Preliminary investigation of the costs of incubation in the Australasian Gannet (Morus serrator) breeding in Port Phillip Bay, Victoria
A. D. Ewing A , F. I. Norman B , S. J. Ward A and A. Bunce CA Department of Zoology, University of Melbourne, Parkville, Vic. 3052, Australia.
B Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, PO Box 137, Heidelberg, Vic. 3084, Australia.
C School of Ecology and Environment, Deakin University, 221 Burwood Highway, Burwood, Vic. 3125, Australia.
D Corresponding author. Email: a.ewing@zoology.unimelb.edu.au
Emu 105(2) 137-144 https://doi.org/10.1071/MU03061
Submitted: 18 December 2003 Accepted: 4 April 2005 Published: 30 June 2005
Abstract
To optimise lifetime reproductive success, individuals must balance current reproductive effort against future reproductive prospects. In birds, incubation and chick-rearing must involve costs, and manipulation of the length of incubation offers an insight into some costs affecting adults. An experiment was conducted at a colony of Australasian Gannets in Port Phillip Bay, Victoria, in which length of incubation was manipulated so that some adults experienced short (10–20 days duration), long (70–80 days) or normal (~45 days) incubation periods. Adults with a manipulated incubation period did not show significant differences in weight change (taken here to reflect cost) during incubation or chick-rearing compared with controls. Manipulation of length of incubation did not significantly affect the hatching success or the growth rate of chicks involved and is not, therefore considered to impose an increased reproductive cost. This suggests that the Australasian Gannet has the capacity to maintain body condition and successfully rear young despite modified duration of incubation.
Introduction
The view that clutch-size in birds evolved to the optimal number of eggs and offspring parents could successfully incubate and raise (Lack 1954, 1968) ultimately resulted in the concept of lifetime reproductive success (LRS) (e.g. Monaghan and Nager 1997). Individual LRS of a bird reflects its life history strategy, where compromises are made between current reproductive effort and future reproductive potential, between raising viable offspring and survival (Stearns 1992). In the elaboration of LRS, compromises may occur (e.g. between parental body condition and the number and quality of offspring) but increasing age may improve an individual’s LRS by enhancing foraging efficiency and maintenance of body condition, improve predator avoidance or increase breeding success as a consequence of cumulative breeding experience (as in the Short-tailed Shearwater (Puffinus tenuirostris), Wooller et al. 1990). Further, it is possible that birds approaching the end of their reproductive life increase reproductive effort at the expense of future survival, by laying earlier, producing larger eggs, increasing investment in offspring or combinations of these (Wooller et al. 1990). Earlier breeding certainly occurs in older, more experienced Southern Fulmars (Fulmarus glacialoides) and Australasian (Morus serrator) and Northern (M. bassana) Gannets (Nelson 1964; Weimerskirch 1990a; Gibbs et al. 2000). In Common Terns (Sterna hirundo), an individual’s breeding potential is related to its body condition, suggesting that reproductive decisions may be determined by individual state rather than age (Wendeln and Becker 1999). Female Lesser Black-backed Gulls (Larus fuscus) provided with additional protein in a year of low food availability produced larger clutches compared to unfed individuals, whereas those fed additional fat did not, and there was an increase in clutch-size in years of good food supply (Bolton et al. 1993). However, using an induced increase in egg production, Nager et al. (2001) found a direct correlation between current egg production and future fitness in females, indicating that increased parental effort can have a detrimental effect on productivity.
Costs of incubation may involve physiological and behavioural components. In general, birds do not adjust body temperature to regulate transfer of body heat but rather modify incubating behaviour in response to external egg temperature (e.g. O’Connor 1984). The addition of an egg at mid-incubation to the single-egg clutch of the Australasian Gannet caused a slight drop in egg temperature, suggesting that changes in clutch-size may affect the ability of parents to incubate successfully (Evans 1995). However, there was minimal impact on parental weight, when sufficient prey was available, or on breeding success of Northern Gannets when an extra chick was added to the nest (Nelson 1964). In the Cape Gannet (M. capensis), parents of twins exhibited reduced parental effort in a breeding seson with apparently reduced prey availability, thus avoiding excessive loss in body condition (Navarro 1991). Australasian Gannets (hereafter, Gannets) are capable of incubating clutches of two, albeit with limited success, but twins had lower weights than singletons (Bunce 2001a).
