Climate change and its impact on Australia’s avifauna
Lynda E. Chambers A , Lesley Hughes B and Michael A. Weston CA Bureau of Meteorology Research Centre, GPO Box 1289K, Melbourne, Vic. 3001, Australia. Email: L.Chambers@bom.gov.au
B Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia. Email: lhughes@rna.bio.mq.edu.au
C Research and Conservation Department, Birds Australia, 415 Riversdale Road, Hawthorn East, Vic. 3123, Australia. Email: m.weston@birdsaustralia.com.au
Emu 105(1) 1-20 https://doi.org/10.1071/MU04033
Submitted: 24 January 2004 Accepted: 11 February 2005 Published: 31 March 2005
Abstract
Relative to the northern hemisphere, little is known about the effect of climate change on southern hemisphere birds, although the impact could be significant. Here we review the effects of climate change on birds that have been documented or predicted, with particular reference to Australian species. Potential impacts include changes in geographic range, movement patterns, morphology, physiology, abundance, phenology and community composition. The evidence suggests that these changes are already happening, both overseas and in Australia, but more research is needed to determine the extent of these impacts and how to conserve birds in the face of climate change. Management options include promoting adaptation and resilience, intensive management of sensitive species, and improved planning for mitigation techniques and monitoring.
Introduction
Surface air temperatures have increased approximately 0.6°C globally since the mid-nineteenth century and the magnitude of this warming is the largest of any century in the last 1000 years (Intergovernmental Panel on Climate Change (IPCC) 2001b). The 1990s were the warmest decade, and 1998 the warmest year, on record, both globally and in Australia. There is widespread acceptance, in both the scientific and general communities, that this warming trend, together with changes in sea level and rainfall, is now discernible above natural decadal- and century-scale variability and that anthropogenic production of greenhouse gases is largely responsible (IPCC 2001b). Evidence is also mounting that anomalously high temperatures in the twentieth century have been associated with changes in many physical and biological systems, including accelerated glacial retreat, thawing of permafrost, lengthening of growing seasons, and alterations in the phenology of flowering, breeding and migration of many species (Hughes 2000; Walther et al. 2002; Parmesan and Yohe 2003; Root et al. 2003).
Surprisingly, a recent IPCC report (IPCC 2001a) lists no avian studies associated with regional temperature change for the Australian region. The report lists changes in 388 bird species that were associated with regional temperature change in areas outside Australia.
The aims of this review are to: (1) briefly summarise recent climatic trends apparent both globally and in Australia; (2) describe how these trends have been associated with changes in the distribution, abundance and phenology of birds in Australia and elsewhere; and (3) highlight some directions for future study and monitoring to improve predictions as to how climate change may affect birds, and therefore improve long-term conservation efforts for the Australian avifauna. The review encompasses birds both from continental Australia and those that occupy Australian territories, including Antarctica, at some stage in their life cycle.
Climate trends: past and future
Consistent with global trends, Australia’s continental average temperature increased 0.7°C from 1910 to 1999, with most of the increase recorded since 1950 (Collins et al. 2000; Nicholls 2003); 1998 was also Australia’s warmest year, and 2002 and 2003 the fifth and sixth warmest (National Climate Centre (NCC) 2004). The 1990s was the warmest decade and the 1980s the second warmest on record (Collins 2000). Recent warming has been greatest in winter and spring (Hulme and Sheard 1999). Night-time temperatures have increased more than daytime temperatures (Suppiah et al. 2001; Lenten and Moosa 2003), consistent with global trends (Easterling et al. 1997). Occurrences of extreme warm events have generally increased over the period 1957–96, while the numbers of extreme cool events have decreased (Plummer et al. 1999; Collins et al. 2000). Warmer temperatures have apparently reduced the frequency and duration of frosts (Stone et al. 1996) and may have contributed to the severity of the 2002 drought (Karoly et al. 2003; Nicholls 2004). Sea-surface temperatures (SST) in many tropical regions have increased almost 1°C over the past century (Hoegh-Guldberg 1999). The Antarctic region has also warmed over the last half century, particularly around the Antarctic Peninsula, leading to a retreat in the ice shelves (IPCC 2001c).
