 2003; Cheke and Tratalos 2007). Furthermore there are well-known long-distance seasonal round trips that are clearly not migratory, such as the multi-day foraging excursions of albatrosses and petrels (e.g. Weimerskirch et al. 1994). These foraging trips are sometimes specifically excluded from definitions of migration but without clear criteria for such exclusion (e.g. Salewski and Bruderer 2007). The variability in the pathways of southern hemisphere birds in particular makes it desirable to have criteria for defining migration independent of pathway.
I have discussed extensively the need for a behavioural definition of migration as distinct from its ecological function because natural selection acts on variation in the movement behaviour of individuals and the syndrome of its associated physiological and life-history traits (see also Bell 2000; Sih et al. 2004; Jahn et al. 2006). This behavioural perspective and the details of its rationale are covered more formally and at greater length elsewhere (Kennedy 1985; Dingle 1996, 2006; Dingle and Drake 2007). I provide a brief summary here as it applies to the variation in southern hemisphere migration as discussed in this review.
By focusing specifically on the behaviour of individual migrants, we can ask what particular characteristics of migration distinguish it from other movements. Several are apparent. Many observers of migratory birds in passage have noted how undistracted they seem (Dingle 1996, and references therein). Except when low on fat reserves, and therefore not in a migratory state (Kennedy 1985), migrants do not stop to feed, even in the presence of rich food sources. Many migrants fly at night, foregoing sleep to proceed with their journeys. There are many temporary metabolic shifts or changes in gut morphology that add energy (primarily fat) and reduce weight (gut shrinkage) (Piersma and Lindstrom 1997; McWilliams and Karasov 2005; Ramenofsky and Wingfield 2007). Flight is along continuous tracks and sometimes at altitudes that contrast sharply with the haphazard, brief, localised movements of daily life, such as foraging (e.g. Winkler 2006). A behavioural definition of migration thus must incorporate these traits and exclude other types of movement. Briefly, then, migration is undistracted and largely straightened-out movement with temporary inhibition of the ‘station-keeping’ responses that accompany maintenance and reproduction (Kennedy 1985; Dingle 1996; Dingle and Drake 2007). This inhibition likely also primes responses that terminate migration. Note that routes and distances travelled are important ecological outcomes or functions of the behaviours that produce migration (and provide important feedback from selection), but they do not define migration (see Gatehouse 1987 and Dingle and Drake 2007 for full discussions of behaviour v. ecological outcomes). To the extent allowed by observations and data, I use this behavioural definition as a rule of thumb to determine if particular species or groups of southern hemisphere birds are in fact migrants. Where this is not possible, I accept at face value statements regarding migratory status. Finally, although the term ‘austral migrant’ is often used to mean migrants in South America (e.g. Chesser 1994; Jahn et al. 2004), I follow Hayes (1995) and use it here as a term for migration by birds breeding anywhere within the southern hemisphere.
Studies of phylogenies fail to reveal a deeply embedded ancestral pattern to migration (Piersma et al. 2005). Rather it is remarkably flexible with migratory syndromes arising as needed by incorporating or modifying traits that already exist, like flight (Dingle 2006), and bearing little relation to relatedness among species or even of subspecies within lineages (Helbig 2003; Leisler and Winkler 2003; Outlaw et al. 2003; Joseph 2005; Outlaw and Voelker 2006). It is a threshold trait that can respond proximately to environmental variation (Rappole et al. 2003) with the threshold at which it becomes advantageous set by natural selection (Sutherland 1998; Pulido 2007; Roff and Fairbairn 2007). When phylogeny does influence migration, it tends to act through other aspects of behaviour and ecology, most notably through habitat and foraging associations (e.g. Böhning-Gaese and Oberrath 2003; Boyle and Conway 2007).
Overview: migration in the southern hemisphere
Northern hemisphere breeders that overwinter in the southern hemisphere
Shorebirds
Shorebirds that breed in the northern hemisphere migrate to all three southern continents. Most species are strong flyers capable of long over-water flights. Some Bar-tailed Godwits (Limosa lapponica), for example, fly non-stop for up to 11 000 km from Asia or Alaska to Australia or New Zealand (Piersma and Gill 1998; Wilson et al. 2007; Battley 2008; Battley et al. 2008). Many species make use of widely scattered islands while in transit (White 1975). Shorebirds are thus not likely to be limited in travel by ocean crossings or other barriers. They occupy sites in coastal areas or in interior plains and wetlands, habitats that are readily available over all the land masses under consideration. The occurrence of shorebirds as long-distance migrants from the north is thus not surprising.
Many species of shorebird are widespread breeders across the tundra regions of northern high latitudes. One result is considerable overlap among continents of species and genera migrating into the southern hemisphere to spend northern winters (Hayman et al. 1986; Clements 2007). Similar numbers of species regularly travel to the three southern continents, with 33–35 species migrating to Australasia (Dingle 2004), 26–30 to southern Africa (Sinclair et al. 2002), and 24–26 to southern South America (Hayman et al. 1986; Clements 2007). Three American species that breed primarily in Alaska, the Wandering Tattler (Tringa incana), the Pacific Golden Plover (Pluvialis fulva), and the Bristle-thighed Curlew (Numenius tahitiensis) migrate to the islands of the Pacific, including New Zealand (Hayman et al. 1986). Numbers of species migrating to each continent are not exact because a few are so rare that it is not clear whether they are rare but regular or only casual travellers or vagrants to the southern regions.
Five species migrate to all three southern continents: Grey (also known as Black-bellied) Plover (Charadrius squatarola), Whimbrel (Numenius phaeopus), Ruddy Turnstone (Arenaria interpres), Sanderling (Calidris alba) and Pectoral Sandpiper (Calidris melanotos). The Pectoral Sandpiper is interesting because the great majority of the Siberian population joins Nearctic birds to winter in South America; only a few migrate to Africa or Australasia (Hayman et al. 1986). In several genera there are in the New World substitutions of species corresponding to Old World counterparts. This is the case for some smaller plovers (Charadrius), Actitis sandpipers, species of Tringa sandpipers and the smaller Calidris stints, godwits (Limosa) and curlews (Numenius). Thus for any given southern continent the shorebird wintering avifauna looks more or less like that of the other two continents. This no doubt reflects the similar ecology and behaviour of shorebird species, leading to worldwide colonisation of widespread habitats.
The particular continental assemblage of wintering shorebirds comes largely from those breeding in the northern hemisphere region centred most directly to the north. The much greater longitudinal spread of the breeding grounds means there is a funnelling of migration routes to the narrower southern wintering areas and often high concentrations of birds at favoured sites like Roebuck Bay in Western Australia, San Antonio Oeste in Argentina, and the lagoons around Walvis Bay in Namibia (Priest et al. 2002; Hockey et al. 2003; González et al. 2006). The migratory divides that separate the flyways reflect geography, with birds migrating to the nearest wintering areas to the south, even Alaskan species migrating to Pacific islands. There are interesting exceptions, like the Pectoral Sandpiper noted above and the Ruddy Turnstone, in which birds from Greenland and north-eastern Canada cross the Atlantic to migrate south through western Europe (Hayman et al. 1986). These are presumably ancestral pathways that have not been under selection to change.
The migratory divide for shorebirds breeding across northern Eurasia is approximately in the centre of that continent, although with an easterly bias. In Australasia most of the wintering shorebirds appear to breed in north-eastern Eurasia and to some extent in far north-western Alaska (Lane 1987; Dingle 2004). Birds breeding to the west migrate to Africa, India and the Indian Ocean islands in the southern hemisphere (Hayman et al. 1986). The eastern source regions are clearly apparent for species that breed more or less exclusively in north-eastern Siberia and northern China, such as the Eastern Curlew (Numenius madagascariensis), or in Japan and adjacent islands like Latham’s Snipe (Gallinago hardwickii) (Blakers et al. 1984). Biometrical data from the Grey Plover suggest that birds banded in Australia breed in Siberia east of the Lena River (Minton and Serra 2001). Bar-tailed Godwits winter widely from southern China to New Zealand. The race baueri from northern and western Alaska migrates across the Pacific to eastern New Guinea, eastern Australia and New Zealand (Blakers et al. 1984; Hayman et al. 1986; Wilson et al. 2007), whereas the race menzbieri breeding in north-eastern Russia migrates to Western Australia (Wilson et al. 2007). Other examples migrating from eastern Siberia to Australia include Ruddy Turnstone, Whimbrel and Black-tailed Godwit (Limosa limosa) (Blakers et al. 1984).
Land and freshwater birds
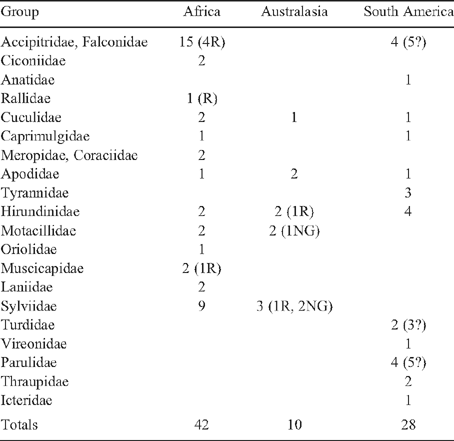
In contrast to shorebirds, the numbers of wintering migrant species of land and freshwater birds do differ among continents. The three regions are compared in Table 1. The largest number of northern hemisphere breeders migrating to the southern hemisphere occurs in southern Africa, with 42 species compared with 10 and 28 species in Australasia and southern South America respectively. One species, the Barn Swallow (Hirundo rustica), is represented on all three continents. The two most numerous migrants to Africa are the raptors and the Old World warblers. Fifteen species of raptor migrate to southern Africa, although four of these are rare; greatest representation is by soaring hawks and eagles (Accipitridae) and strong-flying falcons (Falconidae), including Eleonora’s Falcon (Falco eleonorae) to Madagascar (Bildstein 2006). Some of the migrations are spectacular. The Amur Falcon (Falco amurensis), for example, migrates from eastern Eurasia, including an open-water flight of several thousand kilometres over the Indian Ocean (Fry 1992). Albeit rare in southern Africa (they are common in East Africa), a few Northern Wheatears (Oenanthe oenanthe) do reach there on journeys from as far east as western Alaska, and Willow Warblers (Phylloscopus trochilus) can come from east of 75°E in Siberia as well as from the western Palaearctic (Fry 1992; Pearson and Lack 1992). Like shorebirds, most wintering migrants to Africa breed in the more western parts of Eurasia.