Foraging time is reduced during incubation and brooding and adults may lose body weight at an increased rate (e.g. Grant 1984). In procellariids, there is a significant negative relationship between body weight and percentage of weight loss per day during incubation (Croxall 1982), with Southern Fulmars (~1 kg) losing ~4.5% of body weight per day, compared with 0.9% in the Wandering Albatross (Diomedea exulans) (~10 kg) (Croxall and Ricketts 1983; Weimerskirch 1990b). Although weight is often regained during foraging between incubation shifts, overall energy costs while rearing chicks are higher than at other times (Huin 1997; Wendeln and Becker 1999). Additionally, since foraging time is correlated with the length of the incubation shift in many seabirds, long foraging trips and incubation shifts may result in significantly reduced hatching success (e.g. Dearborn 2001). In experimental manipulation of the length of incubation, measurable effects on breeding success of the parent birds have been found within a season. Thus, extension of incubation in the European Storm-Petrel (Hydrobates pelagicus) resulted in reduced hatching success and it was presumed that eggs successfully incubated by parents with a prolonged incubation were attended by higher quality pairs, whereas eggs that failed were attended by lower quality pairs (Minguez 1998). But, since parents experiencing lengthened incubation laid earlier, subsequent hatching success may have reflected the presence of more experienced adults, which often lay earlier, perhaps being in better body condition (e.g. Hipfner 1997; Wendeln and Becker 1999). In a study of European Shags (Phalacrocorax aristotelis), Daunt et al. (1999) swapped eggs between nests to standardise the effects of age of parents in an investigation of age-specific reproduction. In this process they also adjusted the length of incubation experienced by parents by ~33% and found that age of pairs was the main factor influencing breeding success. Stearns (1992) predicted that any long-lived seabird subject to increased reproductive costs would ensure its own survival before that of its egg or chick, and perhaps even abandon breeding attempts in some years (e.g. Wernham and Bryant 1998).
This study aimed to determine the costs, in terms of reproductive success, of shorter or longer incubation on condition of adult Gannets and their breeding performance and on the growth rate of chicks.
Methods
Study site
This study was conducted at Popes Eye Marine Reserve (hereafter Popes Eye, 38°16′42′′S, 144°41′48′′E), ~3 km off Queenscliff, and just north-east of the entrance to Port Phillip Bay, Victoria, Australia. Popes Eye includes an artificial basalt rock annulus, ~70 m in diameter, with wooden platforms (~65 m2 in area), a walkway, and navigational aids on a tower installed on a concrete structure. Some 160 pairs of Gannets were nesting at Popes Eye during this study, mainly on the platforms and walkway (~150 pairs), with a small number breeding on the concrete structure and on rocks immediately north and south of the platform. The timing of breeding, breeding success and diet of Gannets at this colony has been regularly monitored, as have aspects of the growth of the colony and site-fidelity of adults (see Norman and Menkhorst 1995; Norman et al. 1998; Gibbs et al. 2000; Bunce 2001a, 2001b; Norman 2001; Bunce et al. 2002, 2005, who also provide further details of the site and its location).
Breeding phenology and success
Although visits to Popes Eye were made on average every 7 days, they ranged from 1 to 18 days apart as it was not possible to visit in unfavourable weather. Thirty-four visits were made between 26 July 2001 and 25 February 2002, allowing monitoring of the progress of breeding. Some nests had been formed by 26 July, but no new nests were built after late November. Nests were located on a sketch map as they were built, and the appearance of eggs (and replacements if lost), progress of incubation, hatching and chick-rearing were followed throughout the study. At first encounter, eggs were individually numbered using a permanent marker pen. Losses of eggs and chicks were noted, and the proportion of eggs laid that hatched (hatching success) and number of chicks raised to 60 days recorded. On each visit, contents of nests on the platform, walkway and concrete structure (collectively termed the colony) were examined by gently lifting the sitting adult. For larger chicks, visual observations usually sufficed.