Although there has been no significant, continental-average trend in Australian rainfall since 1910, significant regional and seasonal trends have occurred. Annual total rainfall has risen by about 15% in New South Wales, South Australia, Victoria and particularly in north-western Australia. South-western Western Australia has become 15–20% drier, with a sudden decrease in rainfall occurring in the mid-1970s (Indian Ocean Climate Initiative (IOCI) 2002). Increases in both heavy rainfall events and the number of rain-days in many regions have been recorded. On average, the number of wet days has increased by 10% (despite the decline by 10% in south-western Western Australia), although this figure rises to nearly 20% in parts of New South Wales and the Northern Territory (Hennessy et al. 1999; Haylock and Nicholls 2000). Heavy rainfall events in summer have increased in the east and north of Australia, with decreases in south-western Western Australia (Hennessy et al. 1999; IOCI 2002).
The frequency and duration of El Niño events, a phenomenon associated with drought in Australia, have apparently increased over the past few decades, although the association of this trend with global warming is still being debated (Trenberth and Hoar 1997; Allan and D’Arrigo 1999). In addition to possible changes in the frequency of El Niño–Southern Oscillation (ENSO), the relationship between ENSO, annual temperature and rainfall also changed in the early 1970s (Nicholls et al. 1996; Power et al. 1998). Since then, for any value of the Southern Oscillation Index (SOI) or rainfall, maximum temperature has tended to be higher than previously recorded. Likewise rainfall, for any value of SOI, has tended to be greater than would have been expected for such an SOI value previously. Nicholls et al. (1996) concluded that differential warming in the Indian Ocean, relative to the Pacific Ocean, may be the cause of these changes.
Sea-level trends around Australia vary regionally but most monitored locations have recorded slight increases (average 0.3 mm p.a. during the twentieth Century) compared to 0.8 mm p.a. for the Pacific and 1–2 mm p.a. globally (Mitchell et al. 2000; IPCC 2001b).
The most recently published climate change projections for Australia produced by the Climate Impact Unit of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) suggest that by 2030, annual average temperatures will be 0.4–2.0°C higher (relative to 1990), with slightly less warming in some coastal areas and Tasmania and the potential for greater warming in north-western Australia. By 2070, annual average temperatures may increase by 1.0–6.0°C (Commonwealth Scientific and Industrial Research Organisation (CSIRO) 2001a). These climate models also project rainfall changes, but the level of uncertainty associated with rainfall projections is greater than that for temperature. The scenarios suggest large areas of the mainland will experience significant decreases in rainfall and increases in evaporation during the twenty-first century. Most of the models also simulate an increase in extreme daily rainfall that is likely to be associated with increased flooding. Overall, the scenarios emphasise an increase in variability and reduced predictability for Australia’s climate, with increased incidence and intensity of extreme events such as fires, floods, droughts and tropical storms (e.g. Williams et al. 2001; Cary 2002; Walsh et al. 2004). Modelling also indicates there are likely to be significant reductions in snow cover and the length of the snow season in alpine areas (Whetton 1998; Hennessy et al. 2003). Further details of both recent climatic trends and those projected for the future can be found in CSIRO (2001a, 2001b), Hughes (2003a), Pittock (2003) and references therein.
Potential impacts of climate change on birds
Climate change is expected to have profound and complex impacts on virtually all species. Potential direct effects, with particular reference to birds, can be categorised as follows:
-
Changes in the distribution of species, both latitudinal and altitudinal. As climatic zones move poleward and upward in elevation, changes in geographic ranges of mobile species such as birds are expected. Montane species are predicted to be particularly vulnerable because the area of suitable habitat becomes progressively reduced with increasing altitude (Peters and Darling 1985). However, species inhabiting low-relief areas like plains may also suffer reductions in range as the climate warms because they will have to shift their range proportionately further to maintain their climate envelope (Peterson 2003). Predicted rises in sea level will affect coastal nesting birds as well as birds found in coastal wetlands or mangrove regions that may become inundated with sea water (Galbraith et al. 2002; Smart and Gill 2003). Species that use coastal habitats for other purposes, such as foraging or roosting, may also have to move elsewhere as sea levels rise.