Both Australasia and southern South America are depauperate in northern migrants compared with southern Africa (Table 1). Most conspicuous by their absence are migrant raptors. None migrates to Australasia from Asia, not even powerful flyers like the Peregrine Falcon (Falco peregrinus), although that species is resident in Australia. Only four, possibly five, do so in South America: Swainson’s Hawk (Buteo swainsoni) and the Peregrine Falcon seem to migrate the farthest south, and both have been tracked by satellite to the pampas of Argentina (Fuller et al. 1998; Bildstein and Zalles 2005; Bechard et al. 2006). Cuckoos (Cuculidae), swifts (Apodidae) and swallows (Hirundinidae) occur on all three continents, and groups like flycatchers and warblers are represented by a few ecologically similar species from different families (e.g. Old World flycatchers (Muscicapidae) and tyrant-flycatchers (Tyrannidae)). Migrants are even more underrepresented in Australasia when considering the fact that three species migrate only to New Guinea and two more are rare vagrants (Christidis and Boles 2008). Even the ubiquitous Barn Swallow normally migrates no farther than the north-western parts of Australia and is not particularly common. Only two aerial predators, the Fork-tailed Swift (Apus pacificus) and White-throated Needletail (Hirundapus caudacutus), migrate as far south as Tasmania. The Needletail is confined mostly to higher rainfall regions east and south of the Great Dividing Range, whereas the Fork-tailed Swift occurs over most of Australia (Barrett et al. 2003; Dingle 2004; Higgins et al. 2006).
Within regions there is attenuation of northern migrants towards the southern margins. In Australasia, the few species are represented primarily or exclusively in New Guinea or along the northern rim of Australia. Other than the swifts, the only species reaching even mid-continent is the Oriental Cuckoo (Cuculus optatus) (Pizzey and Knight 2007). In southern Africa abundances are lower in the drier western regions as well as in the south (Underhill et al. 1992). Three exceptions to the thinning of populations in the south of Africa are the Barn Swallow, the Common (Western Steppe) Buzzard (Buteo buteo vulpinus), and the White Stork (Ciconia ciconia). The first is evenly distributed throughout the region except for reduced densities in the drier north-west, while the Stork and the Buzzard actually increase in numbers towards the south (Underhill et al. 1992). The White Stork is also interesting because it maintains a small breeding population in the Western Cape and many migrants remain during the southern winter. The limited data for South America indicate higher densities of migrants along a corridor on the eastern slope of the Andes relative to farther east in addition to an attenuation of numbers to the south (Chesser 2005).
Why do so few Holarctic migrants migrate to the southern hemisphere and why the contrast among continents?
The few northern hemisphere breeders that migrate to higher southern latitudes (Table 1) are a very small proportion of the available pool of migrant species. From the Palaearctic, for example, over 200 species of land and freshwater birds migrate south for the winter (Fry 1992), yet only ~42 of these reach southern Africa and only 10 reach Australasia. In the Palaearctic–African system, 70 or so species migrate usually only as far south as Mediterranean Europe or the North African coastal lowlands. Some 130 species winter south of the Sahara (Fry 1992), of which 89 are recorded as wintering in East Africa (Pearson and Lack 1992). Thus, only about one-third of the total in sub-Saharan Africa or half of those in East Africa migrate to regions south of the Kunene–Zambezi River line, and in the eastern Palaearctic the overwhelming majority of species winter in South-east Asia or the Indian Subcontinent, migrating no farther south than the islands of the Sunda Shelf (Dingle 2004). In the Nearctic, a similarly large pool of migrants winters mostly in Central America, the Caribbean islands, and northern South America (De Graaf and Rappole 1995). The 28 species migrating to latitudes south of the Amazon forests represent only ~15% of the migrant pool.
I now examine five broad hypotheses to account for the small numbers of northern hemisphere breeders reaching the southern hemisphere: area, distance from the breeding grounds, barriers, available habitat, and history.
Area
In discussions of latitudinal gradients of species richness, the so-called ‘area model’ has received much attention (Rosenzweig 1995). The model assumes that the largest ecoclimatic area, the tropics on both sides of the Equator, will generate the greatest species diversification because greater range sizes with more niches create more opportunities for speciation. In spite of caveats, the model is still considered important, although not a sufficient explanation for latitudinal patterns in taxon richness (Chown and Gaston 2000). Given that the southern hemisphere land area under consideration is small relative to the northern hemisphere and tropics and might therefore have fewer niches for wintering species, does its reduced size contribute to the marked reduction in the taxon richness of wintering migrants?.
In the western Palaearctic–African system the lowlands on both sides of the Mediterranean and Africa between the southern edge of the Sahara and the Kunene–Zambezi line comprise almost four times the land area south of the two rivers. (I exclude the Sahara as unsuitable for wintering migrants.) As the northern area contains over four times the number of wintering migrant species, there is a rough correspondence between land area and number of Palaearctic migrants. However, if one considers just the area between southern Africa and the Equator, which about doubles the area north of far southern wintering grounds, some 130 species are accounted for, tripling rather than doubling the number of wintering migrants. Thus there is no compelling evidence that area per se is an explanation for the difference in number of migrant species occurring, although it cannot be completely excluded. In both Australasia and the New World the wintering areas under consideration are smaller than the wintering areas to the north, but the differences are not great enough to account for the marked reductions in numbers of wintering species. Again, area alone seems an insufficient explanation for species subtraction.
Distance
All the southern regions are obviously farther from the breeding grounds than are the wintering areas to their north. Species subtraction does occur with increased migration distance, and at least in Africa this is a statistically significant relationship (Hockey 2000). There is even more rapid attenuation in South America and Australasia. Given the migratory capabilities of most species, however, it is not likely that the added effort to reach the more southerly wintering grounds would be a limiting factor. Many Nearctic species fly for long distances over the Atlantic or the Caribbean, and in the western Palaearctic flights over the Sahara are common (Dingle 1996 and references therein). Intermittent shorter flights could easily carry migrants the added distance south in sub-Saharan Africa. Distance is probably a surrogate for other more direct causative factors contributing to the attenuation of species numbers, although it probably interacts with some of these.
Barriers
For each of the southern continents there is at least one apparent barrier between northern breeding areas and southern wintering areas. In the New World a barrier is formed by the Gulf of Mexico and Caribbean Sea, in Africa by the Mediterranean and the Sahara (and in West Africa by the Gulf of Guinea), and in Australasia by the South China and Philippine Seas and water gaps among the islands of the Sunda Shelf and Wallacea. The potential of tropical rainforests as barriers in the three regions will be discussed in the next section on habitats.
Migrants from the Nearctic and the western Palaearctic can cross barriers along their migration pathways. Nearctic migrants not only cross the Caribbean and Gulf of Mexico (Gauthreaux 1991) but in the autumn many fly out over the Atlantic around the Bermuda high-pressure cell before making landfall in the Antilles or on the northern coast of South America (Richardson 1985, 1990; Williams and Williams 1990). They are aided by the tail winds generally present at the time of migration, whether in spring or autumn (summarised in Dingle 1996). Because they use soaring flight to aid migration, New World raptors avoid over-water crossings where updrafts are largely absent and migrate instead through Central America with often spectacular concentrations along the Caribbean coastline and through the Isthmus of Panama (Kerlinger 1989; Bildstein and Zalles 2005; Bildstein 2006). In the western Palaearctic, raptors and other soaring migrants like storks likewise avoid over-water crossings by flying around the Mediterranean at both ends, again with mass movement at sites such as Gibralter and the Bosporus (Bildstein 2006).
The passage of smaller migratory birds to Africa is varied and complex, but they possess mechanisms to deal with the Mediterranean and the Sahara. Many species fly around the Mediterranean but some cross directly, probably by island hopping (as indeed do some raptors). The Sahara is crossed with a combination of favourable winds and rest stops either at oases or probably more often just seeking shade to avoid prohibitively high daytime temperatures (Bairlein 1988, 1992; Biebach 1992; Dingle 1996). Suffice it to say, in both the Nearctic and the western Palaearctic migratory birds engage the necessary tactics to deal with barriers imposed, so these are not responsible for the subtraction of species in southern Africa or South America. The attenuation occurs primarily after major barriers have been crossed.
In Australasia, the oceanic barriers between this region and Asia probably do contribute to the large species subtraction in the eastern Palaearctic migrants (Dingle 2004). Raptors, owing to their need to soar, would be the most obvious species inhibited from journeying to Australasia by ocean crossings, and none do so (Table 1), even in well-known migrants like the Chinese (Accipiter soloensis) and Japanese (Accipiter gularis) Sparrowhawks (Bildstein and Zalles 2005) and the Peregrine Falcon. Where over-water flights by migrants occur, they are associated with assisting tail winds, as noted for the Nearctic. For birds migrating on a predominantly north–south axis from north-eastern Eurasia to South-east Asia, an over-water crossing to Australasia would require a prominent ‘left turn’, followed by a ‘right turn’ for any proceeding on to Australia. Such dramatic changes in direction along migration routes are exceptional at the least. In this part of the world any such route is also hindered by wind patterns. Immediately north and south of the Equator autumn migrants would be flying into easterly trade winds, and along the Equator they would be experiencing the still air of the ‘doldrums.’ In either case there would either be no wind assistance or wind opposition when migrating to wintering grounds in Australasia. An evolutionary shift in migration pathway requiring an extension of journeys, two orthogonal changes in direction, and long flights over water against prevailing winds seems unlikely. In this case, then, a barrier probably contributes to species subtraction.