Laying dates were estimated (to within 5 days) by considering time elapsed between visits and the extent of egg-staining. Recently laid eggs (1–2 days old) were a clean china-blue colour with a few streaks of blood whereas older eggs (3–5+ days) were progressively stained by guano or nesting material. When the interval between visits exceeded 5 days, laying dates were estimated, and confirmed or adjusted by back-dating 45 days from the date of hatching (average incubation in this study was 45 days (range 40–50, n = 105), cf. mean of 44.1 in Wingham 1984a). Establishment of laying dates provided a pool of known-age eggs from which nests could be chosen for experimental purposes and compared with results from other nests used as controls.
Manipulation of length of incubation
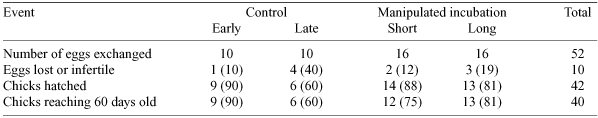
Two experimental groups of nests and attendant adults were established to investigate the costs of incubation, in terms of adult body weight and ability to rear chicks. To establish the two groups, 16 eggs laid early in the breeding period (from mid-September to early October) and incubated for ~30 (± 2) days, were exchanged with 16 freshly laid eggs (laid mid- to late October and early November). Thus, adults that had laid earlier had to incubate for a total of 70–80 days before their replacement eggs hatched (= ‘long incubation’), and adults that laid later only incubated for 10–20 days (= ‘short incubation’). When choosing eggs that had already been incubated for ~30 days for an exchange, care was taken not to use eggs with cracks or emitting ‘pipping’ noises (indicating imminent hatching).
Two control groups were established, that had their eggs exchanged but normal periods of incubation (~45 days). These were also examined for any significant differences in adult weight or chick growth in pairs that began breeding at different times, thus controlling for factors other than length of incubation (such as environmental changes). For logistical reasons, governed by availability of eggs, the timing of laying in the control groups was a little different. To establish the ‘early control’ group, five pairs of nests that held eggs laid at similar times (within 2 days) in early to mid-September had eggs interchanged. In the ‘late control’ group, similar exchanges were repeated using five pairs of nests with eggs laid in late September to mid-October (Table 1). In both control groups, eggs were exchanged in the early stages of incubation (usually within a week of laying). The four ‘treatment groups’ below relate to early and late controls, and to the clutches involved in short and long incubation (see Table 1).
In all experimental and control nests, the embryo experienced an incubation period of normal length. Further, eggs were only exchanged between nests in the more central areas of the colony where birds tend to be older and more successful (e.g. Gibbs et al. 2000; Bunce et al. 2005).
Weights and measurements
Chicks of control and experimental pairs were monitored for at least 60 days after hatching. On each visit, attempts were made to measure bill length (dial callipers, 200 mm ± 0.1 mm) and body weight (spring balance, 0–1 kg ± 5 g, or 1–5 kg ± 25 g) of as many chicks as possible, such that each chick was weighed at least five times between hatching and ~60 days old. Previous studies at Popes Eye and elsewhere have shown that growth of Gannet chicks is relatively slow to 20 days of age, and then becomes rapid and almost linear to 60 days old, followed by a decrease in growth rate (Wingham 1984b; Gibbs et al. 2000). As a consequence, particular attention was paid to the size of chicks half-way through (at 40 days old) and at the end of (at 60 days old) the linear growth phase.
Since chicks became mobile when ~35–40 days old, and often moved away from nest sites, they were banded with coloured, numbered bands supplied by the Australian Bird and Bat Banding Scheme (ABBBS) when ~21 days old to enable easy identification. Visual checking of the band colour allowed more efficient catching of target chicks and minimised disturbance.