-
Changed movement patterns. As the climate warms, some species that currently migrate between habitats may either shorten their migration distance, or even stop migrating altogether, becoming year-round residents (Jenni and Kery 2003). Alteration of resources in time and space may also change the movement patterns of nomadic species.
-
Changes in abundance of species, including some local extinctions. Fragmentation and reductions in populations are expected under most climate-change scenarios (e.g. Williams et al. 2003). This may occur via a variety of mechanisms including direct mortality from climatic extremes, or reduced reproductive success. Reduction in population size may result in reduced genetic diversity (Willott and Thomas 2001). Stresses imposed by climate change will interact with other threatening processes in such a way as to magnify the impact on populations. For example, climate change may reduce the reproductive success of populations already fragmented by habitat loss, and so may further reduce the viability of those populations.
-
Changes in phenology. Temperature is an important cue for many phenological events. Changes in arrival dates of migratory birds have been linked to temperature and may be related to changes in weather systems en route (Sparks and Menzel 2002). Other phenological changes that could be expected include earlier, or in some cases later, laying dates, changes in the timing of territorial formation, and changes in the length of incubation periods (e.g. Crick and Sparks 1999; Dunn and Winkler 1999; Barlein and Winkel 2001; Winkler et al. 2002; Cresswell and McCleery 2003); these will also alter the timing of breeding in general. For migratory birds, the timing of arrival on breeding territories and over-wintering grounds is thought to be a key determinant of reproductive success, survivorship and fitness. Short-distance migrants may benefit from global warming by breeding earlier and over a longer period, by shortening their migration distance and by experiencing better conditions in the breeding area after the breeding season (Jenni and Kery 2003). In contrast, long-distance migrants may not obtain the same benefits from warming. The start of their reproduction may be constrained by a less variable spring arrival date, dictated by endogenous rhythm (Sæther 2000; Both and Visser 2001). Further, resident birds may benefit from warmer winters and impose increasing competitive pressure on migrants (Lemoine and Bohning-Gaese 2003). There is therefore likely to be considerable asymmetry in the effects of climate change on long- and short-distance migrants, with an increasing advantage for short-distance migrants.
-
Changes in community composition. Species-specific contractions and expansions in range, changes in the abundance of some birds and habitat types, together with alterations in phenology, will result in many new species interactions and resulting changes in the structure and composition of present-day communities.
-
Changes in physiology, morphology and behaviour. As global temperatures increase, changes may occur in the morphology of species, such as decreases in body size (Bergmann’s Rule; Bird 1999; Aston 2002). Species may also need to alter their physiology, morphology, or behaviour, or combinations of these, to maintain body temperature and adapt to a changed water balance.
In addition to these direct impacts, many birds will also be affected indirectly via the impacts of climate change on other species and on various aspects of their habitat and food supply. For example, climate change impacts on vegetation (via changes in temperature, rainfall, fire regimes or atmospheric CO2) may affect the quality and connectivity of bird habitat. Climatic effects on the timing of flowering and fruiting in plants or emergence in insects will affect the food supply of many bird species. Increased levels of atmospheric CO2, may also reduce nitrogen concentrations in plant tissue, thereby affecting populations of insect prey (Whittaker 1999). Changes in land-use and human activity, such as agriculture, related to climate change, may also indirectly impact on bird species.
Evidence for changes already: the global picture
The long tradition of biological monitoring in the northern hemisphere, particularly in Europe and Britain, has provided a mounting body of evidence that many species, including birds, have already responded to recent warming trends (reviewed in Hughes 2000; Walther et al. 2002; Parmesan and Yohe 2003; Root et al. 2003).