Habitats
Habitats influence migration flyways both en route and at destinations. Several authors have noted differences in the primary habitat associations of migrants among the three major flyways (e.g. Leisler 1992; Mönkkönen et al. 1992; Hockey 2000, 2005; Boyle and Conway 2007), thus raising the question of what influences habitats may have in the different southern continents. In the western Palaearctic most migrants are denizens of early successional stages and more open habitats. In contrast, in the Nearctic most long-distance migrants to South America are forest-associated species, albeit more likely to occur in more climatically exposed ‘unbuffered’ forest edge habitats (Chesser and Levey 1998). Asian migrants tend to predominate in unbuffered forest or woodland and in the wetter of more open areas. Old World migrants are similar to each other in being habitat selective, occurring in preferred habitats even when these are of smaller total area. Nearctic migrants tend to occur in habitats in proportion to their availability (Hockey 2005). As Hockey (2005) points out, however, we have not resolved how to describe habitats in a way that has predictive power.
Even with limitations to describing habitats, it still seems that habitat has an effect on the numbers of northern hemisphere breeding species migrating to more southern regions. In Africa, suitable open habitats of the sort preferred by migrants occur throughout the eastern half of the continent south of the Sahara. There is a progression of increasing suitability from north to south as a function of the timing of rainfall caused by the passage of the Inter-Tropical Convergence Zone (Pearson and Lack 1992). As migrants move through eastern Africa with the rains, it can take five months, beginning in September, to reach the far south; there is attenuation of species as a result. In this case habitat acts mainly as a filter, with the suitable habitats of more northerly areas progressively removing species as the wave of migrants moves south (Pearson and Lack 1992). Even though there are suitable wintering habitats in the south, many migrants never reach them. The conspicuous exceptions are the raptors whose ability to cover long distances by energy-efficient soaring makes filtering less likely, and the southern veldts and savannas provide them with excellent conditions once they arrive.
A similar filtering effect probably occurs in the New World. Central America, the Caribbean islands, and northern South America provide extensive areas of the unbuffered forest edge and semi-open habitat favoured by Nearctic breeding migrants. These habitats extend southward along the eastern slopes of the Andes providing a corridor that does allow some forest-edge migrants to penetrate at least as far as Bolivia. The open habitats of South America are generally unsuitable for northern migrants, again with the exception of raptors and some swallows, and so do not provide wintering opportunities. A combination of habitat filtering and unsuitable habitats thus seems to prevent most Nearctic migrants from reaching southern South America.
In Australasia there is little additional habitat suitable for migrants (Dingle 2004). The forests of New Guinea and north-eastern Australia cover a small area relative to those in South-east Asia and the islands of Sunda Shelf. Asian forests can thus act as an effective filter, removing migrants before they reach the reduced areas of forest farther east. The forests of eastern Java and Wallacea are drier than those to the west and may pose a barrier to some migrants (Mayr 1944), although few land bird migrants even occur in Wallacea (White 1976, 1977). The large open areas on the Australian continent itself are semi-arid or arid whereas those occupied by Palaearctic migrants are much more mesic. The shrublands, open woodlands and deserts of Australia thus do not provide habitat suitable to northern migrants. Filtering and the absence of wintering habitat in Australasia are probably both significant factors in the virtual absence of Palaearctic migrants wintering there.
History
The flexibility of migration (Rappole et al. 2003) and the absence of a deeply embedded ancestral pattern (Piersma et al. 2005) mean that many migratory pathways observed today are likely to be of geologically recent origin. Nevertheless, even in the fairly recent past, characterised by repeated glaciations, the continents have varied in shape and extent with changes in sea level. This was particularly so with the South-east Asian–Australasian region, as the Sunda Shelf and the Australasian continental shelf were repeatedly exposed and flooded (Johnson 2004). Much of Africa was wetter for periods, including what is now the Sahara. The drying out of the Sahara does not seem to have interposed an insurmountable barrier to Palaearctic–Africa migrants (above), but rising sea levels may have been a factor interrupting a Palaearctic–Australasian flyway. Clearly some Asian species colonised Australasia, because several migrants like the cuckoos and swallows, the Dollarbird (Eurystomus orientalis) and the Rainbow Bee-eater (Merops ornatus) are of Asian origin. Some are closely related to or are the same species as Asian counterparts, but they do not leave Australasia on migration (Dingle 2004; and above). Rising sea levels and changes in climate following periods of glaciation may have contributed to isolating them in Australasia because they do not now cross the Wallacean gap. Particularly interesting is the Peregrine Falcon, highly migratory in the northern hemisphere, including Asia, but sedentary in Australia (Marchant and Higgins 1993). Australia’s two subspecies of Peregrine, and one in New Caledonia (Clements 2007), are presumably descended from ancestors arriving as migrants from Asia – a form of ‘migratory dosing’ as described by Bildstein and Zalles (2005).
In the New World, the origins of some present migration routes may go further back in time. The dominant group of North American breeding migrants is the parulid warblers (Parulidae). The Mexican Plateau is the likely staging area for the evolution of migration in this, and probably other, groups of North American migrants, with migration arising as a consequence of a balance between competition with residents and time available for breeding if migrating (Cox 1985). A question is then why migrate only to the north to breed and not also to the south? The answer may lie in competition. The parulids in particular are largely foliage gleaners, and tropical regions occupied in the non-breeding season contain many breeding species of similar ecological habits, like the foliage gleaning tyrannids (Chesser 2005). Mortality data indicate that tropical habitats can support many more non-breeders than breeders (see Dingle 1996 for summary) so that migrants can be maintained but competition from breeding residents may prevent them from breeding (but see Salewski and Bruderer 2007 for an alternative view). The forest habitat requirements of these northern hemisphere breeders would prevent them from migrating to the more open areas south of Amazonia. These open and semi-open areas contain their own breeding species migrating north to the tropics to winter so that competition in this region may supplement habitat requirements in deterring occupation by wintering northern migrants.
To summarise, travel distance and area per se are probably not significant factors in the paucity of Holarctic migrants in the southern hemisphere. Barriers such as ocean or desert are probably not major causes of this paucity in the Nearctic or the western Palaearctic. For Australasia, however, the combination of water gaps, the location well east of Asia and the prevailing wind patterns in the vicinity of the Equator means that a barrier is probably important. Historically this barrier is ancient, and it broadened again when sea levels last rose, isolating Asian species that had managed to colonise Australasia when the sea level was low. In all three southern continents filtering by habitats between breeding and southern wintering areas reduces the number of migrants. In South America and Australasia there are also fewer wintering habitats to attract them. In the New World it is possible that competition also had a role historically in deterring northern migrants from wintering in southern South America.
Austral migration – migration within the southern hemisphere
Comparisons among bird groups and continents
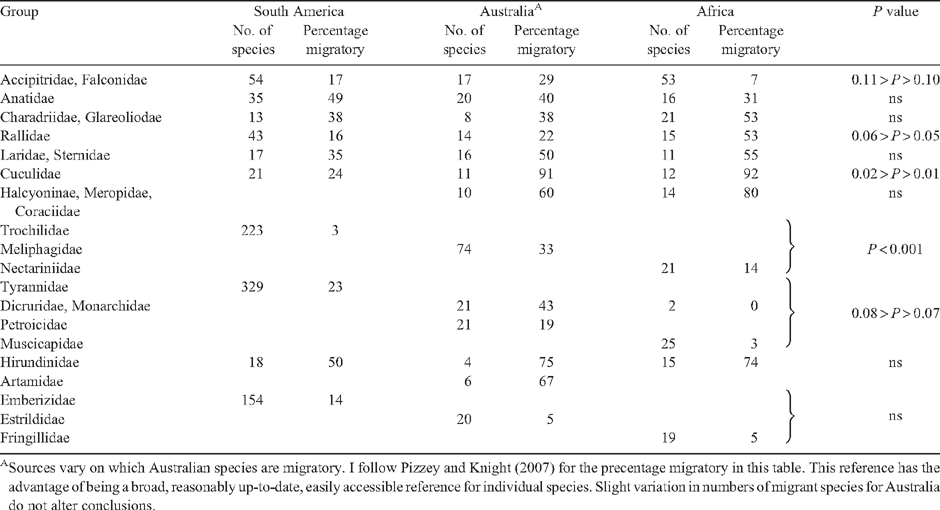
Many southern hemisphere species breeding in the regions under consideration migrate entirely or almost entirely within the southern hemisphere itself. The major groups of birds with such austral migration are listed in Table 2, which indicates the number of species in each group and the percentage of those species that are austral migrants. Because samples would not allow meaningful comparisons, groups or families with fewer than 10 species on any continent, large groups with no or only one or two austral migrants, and migrants that are confined to a single continent without ecological counterparts migrating on the others (as in the parrots and cockatoos (Psittaciformes) of Australia) are excluded from the Table. In some groups confined to a single continent, like the hummingbirds (Trochilidae) of South America, comparisons are made with birds of similar ecology and foraging habits on the other continents, in this case sunbirds (Nectariniidae) in Africa and honeyeaters (Meliphagidae) in Australasia both likewise largely nectarivorous (all three families are also partly insectivorous). There are some obvious limitations to doing this, for example the disregard of possible phylogenetic or body size constraints, but such comparisons seem appropriate for a broad overview such as this. Birds breeding primarily in the forests of New Guinea and the Congo and Amazon Basins are excluded because these areas are dominated by trans-equatorial forest avifaunas that are not migratory. Thus with respect to Australasia, comparisons include Australia only. I test for statistical significance among continents using a simple χ2 contingency analysis. Owing to difficulties of categorisation and the limited sample sizes in some cases, however, the results of such tests are probably best taken as guidelines only. The same limitations in data quality make more sophisticated statistical tests of doubtful value.