Attempts were made to catch one or both adults attending individual study nests (both adults incubate, brood and attend growing chicks) to determine their weight at or soon after laying. Where possible, attending parents were captured and weighed (spring balance, 0–5 kg ± 25 g) each time its chick was being measured, especially at, or close to, hatching, and 40 days and 60 days after hatching. In this study it was assumed that weight changes between consecutive weighings were linear. Some adults and chicks regurgitated food (typically fish) when being caught or handled. To minimise the effects our disturbance may have had on results, such regurgitates were collected and their weight later added to the individual’s body weight, and these adjusted weights were used in calculations below.
In this monomorphic species, it is not possible to sex birds by external morphology, so it was assumed that males and females had an equal opportunity of capture, and sex-effects were not investigated. Bands were read when present, but otherwise adults were marked with a numbered ABBBS band before release.
Assessment of effect of incubation manipulation
Four measures of the effect of incubation manipulation were monitored: changes in adult weight during incubation and following hatching, growth rates of chicks, and weights of chicks at 40 and 60 days old. Since weights of adults and chicks were rarely obtained at exactly 40 or 60 days after hatching, these were estimated by assuming linear weight change between measurements. Adult weights at hatching were determined in a similar way.
Growth-curves were calculated for each chick using weight measurements (at least five in each case) spread across the period of growth to 60 days old. A Gompertz equation (Kaufmann 1981) was fitted to each growth-curve, with the form:
Weight = A*EXP {–EXP [–K(Age – I)]}
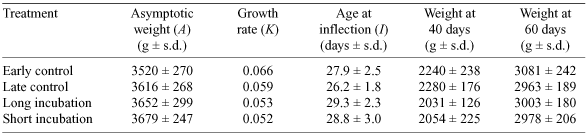
where A = asymptotic weight, K = growth rate, I = age at point of inflection of curve (the point at which the increasing growth rate trend begins to decrease towards the maximum weight). The Gompertz equation was used since it is the most appropriate curve to fit growth data for Gannet chicks (Wingham 1984b; Navarro 1991; Gibbs et al. 2000; Bunce 2001a). The mean parameters (A, K, and I) within treatment groups were compared using ANOVA. Comparisons between groups were also made using estimated chick weights at 40 and 60 days after hatching.
Statistical analyses
Data were analysed using Systat (Wilkinson 1997) or following Quinn and Keough (2002). Means ± standard deviations are given below.
Results
Laying and hatching success
A total of 150 pairs of Gannets built nests on the wooden platform and concrete structure at Popes Eye during this study. For 140 first eggs laid between 20 August and 17 December, the mean laying date was 23 September (± 42 days) and the median 12 September. The other ten nests apparently never contained an egg. By the end of this study, 19 nests had disappeared, and 56 (40%) of the 140 first eggs were lost, abandoned, or infertile. At 31 of the 56 unsuccessful nests (55%), Gannets laid a replacement egg, ten of which (33%) failed to hatch.
Hatching success was high in the four treatment groups (60–90%; Table 2) and did not differ across them (χ2 = 3.87, d.f. = 3, n = 52, P > 0.05), so the two experimental egg exchanges did not significantly reduce hatching success. Results for the two control groups were also compared, as were those for the experimental groups, again with no significant differences (χ2 = 2.4, 0.237, d.f. = 1, n = 20, 32, respectively; both P > 0.05). However, the hatching success for all treatment groups (81%, 42 of 52 of eggs) was significantly higher than that of other nests at the colony (53%, 63 of 119 (χ2 = 45.51, d.f. = 1, n = 171, P < 0.05)). Therefore, significantly more eggs in the treatment groups hatched than in non-manipulated nests, and exchanging eggs did not reduce hatching success.