Changes in distribution
The poleward extension of bird distributions has been documented for several species (e.g. Johnson 1994; Thomas and Lennon 1999, Valiela and Bowen 2003), and some changes have been relatively spectacular, such as the invasion of the British Isles by a former scarce vagrant, the Little Egret (Egretta garzetta) (Musgrove 2002). Shifts of species such as the Golden-crowned Warbler (Basileuterus culicivorus), the Lesser Greenlet (Hylophilus decurtatus) and the Keel-billed Toucan (Ramphastos sulfuratus) to higher elevations have also been documented (Pounds et al. 1999).
Changes in phenology
Increasingly warmer springs have been associated with earlier laying dates for many bird species (e.g. Crick et al. 1997; McCleery and Perrins 1998; Crick and Sparks 1999; Dunn and Winkler 1999; Scharlemann 2001; Barlein and Winkel 2001; Coppack and Both 2002; Sanz 2002; Winkler et al. 2002; Evans et al. 2003; Sergio 2003a). Although this would seem favourable to birds by providing a longer breeding season with the possibility of multiple broods, it can lead to disadvantages if other aspects of the ecosystem, such as the availability of food resources, are not affected in the same way. While the populations of some species have maintained the synchronisation of hatching date and food supply by significantly increasing their incubation period (e.g. Cresswell and McCleery 2003), mismatching of hatching and peak food availability has already affected reproductive success in others (Visser et al. 1998; Both and Visser 2001; Coppack and Both 2002; Drent et al. 2003; Sanz 2003).
Advances in the arrival and departure dates of Northern Hemisphere migratory birds have been documented (e.g. Ahas 1999; Sparks and Menzel 2002; Butler 2003; Cotton 2003; Hubalek 2003; Huppop and Huppop 2003; Ptaszyk et al. 2003) although trends towards later arrivals have also been found in a few species (e.g. Peñuelas et al. 2002). In some European examples, advances have been linked to warming in both the tropical wintering areas (such as sub-Saharan Africa) as well as those in temperate breeding areas (Cotton 2003). Arrival dates for some short-distance migrants appear to have been affected more than those of long-distance migratory species, possibly because short-distance migrants can respond to meteorological cues (such as temperature), whereas long-distance migrants rely more on photoperiod as a cue for migration (Butler 2003). Not all migratory species have shown advances, with some species showing significant delays. In particular, species with a variable rather than a fixed number of broods per year have delayed passage, possibly because they make more breeding attempts (Jenni and Kery 2003). Declines in the proportion of long-distance migrants in some European avian communities have also been recorded (Lemoine and Bohning-Gaese 2003).
Asynchrony between phenology at low and high elevations may also have negative impacts for altitudinal migrants. American Robins (Turdus migratorius), for example, initiate their spring migration to higher elevations earlier, presumably as a result of environmental cues in the over-wintering grounds. They consequently arrive about 14 days earlier at high altitudes in the Rocky Mountains than 20 years ago. The date of snowmelt at the higher elevation, however, has not advanced and the birds have had to wait progressively longer for food to become available (Inouye et al. 2000).
Changes in abundance and population dynamics
Impacts of recent warming on abundance are particularly difficult to disentangle from impacts of other processes such as habitat loss. Increasing air and sea temperatures, and changes in the extent of sea-ice, have been associated with declines in apex marine predators (seabirds and seals) (Veit et al. 1996, 1997; Veit and Hyrenbach 2001; Croxall 1992; Weimerskirch et al. 2003). Warming appears to have favoured some populations of Antarctic seabirds (e.g. Taylor and Wilson 1990; Fraser et al. 1992; Ainley 2002; Bergstrom 2003) but not others (Cunningham and Moors 1994; Kaiser 1997; Barbraud and Weimerskirch 2001; Woehler et al. 2001; Ainley 2002). Population trajectories (and breeding phenology) in several species of land birds have been associated with long-term changes in large-scale meteorological phenomena, such as the Pacific decadal oscillation (Ballard et al. 2003), the North Atlantic oscillation (Forchhammer et al. 1998; Stenseth et al. 2002; Jones et al. 2003) and the ENSO (Boersma 1998; Stenseth et al. 2002); the precise nature of the influence of climate change on these events is currently unknown. Examination of population trends in 77 species of common birds in France over the period 1989–2002 indicated that species with northern breeding distributions were more likely to have declined than those with southern distributions (Julliard et al. 2004). This is consistent with expected population changes resulting from climate change, in addition to other potential causes of decline such as habitat loss.