Starting with the Accipitridae, there are more species present in Africa and South America than in Australia. A likely reason for this difference is that the extensive mesic plains and savanna woodlands of the two larger continents provide habitat that will maintain more raptor species than the much drier woodlands and deserts of Australia. It is also possible that over geological time there were fewer invasions and subsequent colonising of Australia by precursors, a consequence of the relative isolation of the continent following the break-up of Gondwana and the difficulty, discussed above, of reaching the continent from the north (as reflected by Wallace’s Line). The apparent difference among the continents in the proportion of austral migrant raptors is significant only at approximately the 10% level. To the extent that this apparent difference might be real, the cooler temperatures in the southern areas of South America and Australia may promote some northward autumn movement by depressing winter food supplies. With adequate rainfall, some measure of cooler temperatures best predicts proportion of migrants (Newton and Dale 1996a, 1996b; Dingle et al. 2000; Hockey 2000). Factors that promote raptor abundance in Africa may act against migration somewhat. In Australia there may be some movement towards the coast in dry periods, as with the Whistling Kite (Haliastur sphenurus) (Marchant and Higgins 1993; Pizzey and Knight 2007).
Among waterfowl (Anatidae) migration is fairly common, but differences in the proportion of migrants among continents are not statistically significant. The most likely explanation for the high proportion of migrants in South America is again low winter temperatures in temperate regions. In the southernmost areas temperatures regularly dip below freezing and could cause smaller waterbodies to ice over. Unfortunately there is no detailed information on the movement patterns of South American waterfowl. Inland Australia is subject to variable and unpredictable rainfall, affecting the spatial and temporal availability of wetlands for breeding waterfowl (Kingsford and Norman 2002; Dingle 2004; Roshier et al. 2008). Eight species, or 40% of the Australian total, make often extensive but irregular migrations among wetland sites. The Grey Teal (Anas gracilis) is wide-ranging not only over Australia, but also to New Guinea, New Zealand and New Caledonia (Blakers et al. 1984; Dingle 2004). Travelling between seasonal wetlands probably occurs in Africa as well, where 31% of species are apparently migratory.
A satellite-tracking study of the Grey Teal of Australia reveals that it has an interesting set of movements (Roshier et al. 2008). These ducks evidently respond to cues such as rainfall that occur far from their current location. Some undertook long flights to regions of little surface water, which seemed to be a form of prospecting. Roshier and colleagues suggest that these flights are similar to what I have called ‘ranging behaviour’ or facultative exploratory movements to locate habitat outside the daily home-range but within the same habitat region (Dingle 1996). Other individuals flew across diverse and numerous wetlands, bypassing apparently suitable habitats, flights characteristic of true migratory behaviour (Dingle 1996, 2006; Dingle and Drake 2007; and ‘Defining migration’ above). Further analysis of this mixture of movements could provide insights into the cues and resources triggering a switch to migration from other types of movement.
Plovers (Charadriidae), rails (Rallidae), and gulls and terns (Laridae) also associate with wetlands, but primarily with shallower waterbodies and their margins and, among the plovers, with some drier uplands. Only in the rallids is there a conspicuous difference among the continents in migratory frequency. Only 16% of the diverse South American rallids are migratory, only 22% of the Australian birds, but 52% of the African. Among plovers and gulls and terns the frequency of migrants is uniformly fairly high on all three continents with no significant difference among regions.
Why the difference between Africa and the other two continents in migratory rallids? If rallid migrations were mainly a consequence of ephemeral wetlands, one might expect a higher proportion of migrants in Australia. Australian rallids, however, are distributed primarily coastally and subcoastally where wetlands are largely permanent (Barrett et al. 2003). Inland species of temporary wetlands like the Black-tailed Native-hen (Tribonyx ventralis) are irruptive in dry years (Matheson 1978; Dingle 2004). It would be interesting to know if they use a combination of ranging and migration as is apparently the case with the Grey Teal. South American species, too, generally prefer permanent wetlands, and cold in the south is probably a major factor that influences migration in species like the three coots (Fulica) whose ranges include Tierra del Fuego (Clements 2007). The significantly higher frequency of migration in African rallids is probably a response to variable wetlands, but the even more extreme variability of arid zone wetlands in Australia may limit their use on that continent (Puckridge et al. 1998; Roshier et al. 2001). More extensive analysis of habitat use in rallids and other wetland groups with respect to migration, both austral and Holarctic, should prove revealing. Note also that many Australian herons and egrets (Ardeidae), likewise occupants of ephemeral wetlands, are migratory (Dingle 2004), including some of the same cosmopolitan species that do not migrate in Africa or South America.
A big difference among continents occurs in cuckoos, with 24% of species austral migrants in South America but 90% are migratory in both Africa and Australia. The one Madagascar and two of the New Zealand cuckoos are also migrants (Heather and Robertson 1997; Sinclair and Langrand 1998). South American species are an independent radiation, whereas the African and Australian species are closely related and of probable Asian origin (e.g. several species of Chrysococcyx and Chalcites). The taxonomic and ecological overlap among the Old World cuckoos probably contributes to the similarity in migration frequencies and likely acts via habitat and foraging preferences (Hockey 2000). Migration in cuckoos increases along a gradient of increasing habitat aridity, and the Old World species occupy more open, drier habitats than those of the New World. The latter are primarily denizens of forest interiors where migrants are few in any austral group (Hockey 2000, 2005). Several South American cuckoos are ground dwellers or occupants of scrub or long grass (e.g. anis (Crotophaga)) and non-migrants of the Old World (e.g. coucals (Centropus)) occupy similar scrub habitats.
Halcyonid kingfishers (Halcyonidae), rollers (Coraciidae) and bee-eaters (Meropidae) are all sit-and-wait predators of large insects, and there are large proportions of migrants in this group in both Africa (including Madagascar) and Australia. Like the cuckoos of the same regions, they tend to occur in drier, more open habitats rather than forests. None of these families occurs in South America where the non-passerine sit-and-wait predators of large insects are families like puffbirds (Bucconidae), jacamars (Galbulidae) and motmots (Momotidae). These New World birds, like the South American cuckoos, are primarily interior tropical forest denizens, a habitat in which migration is rare.
The nectar-feeding guild reaches its highest diversity in the South American hummingbirds, with 233 species considered here (Chesser 1994; Table 2). Most species are small, including some of the smallest birds known, and display high metabolic rates to meet energy demands of flight. Most Nearctic species are highly migratory over long distances (Clements 2007), but only 3% of South American species are austral migrants. This may be because the ‘non-migrant’ species are capable of energy-conserving nocturnal torpor or are short-distance altitudinal migrants (Rappole and Schuchmann 2003), and so can survive as residents or by travelling only short distances so long as daytime temperatures permit nectar production by the flowers on which they feed. The honeyeaters of Australia and the sunbirds of Africa, likewise nectar feeders, are 33% and 14% migratory, respectively; in both cases higher than hummingbirds. The difference across continents is highly significant. The tendency of these latter groups to occur in drier habitats with patchier resources may contribute to the difference from hummingbirds. So too might differences in physiology and foraging ecology, both of which merit further study with respect to austral migration (Munro 2003; and below).
Although the hummingbirds of the New World are highly diverse, the number of species does not match the dramatic diversification of the tyrant-flycatchers, the world’s largest bird family with 435 species (Clements 2007). Of these, 329 occur in South America (Table 2). Several authors have noted that this richness of species means they contribute the most species to the austral migrant avifauna (Chesser 1994, 1998, 2005; Stotz et al. 1996; Jahn et al. 2004; Joseph 2005), some 76 out of the total of 329 species (Chesser 1994). A scan of Table 2, however, reveals that the proportion of austral migrant tyrant-flycatchers (23%) is not excessively high. I compare them with the monarch flycatchers and their allies (Monarchidae) and the Australasian robins (Petroicidae) of Australia and the drongos (Dicruridae), chats and Old World flycatchers (Muscicapidae) of Africa. Many Australasian robins (Robinson 1992) and African chats are ground foragers like the ground tyrants (Tyrannidae) of South America. These latter are a significant number of the migrants among the tyrant-flycatchers (Chesser 1994), and the Australasian robins match the tyrannids in migration frequency. The chats of Africa are particularly similar to the ground tyrants, but in contrast are largely sedentary. These chats inhabit areas at lower latitudes than much of South America and that are drier but less subject to winter cold. These milder temperatures may be a factor contributing to their lack of movement. Likewise, neither of the southern African drongos is migratory. In South America the proportion of migrants among breeding tyrannids was strongly associated with mean temperature of the coldest month and relative annual temperature range, indicating the possible importance of cool winter temperatures to the ‘flycatcher’ guild in general (Chesser 1998).
Aerial insectivores that hunt above the canopy rather than by sallying from perches are frequent austral migrants. Thus swallows vary from 50% migratory in South America to 74% and 75% in Africa and Australia (Table 2). The similar Australian woodswallows (Artamidae) are 67% migratory. The swifts match swallows in migration frequency, at least in Africa where more than 50% are either Holarctic or austral migrants (Hockey 2000). The two Holarctic species that migrate farthest into Australia are both swifts (Dingle 2004; and above). The one endemic swift in Australia and those in New Guinea inhabit tropical forests and are not migratory (Beehler et al. 1986; Pizzey and Knight 2007). Nightjars are not listed in Table 2 because there are so few species, but they are also aerial insectivores. Four of six in Africa and two of three in Australia are austral migrants, but only five of 27 (19%) in South America migrate. Many South American species occur in forest, whereas the Old World species prefer more open habitats, possibly accounting for differences in migration frequency.
The last group in Table 2 consists of three families of mostly seed-eating ‘finches’ (Emberizidae, Estrildae, Fringillidae), few of which are migrants. The highest proportion, 14%, occurs in South America with most migrants being small seed-eaters in these three families. The generally low migration frequency among these seed-eaters has been noted by Hockey (2000, 2005), although many have been reported as ‘nomads’ (Dean 2004). These nomads have not been examined carefully for characteristics of migration or ranging syndromes (Dingle 2004; Dingle and Drake 2007). Such an examination is clearly desirable in view of the fact that ‘nomads’ like crossbills (Loxia) and queleas (Quelea) are clearly migrants (Newton 2006; Cheke and Tratalos 2007; and below).