|
Changes in adult weight
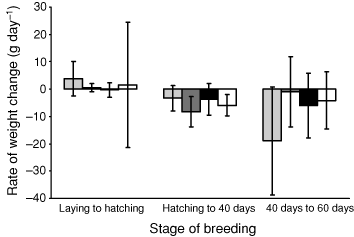
Adults in the ‘long incubation’ group had a mean body weight of 3100 g (Table 3) at laying, and lost weight at a rate of 0.3 g per day, amounting to a mean total loss of 22 g (~0.7% of initial body weight) in a period of 70–80 days to hatching. Adults of all other treatment groups showed an increase in mean body weight during incubation of 0.5–3.7 g per day (Table 3). Adults of ‘late control’ and ‘short incubation’ groups showed increases in mean body weight of 0.9% and 0.8% of initial body weight respectively. Adults of the ‘early control’ group had a mean body weight of 2960 g at laying (Table 3), and showed a mean increase in weight of 165 g (5.6%) at hatching. As actual weight changes, however, these differences were not significant between treatment groups during incubation (ANOVA, F3,20 = 0.120, P > 0.05).
Adults from all treatment groups showed a mean decrease in weight while rearing chicks (though variation was high; Fig. 1), but rates of change of weight from hatching to 40 days after hatching (ANOVA, F3,20 = 0.849, P > 0.05) or 60 days after hatching (ANOVA, F3,17 = 0.217, P > 0.05), and from 40 to 60 days after hatching (ANOVA, F3,28 = 1.958, P > 0.05), showed no significant differences between treatments. The early control group lost a mean of 19 g per day (± 20 g) in the period 40–60 days after hatching, and the late control group lost a mean of 1 g per day (± 15 g) in the same period. Similarly, there was no significant difference between treatment groups in the rate of loss of weight of adults for the period from laying to 60 days post-hatching (ANOVA, F3,22 = 0.412, n = 26, P > 0.05).
|
Chick weight gain and growth
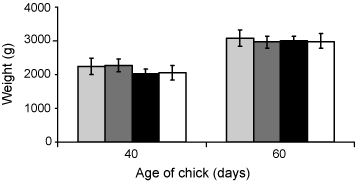
At 40 days old, the mean weights of chicks in treatment groups varied from 2031 g to 2280 g, but at 60 days mean weight of chicks in all treatment groups was close to 3 kg (Fig. 2). Although there were significant differences in weights of chicks among the four treatment groups (ANOVA, F3,36 = 3.877, P < 0.05), a Tukey’s test could not distinguish between the four means. The rates of weight increase from hatching to 40 days old (ANOVA, F3,36 = 1.870, P > 0.05), and from 40 days to 60 days old (ANOVA, F3,36 = 0.549, P > 0.05), were not significantly different. Variation in weights of chicks was reduced at 60 days post-hatching (ANOVA, F3,36 = 1.457, P > 0.05).
|
Weights of chicks increased slowly in the first 20 days after hatching, increased rapidly between 20 and 60 days, and then gradually levelled out. For all chicks, average parameters provided from Gompertz curves were A = 3618 g (± 274), K = 0.059 (± 0.01) and I = 28 days (± 2.7; see Table 4 for details). Although there were no significant differences between groups in asymptotic weights (A) (ANOVA, F3,36 = 1.054, P > 0.05) or age at inflection (I) (ANOVA, F3,36 = 2.238, P > 0.05), there were differences in growth rates (K) (ANOVA, F3,36 = 5.072, P = 0.005). Here, the growth rates of chicks in the early control group were significantly faster than those of chicks in the long and short incubation groups (Tukey’s HSD multiple comparison test, P = 0.011 and P = 0.005, respectively).
|
Discussion
In this study there were no detectable, or consistent, costs of incubation to adult Gannets breeding at Popes Eye in 2000–01 despite artificially extending or shortening the incubation period by an average of ~67%. In addition, there was no persistent, discernible difference in the sizes and growth rates of chicks raised by parents that experienced extended or shortened incubation periods. The absence of observable differences between manipulated nests (long and short incubation) could have arisen if a true cost of incubation was counteracted by an age effect (Daunt et al. 1999), since older Gannets typically lay earlier in a breeding period than younger birds (Gibbs et al. 2000). However, there was also no difference in the performance of Gannets from experimental and control nests, and all nests were chosen from the core area of the Popes Eye colony that contains few younger and inexperienced pairs (e.g. Gibbs et al. 2000; Bunce et al. 2005).