Changes in morphology and physiology
Yom-Tov (2001) examined trends in body mass and tarsus length of five passerine species in museum collections in Israel. Body mass declined significantly in four of the species and tarsus length decreased in two species over the period 1950–99. Yom-Tov concluded that these declines are consistent with changes resulting from warming trends, in accordance with Bergmann’s Rule, which predicts that body size within species decreases in warmer climates. Egg size of the Pied Flycatcher (Ficedula hypoleuca) in Finnish Lapland increased significantly between 1975 and 1993, possibly also owing to warming (Jarvinen 1994).
Changes in behaviour
Observations of breeding colonies of the Antarctic Petrel (Thalassoica antarctica) illustrate how a fairly subtle environmental change can have disastrous consequences for the reproductive success of a prey population by altering the behaviour of a predator (Van Franeker et al. 2001). Snowfall from 1980 to 1996 increased at Casey Station in the Antarctic, leading to large, persistent snowdrifts in areas that had previously remained bare. These snowdrifts allowed Giant Petrels (Macronectes giganteus) to ‘crash land’ at breeding sites of the Antarctic Petrels, a behaviour they were previously unable to display owing to their heavy body size and lack of manoeuvrability around the steep cliffs preferred by Antarctic Petrels. The Giant Petrels preyed upon the Antarctic Petrels, as well as making eggs vulnerable to predation by South Polar Skuas (Catharacta maccormicki). Antarctic Petrel breeding success at this location during the 1996–97 breeding season was reduced to virtually zero as a result.
Observations of foraging behaviour in the Black Kite (Milvus migrans) indicate that compensatory behavioural responses may occur in response to warming (Sergio 2003b). The Kites, which have expanded their ranges northward and have shown progressively earlier laying over recent years, also adjust their flight styles depending on the temperature, such that they spend more time hunting in favourable weather and use flight styles that are less energetically expensive.
Indirect impacts
Birds may also be indirectly affected by the effects of warming on other species. For example, dates of first appearance of mosquitoes have advanced since the mid-1980s on Coats Island in Hudson Bay. Increased mosquito abundance, in combination with high ambient temperatures, has been positively correlated with increased rates of egg loss in Brunnich’s Guillemot (Uria lomvia) (Gaston et al. 2002).
Climate change impacts on Australian birds
In the past there has been a tendency to attribute all changes in bird distributions in Australia, as well as declines and extinctions, to human activities (Serventy 1977). Serventy instead suggests ‘the fundamental cause of most expansion of range and reduction in numbers of animal species in Australia is climatic but modified sometimes by man-made alterations to the environment’.
Concern over the potential impacts of climate change on Australia’s avifauna has become much more prominent in the popular press and scientific literature over the last few years (e.g. Abbott 1999; Duncan et al. 2001; Green and Pickering 2002; Australian Associated Press (AAP) 2003; Beeby 2003a, 2003b, 2003c; Hilbert 2003a, 2003b; Reid 2003a, 2003b; Williams et al. 2003; Brown 2004; Hilbert et al. 2004; Thomas et al. 2004).
There are two main approaches to examining climate change and birds: that based on observational data and that based on modelling results. Observational studies are often based on historical trends in bird records, mainly abundance or the timing of breeding, but some are based on range shifts (e.g. Dunlop 2001; Green and Pickering 2002; Reid 2003b; Roberts 2003; Chambers 2004). Other observational studies are based on current relationships between biological variables and climate; the investigators then hypothesise the likely impact of climate change (e.g. Arnold 1988; Johnson 1998; Duncan et al. 2001; Rowley and Russell 2002; Smithers et al. 2003). Modellers examine the potential impact of climate change by investigating the current relationships between species distributions and climate and by projecting changes in range under various climate-change scenarios (e.g. Bennett et al. 1991; Brereton et al. 1995; Hilbert 2003a; Williams et al. 2003; Hilbert et al. 2004).