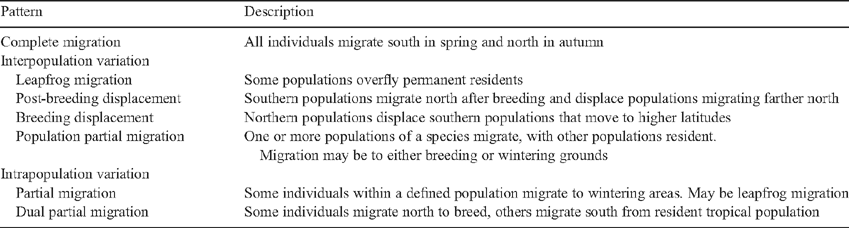
Partial migration
In many species there is intrapopulation variation of migratory behaviour. Two broad categories of such variation occur. In the first, some fraction of the population migrates and the rest is sedentary (reviewed in Dingle 1996). This is known as partial migration. In the second, differential migration, individuals move different distances, often as a function of age or sex. Both differ from interpopulation variation where sedentary and migratory behaviours occur in different populations of a species (Dingle 1996; Cristol et al.1999; Bell 2005). The three phenomena are not mutually exclusive. Austral migrants usually travel for shorter distances than most of their Holarctic counterparts, and non-breeding seasons are generally less severe than in the northern hemisphere. For these reasons, various authors have suggested that selection for migration is weaker (or at least different) and therefore partial migration is particularly common in austral migrants (e.g. Rappole 1995; Chan 2001). Does a comparison of the frequency of partial migrants between north and south bear this out?
A thorough assessment of southern hemisphere partial migration has been carried out only for Australia (Chan 2001) where it is common. Chan estimates that 44% of 155 non-passerine species examined and 33% of 317 passerines are partial migrants. Even in Tasmania where winter conditions are coldest for the region, partial migration occurs in 16 of 20 migratory species (80%), with many individuals remaining over the winter rather than migrating to the mainland. One of the best studied Australian migrants is the Silvereye (Zosterops lateralis; Timaliidae), and it is partially migratory or sedentary over all of its range, with the Tasmanian subspecies exhibiting partial migration. Tasmanian birds are interesting because banding studies reveal that the same breeding individual may be a migrant one year and sedentary the next (Mees 1974). Detailed studies of the meliphagid Eastern Spinebill (Acanthorhynchus tenuirostris) in the New England region of New South Wales reveal that the species is an altitudinal migrant ascending above 1500 m when Banksia collina flowers are blooming in the winter (Ford and Pusey 1982). In contrast, in south-eastern New South Wales, Eastern Spinebills descend in winter to lower altitudes (Tidemann et al. 1988). Not all individuals take part in these movements, so it is a partial migrant. Other populations remain sedentary, so the species is also a population partial migrant (as described in Table 3).
|
In Australia, partial migration thus fulfils predictions that it is common among austral migrants. Many species of African austral migrants are partial migrants (Hockey 2000), hinting that the phenomenon may be common there as well. South America lacks geographical barriers at its centre, a situation presumed to promote partial migration (Chan 2001; Jahn et al. 2004). Indeed, the frequency of partial migrants does seem to be high, with ~70% of austral migrants on that continent reported as being partially or population partially migratory or both (Stotz et al. 1996; Jahn et al. 2004). However, partial migration also seems to be common in the western Palaearctic (Peterson et al. 1993; Berthold 1999) and in the northern areas of the Neotropics. Therefore, although partial migration is common in austral migrants, there is no evidence to suggest it is exceptionally so. In fact early on, Lack (1943–1944) suggested that partial migration was common worldwide. Selection should promote the capability for either migration or residency in individuals of any population inhabiting an area where climatic conditions in the non-breeding season permit residency and high survival in some portion. An early start to breeding and maintenance of favourable territories are some of the advantages postulated to favour such residency (Dingle 1996; Newton 2008), whereas the migratory portion of the population may be ‘making the best of a bad job’ as a result of intraspecific competition (Gillis et al. 2008).
Migration routes and patterns
Migrations occurring on all three southern hemisphere continents display a rich diversity of routes and patterns. Although critical comparisons have yet to be made, the generally milder but drier climates of southern continents seem to have selected for a greater variety of responses to environmental variation than in the northern hemisphere where cold winter temperatures are the overriding influence (Newton and Dale 1996a, 1996b). Temperature is a factor in the southern hemisphere (Chesser 1994, 1998; Joseph 1996, 1997; Hockey 2000), but is less potent and interacts more with other climatic factors such as rainfall (Nix 1976; Hockey 2000; Dingle et al. 2000; Dingle 2004). The consequence of less severe temperatures appears to be a greater diversity of overlapping migration patterns.
Some of the patterns present in South America are given in Table 3. All these patterns, or variants of them, occur in the northern hemisphere, but complete migration, with essentially all individuals leaving the breeding grounds to winter to the south, is prominent, whereas it is but one of several prevalent patterns in South America. Equally prominent in the latter are displacements and variants of partial and population partial migration. Note, however, that as discussed above, partial migration may be common in milder climatic regimes of the northern hemisphere, and there is a need for a quantitative comparison between the two hemispheres. South American migrants further typically display overlapping breeding and wintering ranges. For example, 54 of the 75 or so species of migrant tyrant-flycatcher show this pattern (Chesser 2005).
In addition to the proportion migrating (Chesser 1998), where South American breeding migrants travel to overwinter can be predicted by temperature, specifically daily mean temperatures (DMT) in the months of January and July (Joseph 1996, 1997, 2003). Some 92 migrant passerines breeding in the southern temperate ‘cone’ of South America divide into two groups. The first group, South American temperate tropical (SATT) migrants, moves north to the warm humid regions of northern South America with DMT greater than ~20°C. These migrants breed mostly in the warmer, more northern temperate regions. The second group, South American cool temperate (SACT) migrants, spends the cooler months in regions where DMT is generally <20°C, with the region of DMT between 16°C and 20°C being an area of overlap. There are also complex patterns not readily categorised, especially in groups like the seed-eaters (Emberizidae). The January and July DMT tend to be more similar on average for SATT migrants than for SACT migrants (Joseph 2003). For example, there is no significant difference between temperature profiles of the breeding and non-breeding distributions of two subspecies of the SATT Swainson’s Flycatcher (Myiarchus swainsoni) even though subspecific ranges are distinct (Joseph 2003; Joseph and Stockwell 2000; and below). The SACT and SATT patterns mirror similar patterns in North American migrants (NACT and NATT; Joseph 1997).
Temperature is likely a surrogate for other aspects of a migrant’s ecology (e.g. Newton and Dale 1996a, 1996b). A hint of what these might be is present in migrants in the Monte Desert of Argentina (Cueto et al. 2008). Twelve species were analysed, five SATT migrants and seven SACT. The SATT species were all tyrannids: four of these forage primarily by sallying for flying insects, and one, the White-crested Elaenia (Elaenia albiceps), belongs to a genus that also gleans from leaves and is convergent in foraging habits with North American parulid warblers (Chesser 2005). The SACT species belong to seven families, only two of which are tyrannids. Six of these species, including one of the tyrannids (a monjita, Black-crowned Monjita (Xolmis coronatus)), feed either on the ground or in lower levels of vegetation. A similar tendency for ground foragers to occupy cooler winter habitats occurs in Australian robins (Robinson 1992). Clearly more data are needed, but these studies suggest a possible interesting association between temperature in both breeding and non-breeding ranges and foraging mode or diet or both.
Species that do not show temperature associations between breeding and winter ranges, or ‘niche switchers’, are also interesting. Evidence from North American migrants indicates that differences in temperatures and other climatic variables between breeding and non-breeding ranges result from new niche associations in the breeding range only (Martínez-Meyer et al. 2004; Nakazawa et al. 2004). Therefore breeding season niche switching indicates the direction of seasonal niche evolution in migratory species because the niches of non-breeding populations are highly conserved. Southern hemisphere migration patterns would seem to be rich material for further studies.
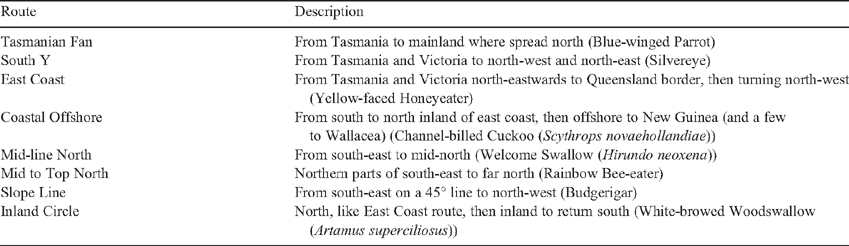
Migration routes in Australia seem to be particularly diverse as a function of the geography and the dry climate. The Great Dividing Range inland from the east coast and the presence of water crossings between Tasmania and the mainland and between the mainland and New Guinea–Wallacea modify pathways (Dingle 2004). Griffioen and Clarke (2002) used sophisticated algorithms to analyse bird atlassing and other data for possible migration routes in eastern Australia. They discerned no less than 19 distinctive patterns that divided approximately between the east coast and inland. A sample of eight of the routes is given in Table 4. Most of the migrant species were partial migrants, confirming Chan (2001). The methods and data could not discern erratic or ‘nomadic’ movement, especially if the distances were less than 200 km. Movements such as those of Red-billed Queleas in southern Africa (see below) would thus likely have been missed.
|
In addition to the broad patterns outlined in Tables 3 and 4, there are interesting patterns and routes followed by individual species. In Africa and Australia there are longitudinal migrants. In Africa both species of flamingo, the Greater (Phoenicopterus ruber) and the Lesser (P. minor), follow this pattern (Hockey 2000), whereas in Australasia the Double-banded Plover (Charadrius bicinctus) breeds in the mountains of New Zealand and migrates westward across the Tasman Sea to winter in coastal Australia (Lane 1987). In the African Oystercatcher (Haemotopus moquini) a portion of the newly fledged young migrate from their birthplace on the southern coast of South Africa around the Cape of Good Hope to lagoons on the coast of Namibia. Here they remain for 2–3 years before returning to their natal area to breed without migrating again (Hockey et al. 2003). These shorebirds are thus once-in-a-lifetime partial migrants.