Parallel studies of the diet of gannets at Popes Eye indicate that the diet in 2001–02 was dominated by Barracouta (Thyrsites atun) and Redbait (Emmelichthys nitidus) (A. D. Ewing, unpublished data) and was not typical of those reported previously (e.g. dominated by Pilchards (Sardinops sagax), Norman and Menkhorst 1995; Bunce 2001b). Otherwise, our results were obtained within a routine breeding period, one with a chronology similar to that found previously (e.g. Norman and Menkhorst 1995; Gibbs et al. 2000; Bunce et al. 2005), and are discussed in the light of present knowledge of the species and its biology.
Adult condition
Changes in adult weight have been used as an index of body condition, and hence general fitness, during particular breeding stages (e.g. Minguez 1998; Weimerskirch 1990b). Indeed, body condition is considered to play a major role in resource allocation between reproduction and survival in seabirds (e.g. Weimerskirch et al. 1997). Wandering Albatrosses lose weight during their long incubation shifts, but regain it on subsequent foraging trips, and both parents maintain body weight throughout incubation (Croxall and Ricketts 1983). Similar results were found in male Great Frigatebirds (Fregata minor; Dearborn 2001). However, loss of body weight during incubation occurs in the Antarctic Petrel (Thalassoica antarctica) and Southern Fulmar (Weimerskirch 1990b; Tveraa et al. 1998). Since these species have restricted breeding periods in the short Antarctic summer (Norman et al. 1992), they may be more susceptible to weight loss during incubation, as they must invest more in a short period of time (see also Weimerskirch 1990a, 1990b). Clarke (2001) also considered that Adélie Penguins (Pygoscelis adeliae) showed a foraging behaviour that reflected a trade-off between provisioning chicks and maintaining food reserves. In other studies of seabirds with longer foraging trips (e.g. Weimerskirch et al. 1997) there may be variable changes in mass, influenced by time since last foraging; such information was not available in this study. Most adult Gannets in the present study showed a slight gain in weight during incubation and the loss recorded in birds of the longer incubation group was insignificant. Further, there was no significant difference in rate of change of weight during incubation between pairs of Gannets that laid early in the breeding period and those that laid later. This suggested that the timing of egg exchanges did not have an additional temporal effect on adult condition and that changes in prey availability or weather conditions were unlikely to have had an influence. Daunt et al. (1999) demonstrated that the length of incubation had little effect on the proportion of hatched eggs that survived in European Shags. However, some of our results were not consistent with other studies of temperate species. For example, in the Thick-billed Murre (Uria lomvia), which lays a single-egg clutch, removal of the egg of birds that laid early in a season induced re-laying; chicks of these manipulated pairs grew faster than chicks of non-manipulated pairs that began breeding later, suggesting some qualities of the parents (e.g. age, experience) had greater influence on breeding success than temporal factors (Hipfner 1997). In the study by Daunt et al. (1999), the effect of parental age on breeding success was independent of temporal factors. Further, it has been suggested birds that lay earlier in a breeding period are often more successful, owing to better reproductive fitness than birds that lay later in a season, and are possibly older, more experienced breeders (Wooller et al. 1990; Weimerskirch 1990a; Heaney and Monaghan 1996). Certainly, older Gannets lay earlier at Popes Eye, and replace more lost eggs, than younger birds (e.g. Gibbs et al. 2000). Our results indicate that in Gannets, adults can sustain or increase their body weight during incubation, even when extended, so it is during chick rearing that differences in parental quality are more likely to be expressed.