A further pattern especially evident in Africa and Australia is that of ‘rich-patch fugitives’ (Ford et al. 1993). These are species that move among sequentially energy-rich patches of habitat. Queleas in Africa are perhaps the most classic example (reviewed in Dingle 1996; Cheke and Tratalos 2007). The spatiotemporal distribution of rainfall determines where and when Red-billed Queleas breed. These birds overfly rain fronts and breed in areas where there is a flush of new grass. This migration can be repeated with up to three breeding episodes in widely separated regions in a season, and it can be extremely complicated owing to variability in the distribution of rainfall (Oschadleus and Underhill 2008). This is a true migration rather than simply nomadic foraging because the birds lay down fat deposits before flight in amounts that correlate with the distances eventually travelled and are undistracted by suitable habitats en route (Ward and Jones 1977).
In Australia, the Regent Honeyeater (Anthochaera phrygia) fits the rich-patch fugitive pattern. This species is largely dependent on nectar from flowering trees in woodlands scattered in south-eastern Australia (Franklin et al. 1989; Ford et al.1993; Geering and French 1998). As was also the case with Red-billed Queleas, repeated nesting can take place at sites far apart, apparently tracking regions providing rich nectar resources (Franklin et al. 1989; summary in Dingle 2004). A second Australian example is the Banded Stilt (Cladorhynchus leucocephalus), which exhibits fugitive status in extreme form. This shorebird species breeds on the large salt lakes of central and western Australia. These lakes fill at lengthy and irregular intervals, and breeding events may be years apart, but massive when they occur. The Stilts migrate to the lakes when they fill and have adaptations for rapid breeding, such as large eggs and rapid growth of chicks (see Dingle 2004 for summary). The Grey Teal discussed above is another possible Australian example.
In South America there are several specialists that move between patches of flowering and seeding bamboo (Stotz et al. 1996). This would place them in the category of rich-patch fugitives, but the extent to which migratory or ranging behaviour are involved is unknown. In the northern hemisphere Red Crossbills (Loxia curvirostra), which are dependent on conifer cone crops for breeding, also fulfil the criteria for migratory rich-patch fugitives (e.g. Newton 2006).
The southern subspecies of Red-billed Quelea (Q. q. lathamii) shows a migratory divide in which divergent but geographically adjacent populations of the species migrate along different routes (Irwin and Irwin 2005). For Red-billed Quelea the divide lies across a south–west to north–east traverse in Botswana and Zimbabwe at an angle of ~27° to the east of the north–south axis (Cheke and Tratalos 2007). Populations spend the dry season on either side of this line and tend to migrate towards it with the onset of the rains. A further migratory divide occurs in Swainson’s Flycatcher of South America (Joseph 2005). There are two migratory subspecies, M. s. swainsoni and M. s. ferocior. The former is not closely related to other subspecies and apparently evolved migration at a different time from the latter. It may even warrant species status. Myiarchus s. ferocior, in contrast, is closely related to non-migratory subspecies within the complex, and its evolution is probably a consequence of rapid range expansion within the species. There are thus layers of spatiotemporal complexity in the evolution of sedentary and migratory subspecies leading possibly to speciation (Joseph et al. 2003; Joseph 2005). Migratory divides have been identified in the northern hemisphere (Bensch et al. 1999; Irwin and Irwin 2005) where physical barriers apparently promote their evolution. It is interesting that they have evolved in the southern hemisphere as well, but in the absence of obvious physical barriers in the zone of separation (e.g. Chesser 1994).
Special characteristics of each continent
In the Afro-tropics there is north–south symmetry of vegetation types, grading from tropical to temperate and centred more or less on the Equator (Hockey 2000). As the Inter-Tropical Convergence Zone moves back and forth with the seasons, the resulting rains promote flushes of vegetation at opposite times on the two sides of the Equator and cause avian species to perform both northward and southward migrations to breed (Hockey 2000). Austral migrants that breed in southern Africa in the southern summer migrate north to the vicinity of the Equator in the autumn and replace conspecifics that now migrate north to breed in the northern spring and summer. Species with bi-directional migration include 10 species of cuckoo, three kingfishers, and a nightjar. Other Afro-tropical migrants divide between species that migrate only north or only south to breed in contrast to those that migrate bi-directionally. Both patterns reflect symmetry of breeding habitats around the Equator.
Habitats suitable for wintering by some species occur on both sides of the Equator in the New World as well. Species that migrate either north or south to breed, with populations replacing each other in tropical South America in the respective non-breeding seasons, do occur, but they are a smaller portion of the species pool than is the case with Africa. Two examples are the Tropical Kingbird (Tyrannus melancholicus) and the Fork-tailed Flycatcher (T. savanna) (Jahn et al. 2006).
Australasia is unique in the isolation of its migration system, because so few migrants reach the region from Asia and none migrates out to Asia (Dingle 2004). The latter situation might be expected of those birds, like honeyeaters, endemic to the region and of ancient origin, but it is equally true of species of Asian origin, such as the cuckoos and raptors like the Peregrine Falcon and Black Kite (Milvus migrans), that are apparent recent invaders (Sibley and Ahlquist 1985). Even species that are included in Asian superspecies, like the Australian subspecies of Eastern Koel (Eudynamys orientalis cyanocephala) and the Pied Imperial-Pigeon (Ducula bicolor), confine their movements to the east of Wallace’s Line. Thus, unlike either Africa or South America, Australasia is not part of a flyway including northern hemisphere continents except for the migration of shorebirds.
A second striking feature of the Australian avifauna is the abundance of parrots and cockatoos (Psittaciformes), with some 52 resident species, relative to other Australian groups (Christidis and Boles 2008). Many species occur in the southern or inland regions where climatic characteristics might favour migration, and indeed several of these species are migratory (Dingle 2004). For example, three species of small parrot migrate from Tasmania to the mainland: the endangered Orange-bellied Parrot (Neophema chrysogaster), the Blue-winged Parrot (N. chrysostoma) and the Swift Parrot (Lathamus discolor). The mainland populations of the Blue-winged Parrot also move northward for the winter. The Swift Parrot wanders as far as southern Queensland following lerp outbreaks (they feed on the sugary exudates) and winter flowering trees (Saunders and Heinsohn 2008) and probably qualifies as a rich-patch fugitive during the non-breeding season. Some populations of the Budgerigar (Melopsittacus undulatus) breed in south-eastern Australia in the early spring followed by migration north-westward (Griffioen and Clarke 2002), but the situation with other populations is more complicated. Wyndham (1981, 1983) postulated three breeding populations of Budgerigar in eastern Australia with movements driven by the availability of grass seed; they are thus also rich-patch fugitives. In all, at least 14 of the 52 parrots in Australia are migratory (Dingle 2004), contrasting with the single migrant in southern Africa (but out of only seven species; Hockey 2000), and the apparent absence of migration in the rich Psittacidae fauna of South America, including those species that occur in the far south (but more data are needed). In addition several inland species of Australian Psittaciformes apparently wander widely, but whether these movements constitute migration or ranging (cf. Roshier et al. 2008) remains to be determined.
A unique characteristic of South America is the dominance in the migratory avifauna of one taxon, the tyrant-flycatchers (as stated earlier, a total of 329 species occurs in South America; Chesser 1994, 1998, 2005), which make up 33% of South American austral migrants (Chesser 1994). Species of the family occur to the wintry southern limits of the continent, and temperature and latitude are the best predictors of migration (Chesser 1998, 2005), consistent with results from the northern hemisphere (Newton and Dale 1996a, 1996b). A few species migrate from drier to moister regions in the winter (Chesser 2005). Relative to four other large South American bird families – the furnariids (Furnariidae: 296 species, and 5% of austral migrants), the hummingbirds (340 species, 3% austral migrants; Table 2), the thamnophilids (Thamnophilidae: 212 species, no migrants), and the tanagers (Thraupidae: 226 species, no austral migrants) – tyrannids have more migrants (23%, Table 2). A combination of a larger migrant fraction compared with other diverse South American bird families plus a high species number thus contributes to the dominance of the tyrannids among South American austral migrants. However, the proportion of migrants among South American tyrannids is not especially high (Table 2). (Note that both hummingbirds and tanagers contain a few non-austral species that migrate to North America to breed, but inclusion of these still results in fewer than 23% migrants in these families.)
South America is also the only southern hemisphere continent that extends well into temperate latitudes. Two consequences are that, first, it has a southern avifauna that is largely migratory and distinct from that of tropical areas to the north and, second, it includes species that breed beyond 50°S and migrate several thousand kilometres, comparable to the intercontinental migrations of northern hemisphere species (Chesser 1994). A lack of geographical barriers in South America, however, means that even the long-distance migrants are still likely to have overlapping breeding and wintering ranges. In further contrast to the Nearctic–Neotropical migration system, austral migrants tend to breed in open or scrubby areas whereas those from the northern hemisphere occur mostly in forest or woodland (Chesser 1994).
Ecophysiology and life history
It is a truism that many physiological adjustments are required to support migratory behaviour (Dingle 1996). These have been extensively studied in northern hemisphere migrants and include mechanisms of orientation, timing of the annual cycle, and metabolic changes to prepare for migratory flight (Munro 2003; Åkesson and Hedenström 2007; Ramenofsky and Wingfield 2007). In these northern migrants, endogenous annual time programs synchronise migratory activity with environmental cues such as photoperiod (Gwinner and Helm 2003). Most understanding of migration physiology comes from nocturnal migrants adjusting to northern hemisphere seasons. There is little research on southern hemisphere species experiencing less severe but less predictable seasonality or that migrate by day rather than at night (Munro 2003). Questions to consider concern similarities or differences between the migratory syndromes of northern and southern hemisphere species and whether these syndromes reflect differences in environments within and between the two hemispheres.
The two southern hemisphere species most intensively studied with respect to ecophysiology are both Australian: the Yellow-faced Honeyeater (Lichenostomus chrysops) and the Silvereye. The honeyeater migrates north-east up the eastern coast and along the Great Divide and associated tablelands of Australia in the autumn until it reaches south-eastern Queensland whereupon it turns north-west. Like northern migrants these birds show increased activity in laboratory cages at the time of migration, change diet (to feeding on more nectar), deposit fat, and orient in Emlen funnels in the direction predicted by the migration pathway, including the change in orientation from north-east to north-west at mid-course (Munro and Wiltschko 1992; Munro et al. 1993; Munro and Munro 1998; Munro 2003). These diurnal migrants use both celestial and magnetic cues for orientation like well-studied nocturnal northern migrants (Wiltschko and Wiltschko 1995). Like the Yellow-faced Honeyeater, the partially migratory Tasmanian population of Silvereyes display migratory restlessness and pre-migratory fattening (Chan 1994, 1995) and orientation appropriate to the season of migration using both celestial and magnetic cues (Wiltschko et al. 1998a, 1998b, 2001). In both species, endogenous control mechanisms regulate migration and the seasonal cycle, as the experiments with migratory restlessness and diet revealed (Munro 2003). Thus with respect to endogenous control and orientation, these southern hemisphere migrants display mechanisms similar to northern hemisphere species, especially those travelling from Europe to Africa (Gwinner and Helm 2003). Both the Yellow-faced Honeyeater and the Silvereye, however, come from fairly predictable environments, similar in many respects to the seasonal environments experienced by well-studied northern migrants. Species from less-predictable environments, like those in the drier regions of southern Africa or interior Australia, are still in need of study (Munro 2003).
The primary cue for synchronising annual rhythms in northern migrants is photoperiod (reviewed in Dingle 1996). Although not explicitly analysed for austral migrants, photoperiod presumably operates as a cue in these birds as well because sensitivity to photoperiod has long been established in avian lineages, including those of the southern hemisphere. For example, North Island Brown Kiwi (Apteryx mantelli), Yellow-eyed Penguin (Megadyptes antipodes), and Kakapo (Strigops habroptilus) of New Zealand all have photoperiodically synchronised breeding cycles similar to those of northern hemisphere species from equivalent latitudes (Cockrem 1995). The photoperiodic response is, however, modifiable by other environmental cues. In two equatorial populations of the Rufous-collared Sparrow (Zonotrichia capensis) separated by only an Andean ridge, the photoperiod is the same but breeding is synchronised to two quite different rainfall regimes (Moore et al. 2005).
A second aspect of possible southern hemisphere adaptations concerns life history traits related to reproduction and mortality. Here, problems caused by the paucity of data on southern hemisphere migrants (Clarke 1997; Russell 2000) are particularly acute. The extensive study of Böhning-Gaese et al. (2000) of European and North American migrants, for example, examined data from 373 and 252 land bird species respectively. Nothing remotely similar is available from the southern hemisphere. With respect to the fitness traits of clutch-size, number of broods and annual fecundity, they found: (1) clutch-size decreased from residents to short-distance migrants (wintering north of the Tropic of Cancer for Nearctic birds or north of the Sahara for European species) to long-distance migrants (wintering south of the Tropic of Cancer or the Sahara); (2) short-distance migrants averaged the most broods per season; and (3) therefore long-distance migrants displayed the lowest fecundities, with residents and short-distance migrants of roughly equal average fecundity. These conclusions were not altered when controlled for body size and phylogeny.
Clarke et al. (2003) examined these traits in the migratory Yellow-faced Honeyeater, a short-distance migrant by northern hemisphere standards, and found that the species produced small, multiple clutches not differing from the fecundity pattern seen in sedentary honeyeaters. This result is thus not consistent with the pattern seen in Europe and North America where migrants tend to produce fewer but larger clutches (Böhning-Gaese et al. 2000). The fairly short period of parental care in the Yellow-faced Honeyeater, however, contrasted with sedentary southern hemisphere species and with the Australian migrants Rufous Whistler (Pachycephala rufiventris) and Leaden Flycatcher (Myiagra rubecula) (Bridges 1994; Russell 2000; Trémont and Ford 2000). Several behavioural characteristics of the Yellow-faced Honeyeaters were similar to those in northern hemisphere migrants. These included: earlier return to breeding habitat and greater territorial fidelity by males relative to females; a high divorce rate between breeding seasons; a 3.5–4 month breeding season (sedentary honeyeaters generally longer); and a fairly short 2–3 week fledging period. These results are interesting, but they pertain only to a single species. There is a clear need for much more data on fitness traits in southern hemisphere migrants of both predictable and unpredictable habitats for comparisons both within and between hemispheres. Such data aid understanding of both proximate and evolutionary aspects of migration syndromes.
Why don’t austral breeders migrate to the northern hemisphere?
The southern hemisphere possesses a much reduced temperate zone, relative to the northern hemisphere, especially in Africa and South America. Austral migrants breeding at higher latitudes on these two continents thus encounter many opportunities to locate suitable wintering habitats and habitat filtering keeps migrants remaining south of the Equator. A few do cross the Equator, but only because their tropical wintering habitats do so (Joseph 1996, 1997; Hockey 2000). This trans-equatorial migration, however, occurs in a small portion of austral migrants, and there is no travel north of the equatorial tropics. Generally mild climates mean that migrants need not travel far to find wintering habitats, contributing to overlapping breeding and non-breeding ranges as already noted for South America (Chesser 1994; Stotz et al. 1996; Jahn et al. 2006). So for reasons of both climate and habitat, austral migrants remain in their own hemisphere when not breeding.
The situation in Australasia differs from Africa and South America for two reasons. First, temperate and tropical land areas are approximately equal. Second, the region has long been geographically isolated and is well separated from the northern hemisphere by longitude and water gaps, as noted above. Nevertheless habitat filtering is still the most likely primary cause preventing austral breeders from migrating to the northern hemisphere (mainland Asia) for the non-breeding season. The geographical separation of Australasia from Asia may further constitute a barrier supplementing or reinforcing habitat filtering as in northern breeders migrating south. Historically the confinement of austral migration within Australasia is probably of long standing, because so few species of Australasian origin have established in mainland Asia; notable examples of species that have are a whistler (Mangrove Whistler (Pachycephala grisola)), a gerygone (Golden-bellied Gerygone (Gerygone sulphurea)) and two woodswallows (Ashy Woodswallow (Artamus fuscus) and White-breasted Woodswallow (A. leucorynchus)) (Clements 2007). The Whistler and Gerygone are mangrove specialists, which could have spread via islands, and the woodswallows are included in the highly migratory aerial predator guild (Table 2). If Australasian species historically migrated outside the region, more should have colonised and left descendants in Asia. In marked contrast, the descendants of many Asian taxa that include migrants are well represented in Australasia (Christidis and Boles 2008).
Conclusions and future directions
The patterns outlined in this review suggest that the southern hemisphere is indeed distinct in many respects from the northern when it comes to avian migration. First, except for shorebirds, few long distance migrants breeding in the northern hemisphere winter in the temperate regions of any southern hemisphere continent (Table 1). The extreme case is Australasia to which only 10 Asian breeders migrate to spend the northern winter, and all but three of these are rare or occupy only the northern fringes of the region (Dingle 2004). Second, no southern hemisphere breeders migrate to the northern hemisphere except for a few species in Africa and South America that occupy tropical wintering habitats extending across the Equator. Third, migration within the southern hemisphere is extensive and varied (Tables 3and 4), and arguably more varied than migration in the northern hemisphere. Several patterns are not classic round trips, with the same routes from breeding to wintering areas from year to year, but they vary with respect to routes and breeding areas (e.g. the Grey Teal, the Budgerigar, and the Red-billed Quelea). Finally, migration within the southern hemisphere seems to be more influenced by aridity and rainfall frequency, especially in Africa and Australasia (Nix 1976; Dingle et al. 2000), although the point at which aridity supersedes temperature as a predictor of migration is unknown. These differences from the northern hemisphere result because there is less land area in the southern hemisphere, and little of it extends into high latitudes. Seasons are still predictable in occurrence, but are both less distinct and less predictable in terms of onset, duration and severity, thus contributing to the variation in migration patterns.
Ornithologists have barely scratched the surface of southern hemisphere migration. In Australasia, for example, far more attention has been devoted to sedentary species than to migratory species (Clarke 1997). We are still mostly at the stage of describing patterns rather than having sufficient data to propose evolutionary hypotheses as to why these patterns exist. Yet the variety present in the southern hemisphere migration systems provides a little exploited resource for exploring the ecology and evolution of migratory behaviour. The flexibility of occurrence, the different degrees expressed, and the fluctuating thresholds of response to the environmental spectrum provide opportunities for dissecting both the nature of selection for migration and the responses to that selection by species of differing behaviour and ecology. Focus on round trips in the classic sense of many northern hemisphere migrations is likely to be counter-productive to a comprehensive understanding. There is too much variation and there are too many possible interactions between behaviour and ecology in determining movement to confine ourselves to only one possible outcome.
Given that the physiological mechanisms to support migration are inherent in birds with no embedded ancient syndrome (Rappole et al. 2003; Piersma et al. 2005), a key question concerns what aspects of the environmental ‘arena’ push maintenance movements into the realm of migration (Dingle and Drake 2007). A focus on the southern hemisphere would seem particularly apt for addressing this question because of the behavioural continuum present in the degree and variety of the migrations of its avifauna. The Grey Teal of Australia, for example, displays movements that can be described as a continuum from prospecting or ranging to migration (Roshier et al. 2008). What factors induce the transition from ranging, which does not display characteristics like the inhibition of responses to suitable habitats, to migration, which does show suppression of such responses? And how do these behaviours integrate with breeding and other aspects of the life history? These sorts of questions could be asked of other rich-patch fugitives in Australasia and on the other continents as well.