The weight loss recorded for Gannets rearing chicks also occurs in other seabirds, chick rearing being a time of increased energy costs for parents (Croxall and Ricketts 1983; Golet et al. 1998; Weimerskirch et al. 2000). At this time, parents must balance effort in rearing offspring with future reproductive potential (e.g. Weimerskirch et al. 1997). Brooding Antarctic Petrels with artificially increased foraging costs decreased food provisioning to their chick and increased feeding intervals to offset the greater costs, resulting in increased chick mortality (Sæther et al. 1993). In Leach’s Storm-Petrel (Oceanodroma leucorhoa), experimentally increasing flight cost did not cause a difference in adult weight between experimental and control birds. However, chicks of manipulated birds had a lower growth rate since parents invested less resources in chicks (Mauck and Grubb 1995). With reduced time for foraging, Gannets with an artificially lengthened period of incubation might be expected to make a trade-off between current and future reproductive effort and success, but we found no evidence of parents losing more weight or reducing investment in their chicks. This lack of response may be facilitated by the flexible foraging strategy of Gannets, in which the diet reflects availability of prey species (Bunce and Norman 2000; Bunce 2001b). Futhermore, Gannets do not spend a lot of time diving each day (Lewis et al. 2002; K. Goyen, personal communication), so have time for more feeding dives each day if necessary.
Since both sexes share incubation and chick care in Gannets, it is unlikely that the manipulations used in this study would differentially affect the sexes. The short incubation and brooding shifts of Gannets (both averaging <16 h; Bunce 2001b), compared with some other seabirds (e.g. procellariids, Warham 1990), probably result in a minimisation of short-term variation in weight of Gannets. The associated frequent foraging trips may also result in minimal longer-term weight loss itself. However, any increased effort by pairs in one breeding period may have a residual cost more obvious in subsequent breeding periods, or when food is limited. In Atlantic Puffins (Fratercula arctica), for example, increased parental effort in the experimental year caused reduced breeding success in the next (Wernham and Bryant 1998), and in the European Storm-Petrel, there was a significant difference in laying dates between pairs that experienced a lengthened incubation and controls in the year following manipulation (Minguez 1998). Although this difference subsequently disappeared, increased parental effort, perhaps not obvious in the experimental period, may result in parents requiring a longer period between breeding efforts to recover body condition (Minguez 1998). Further, increased reproductive effort may have costs other than those reflected by weight (e.g. Nilsson and Svensson 1996) and influence success in the next breeding period.
Chick growth
Chick growth and survival are influenced by parental investment (e.g. Sæther et al. 1993). In maximising LRS, parents must continually balance investment in current offspring against their chances of survival to breed in future. Hence, higher investment by adult Gannets might be expected for the first 60 days after hatching, when chicks grow most rapidly (Wingham 1984b; Gibbs et al. 2000). However, the absence of differences in growth rate, even when considered in two stages, from hatching to 40 days old and from 40 days old to 60 days old, again indicated that food availability was sufficient to maintain condition of both chicks and adults and that the growth rate of chicks was not affected by the length of incubation experienced by their parents.
There were no differences between treatments in the rates at which chicks gained weight after hatching, and though ANOVA indicated differences in the weights of chicks at 40 days old in the different treatment groups, these differences were not detectable in a post hoc Tukey’s test. By 60 days, there was no significant difference in weight between groups. Such results suggest that the chicks of long-incubation parents were not disadvantaged, in weight, compared with those of parents with normal incubation periods. This is supported by the absence of any significant differences in the mean asymptotic weight of chicks from each treatment group. Though chicks show considerable variation in weight at any age, this variation decreases with increasing age (e.g. Wingham 1984b).
Conclusions
Variation in the length of incubation did not have a significant effect on adult Australasian Gannets breeding at Popes Eye Marine Reserve, in the 2001–02 breeding period. In this study, sample sizes were sufficient to detect medium to large changes in adult body weight. Shorter incubation periods could advantage adults by reducing their energy demands, and thereby allow maintenance of body condition. Reid et al. (2000), for example, showed that incubation demands can limit reproductive success, and suggested that there may be a reallocation of resources from incubation to later stages in the same, or future, reproductive attempts. However, in this study there was no apparent reallocation to favour chick growth. Larger samples may be required to detect the effects if they are only small.