Further questions concern ecophysiology and orientation mechanisms. We know that Australian migrants making classic round trips display endogenous rhythms and orient appropriately on their journeys using celestial cues like their northern counterparts (Munro 2003). But what cues are used and what endogenous patterns are present and integrated into the migrations of species like the Red-billed Queleas of Africa, which breed in different places both in the same year and in different years (Cheke and Tratalos 2007)? Are there differences in the life histories with respect to traits like clutch-size or duration of breeding between the SATT and SACT migrants of South America (Joseph 1996, 1997), and if so how might these relate to the evolution and ecology of migration in general (e.g. Dingle 2006)? There is no shortage of queries and no shortage of southern hemisphere migration patterns in which they might be addressed.
There are also important questions concerning phylogeny and historical biogeography that could well be addressed with southern hemisphere migrants. In the absence of ancient embedded syndromes what can phylogeny tell us? A comparative phylogenetic approach applied across families of birds suggests there are ecological characteristics, such as habitat preference and foraging patterns, that have been precursors of migration (Chesser and Levey 1998; Joseph 2005; Boyle and Conway 2007). Such comparative methods have yet to be applied across southern continents, so we have little idea of phylogenetic ‘footprints’ that may occur in their migration systems (Hockey 2000). The complex and variable patterns seen would seem ripe for such analysis. Especially interesting would be reconstructions of distributional shifts that accompanied the evolution of migration. The descendants of Asian species, for example, that now migrate entirely within Australasia pose many questions concerning the extent and the time-frames for such shifts of distribution. Phylogenies can also reveal the finer points of divergence within a clade, such as the two migratory populations of Swainson’s Flycatcher in South America with distinct ancestries (Joseph 2005). Species like Australian Silvereyes with their overlapping migration routes (Griffioen and Clarke 2002) come to mind as appropriate candidates for further applications of molecular and other methods. A similar case can be made for investigating phylogenetic relationships in other Australasian migrants with complex migration routes (Table 3).
To sum up, the highly diverse and flexible behaviours of southern hemisphere avian migrants have too long been largely neglected by the ornithological community. I hope this review is convincing in revealing the range of opportunities available in the ‘lower half’ of the world for examining migration as part of a profile of movement behaviours and for broadening perspectives on so fundamental an aspect of avian life histories.
Acknowledgements
I am grateful to Camilla Myers for the invitation to contribute a Rowley Review and to her and three reviewers whose extensive comments have very much improved the paper. Thanks to George Cox and Jiro Kikkawa for reading and commenting on an early draft. I owe special thanks to Leo Joseph for sending me papers and for his insightful comments on migration in South America in particular. John Wingfield and Tom Hahn provided insight and papers regarding ecophysiology. Sharon Lawler graciously provided work space while I was on a visit to UCDavis. Errors of fact or interpretation are my own.
Åkesson, S. , and Hedenström, A. (2007). How migrants get there: migratory performance and orientation. Bioscience 57, 123–133.
| Crossref | GoogleScholarGoogle Scholar |
Bairlein, F. (1988). How do migratory songbirds cross the Sahara? Trends in Ecology & Evolution 3, 191–194.
| Crossref | GoogleScholarGoogle Scholar |
Battley, P. (2008). Long haul flights godwit style. Wingspan 18(2), 28–30.
Bell, C. P. (2000). Process in the evolution of bird migration and pattern in avian ecogeography. Journal of Avian Biology 31, 258–265.
| Crossref | GoogleScholarGoogle Scholar |
Bensch, S. , Andersson, T. , and Åkesson, S. (1999). Morphological and molecular variation across a migratory divide in Willow Warblers, Phylloscopus trochilus. Evolution 53, 1925–1935.
| Crossref | GoogleScholarGoogle Scholar |
Böhning-Gaese, K. , and Oberrath, R. (2003). Macroecology of habitat choice in long-distance migratory birds. Oecologia 137, 296–303.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Chesser, R. T. , and Levey, D. J. (1998). Austral migrants and the evolution of migration in New World birds: diet, habitat, and migration revisited. American Naturalist 152, 311–319.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Clarke, M. F. (1997). A review of studies of the breeding biology of Australian birds from 1986–1995: biases and consequences. Emu 97, 283–289.
| Crossref | GoogleScholarGoogle Scholar |
Cockrem, J. F. (1995). Timing of seasonal breeding in birds, with particular reference to New Zealand birds. Reproduction, Fertility and Development 7, 1–19.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Dingle, H. (2004). The Australo-Papuan bird migration system: another consequence of Wallace’s Line. Emu 104, 95–108.
| Crossref | GoogleScholarGoogle Scholar |
Ford, H. A. , and Pusey, J. F. (1982). Status and feeding of the Eastern Spinebill Acanthorhynchus tenuirostris in New England National Park, north-eastern N. S. W. Emu 82, 203–211.
Hayes, F. E. (1995). Definitions for migrant birds: what is a Neotropical migrant? Auk 112, 521–523.
Hockey, P. A. R. (2000). Patterns and correlates of bird migrations in sub-Saharan Africa. Emu 100, 401–417.
| Crossref | GoogleScholarGoogle Scholar |
Hockey, P. A. R. , Leseberg, A. , and Loewenthal, D. (2003). Dispersal and migration of juvenile African Black Oystercatchers, Haematopus moquini. Ibis 145, E114–E123.
| Crossref | GoogleScholarGoogle Scholar |
Jahn, A. E. , Levey, D. J. , and Smith, K. G. (2004). Reflections across hemispheres: a system-wide approach to New World bird migration. Auk 121, 1005–1013.
| Crossref | GoogleScholarGoogle Scholar |
Joseph, L. (1996). Preliminary climatic overview of migration patterns in South American austral migrant passerines. Ecotropica 2, 183–193.
Joseph, L. , and Stockwell, D. (2000). Temperature-based models of the migration of Swainson’s Flycatcher Myiarchus swainsoni across South America: a new use for museum specimens of migratory birds. Proceedings of the Academy of Natural Sciences of Philadelphia 150, 293–300.
Kingsford, R. T. , and Norman, F. I. (2002). Australian waterbirds – products of the continent’s ecology. Emu 102, 47–69.
| Crossref | GoogleScholarGoogle Scholar |
Leisler, B. (1992). Habitat selection and coexistence of migrants and Afrotropical residents. Ibis 134(Suppl. 1), 77–82.
Martínez-Meyer, E. , Peterson, A. T. , and Navarro-Sigüenza, A. G. (2004). Evolution of seasonal ecological niches in Passerina buntings (Aves : Cardinalidae). Proceedings of the Royal Society of London. Series B: Biological Sciences 271, 1151–1157.
| Crossref | GoogleScholarGoogle Scholar |
Mees, G. F. (1974). The migration of the Tasmanian race of the Silvereye. Australian Bird Bander 12, 51–54.
Munro, U. , and Munro, J. A. (1998). Migratory restlessness in the Yellow-faced Honeyeater Lichenostomus chrysops (Meliphagidae) an Australian diurnal migrant. Ibis 140, 599–604.
| Crossref | GoogleScholarGoogle Scholar |
Newton, I. (2006). Movement patterns of Common Crossbills Loxia curvirostra in Europe. Ibis 148, 782–788.
| Crossref | GoogleScholarGoogle Scholar |
Newton, I. , and Dale, L. (1996a). Migration patterns in west Palearctic birds. Journal of Animal Ecology 65, 137–146.
| Crossref | GoogleScholarGoogle Scholar |
Piersma, T. , and Gill, R. E. (1998). Guts don’t fly: small digestive organs in obese bar-tailed godwits. Auk 115, 196–203.
Priest, B. , Straw, P. , and Weston, M. (2002). Shorebird conservation in Australia. Wingspan 12(Suppl. 4), I–XVI.
Rappole, J. H. , Helm, B. , and Ramos, M. A. (2003). An integrative framework for understanding the origin and evolution of migration. Journal of Avian Biology 34, 124–128.
| Crossref | GoogleScholarGoogle Scholar |
Robinson, D. (1992). Why do Flame Robins Petroica phoenicia migrate? A comparison between social and feeding ecologies of the Flame Robin and the Scarlet Robin P. multicolor. Corella 16, 1–13.
Roshier, D. A. , Whetton, P. H. , Allan, R. J. , and Robertson, A. I. (2001). Distribution and persistence of temporary wetland habitats in arid Australia in relation to climate. Austral Ecology 26, 371–384.
| Crossref | GoogleScholarGoogle Scholar |
Sutherland, W. J. (1998). Evidence for flexibility and constraint in migration systems. Journal of Avian Biology 29, 441–446.
| Crossref | GoogleScholarGoogle Scholar |
Wilson, J. R. , Nobel, S. , and Minton, C. D. T. (2007). Migration ecology and morphometrics of two Bar-tailed Godwit populations in Australia. Emu 107, 262–274.
| Crossref | GoogleScholarGoogle Scholar |
Wiltschko, W. , Munro, U. , Ford, H. , and Wiltschko, R. (1998a). Effect of a magnetic pulse on the orientation of silvereyes, Zosterops l. lateralis, during spring migration. Journal of Experimental Biology 201, 3257–3261.
| PubMed |

Wiltschko, W. , Wiltschko, R. , Munro, U. , and Ford, H. A. (1998b). Magnetic versus celestial cues: cue–conflict experiments with migrating silvereyes at dusk. Journal of Comparative Physiology. A: Sensory, Neural, and Behavioral Physiology 182, 521–529.
| Crossref | GoogleScholarGoogle Scholar |

Wiltschko, R. , Munro, U. , Ford, H. A. , and Wiltschko, W. (2001). Orientation in migratory birds: time-associated relearning of celestial cues. Animal Behaviour 62, 245–250.
| Crossref | GoogleScholarGoogle Scholar |

Winkler, D. W. (2006). Roosts and migrations of swallows. Hornero 21, 85–97.

Wyndham, E. (1981). Breeding and mortality of Budgerigars Melopsittacus undulatus. Emu 81, 240–243.

Wyndham, E. (1983). Movements and breeding seasons of the Budgerigar. Emu 82, 276–282.