Any costs incurred by longer incubation period did not influence the ability of adults to hatch and rear chicks successfully; adult body weight in treatment groups showed no variation and offspring of manipulated adults did not experience a significant difference in growth rate. The major shift in diet observed in 2001–02 (A. D. Ewing, unpublished data), compared with previous studies, did not affect the ability of adults to breed successfully, as indices of breeding chronology, hatching success and chick growth were similar to those found previously. Experimental manipulation of incubation length apparently had no influence on current reproductive success in the Gannet but the effect on residual reproductive value remains to be measured. Investigation of inter-year costs, in the form of parental effort and breeding success in subsequent breeding seasons, could determine whether the length of the incubation period has an effect on future reproductive success in the Australasian Gannet. Similarly, costs in years when alternative foods are restricted would also warrant examination since it may be that chick growth rates themselves influence reproductive strategies more than parental provisioning ability (Ricklefs 1979, 1992; see also Erikstad et al. 1998, and references therein).
Acknowledgments
We thank Kath Handasyde and Graham Coulson (Department of Zoology, University of Melbourne) for their quite varied and unique assistance during this study. Boat transport to and from the study site was provided routinely by Rod Watson of Queenscliff Marine Station, although staff of Parks Victoria, Queenscliff, also helped out on occasion. Christopher Robertson (Wellington, New Zealand) provided enthusiasm and a novel weighing technique, and a host of volunteers assisted in various ways throughout the project. Rob Day, Mick Keough (Department of Zoology, University of Melbourne) and Michael Scroggie (Arthur Rylah Institute) assisted with statistical analyses. This investigation on Australasian Gannets at Popes Eye Marine Reserve was conducted under permits issued by the University of Melbourne Animal Experimentation Ethics Sub-Committee (Register number 01064) and the Department of Natural Resources and Environment (Permit number 10001510).
Bolton, M. , Monaghan, P. , and Houston, D. C. (1993). Proximate determination of clutch size in Lesser Black-backed Gulls: the roles of food supply and body condition. Canadian Journal of Zoology 71, 273–279.
Heaney, V. , and Monaghan, P. (1996). Optimal allocation of effort between reproductive phases: the trade-off between incubation costs and subsequent brood rearing capacity. Proceedings of the Royal Society of London. Series B. Biological Sciences 263, 1719–1724.
Lewis, S. , Benvenuti, S. , Dall’Antonia, L. , Griffiths, R. , Money, L. , Sherratt, T. N. , Wanless, S. , and Hamer, K. C. (2002). Sex-specific foraging behaviour in a monomorphic seabird. Proceedings of the Royal Society of London. Series B. Biological Sciences 269, 1687–1693.
| Crossref | GoogleScholarGoogle Scholar |
Reid, J. M. , Monaghan, P. , and Ruxton, G. D. (2000). Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proceedings of the Royal Society of London. Series B. Biological Sciences 267, 37–41.
| Crossref | GoogleScholarGoogle Scholar |
Tveraa, T. , Saether, B. E. , Aanes, R. , and Erikstad, K. E. (1998). Regulation of food provisioning in the Antarctic Petrel: the importance of parental body condition and chick mass. Journal of Animal Ecology 67, 699–704.
| Crossref | GoogleScholarGoogle Scholar |
Weimerskirch, H. (1990a). The influence of age and experience on breeding performance of the Antarctic Fulmar, Fulmarus glacialoides. Journal of Animal Ecology 59, 867–875.
Wingham, E. J. (1984a). Breeding biology of the Australasian Gannet Morus serrator (Gray) at Motu Karamarama, Hauraki Gulf, New Zealand. I. The egg. Emu 84, 129–136.

Wingham, E. J. (1984b). Breeding biology of the Australasian Gannet (Morus serrator) (Gray) at Motu Karamarama, Hauraki Gulf, New Zealand. II. Breeding success and chick growth. Emu 84, 211–224.

Wooller, R. D. , Bradley, J. S. , Skira, I. J. , and Serventy, D. L. (1990). Reproductive success of Short-tailed Shearwaters Puffinus tenuirostris in relation to their age and breeding experience. Journal of Animal Ecology 59, 161–170.