Expected changes
Most Australian studies investigating the potential effects of climate change on avifauna have focussed on range shifts under various future climate scenarios. One of the earliest comprehensive studies was that of Bennett et al. (1991) who used the Atlas of Victorian Wildlife (Department of Sustainability and Environment) and the computer package BIOCLIM (a bioclimatic analysis and prediction system; Busby 1988, 1991; Nix 1986) to examine the potential effects of climate change on 40 species of Victorian vertebrate fauna (17 of which were birds; see Table 1). Six climate-change scenarios were considered.

|
According to current CSIRO projections (Australian Greenhouse Office (AGO) 2002; Whetton et al. 2002), of the six scenarios considered by Bennett et al. (1991), the one representing a 3°C temperature rise and a 10% decrease in rainfall in all months is the most realistic for south-eastern Australia, as projected changes involved decreases in annual rainfall and increases in temperature of the order of 0.7–5.0°C by the year 2070. As there is less certainty in the rainfall projections, Bennett et al.’s conclusions for this scenario, and for a 3°C temperature rise with no change in rainfall, are shown in Table 1. The bioclimates of all bird species examined were predicted to decrease in distribution with a 3°C temperature increase, regardless of rainfall changes. The bioclimates of at least three bird species were projected to disappear from Victoria: Western Whipbird (Psophodes nigrogularis), Mallee Emu-wren (Stipiturus mallee), and the Helmeted Honeyeater (Lichenostomus melanops cassidix). Other species predicted to be severely affected were: the Malleefowl (Leipoa ocellata), Red-tailed Black-Cockatoo (Calyptorynchus banksii graptogyne), Orange-bellied Parrot (Neophema chrysogaster), Ground Parrot (Pezoporus wallicus), Rufous Bristlebird (Dasyornis broadbenti), and the Red-lored Whistler (Pachycephala rufogularis). In a subsequent report, Brereton et al. (1995) noted that some of the species most sensitive to climate change were the lowland mallee and grassland species, together with those with restricted habitats or narrow habitat requirements. Brereton et al. (1995) found that the species less affected by climate change were the forest dwellers, probably owing to the wide altitudinal range of forest regions in south-eastern Australia. Mixed responses were found for woodland and dry forest species.
Interpretation of studies such as that of Bennett et al. (1991) and Brereton et al. (1995) need to take into account a number of assumptions that underlie such modelling exercises. Importantly, the models assume that the present-day distribution of a species is in equilibrium with present-day climate, and that climate is the most important factor determining the distribution. It is likely, however, that many species do not currently occupy the full range of potentially suitable climatic envelopes, because of non-climatic factors such as the presence of natural enemies, competition, or human-induced habitat loss. Applying models to these species underestimates the ‘climatic envelope’ they may tolerate. The use of historical, rather than current, ranges may help, although there may be other issues associated with the level of confidence of the historical range and with regions that may have already started to experience climate change. The habitat required by a species may not move as expected under climate change and there may be time delays involved, as plants are generally slower to disperse. Similarly, defining a climatic envelope within a non-biological distribution (i.e. a subset of a distribution, such as that contained within a state) may underestimate climatic tolerance. Underestimating the tolerance of species to climate will result in overly pessimistic predictions. For more detailed reviews of the use and abuse of bioclimatic models, see Baker et al. (2000) or Pearson and Dawson (2003).
A species of the wet tropics, the Golden Bowerbird (Prionodura newtoniana) is also predicted to be severely affected by climate change (Hilbert 2003a; Hilbert et al. 2004). With a 1°C warming and 10% decrease in rainfall, the habitat of the Bowerbird is expected to decrease to 63% of its current size. If temperatures rise by 3°C, with a 10% decrease in rainfall, modelling using artificial neural networks indicates that the potential habitat size may contract from 1564 km2 in several patches to 37 km2 (Hilbert et al. 2004). Other fauna endemic to the region are also expected to be negatively affected by climate change because species restricted to ‘island-like’ upland areas have limited ability to disperse to more suitable climates (Williams et al. 2003). Under global warming, soil-moisture levels in most regions are also expected to fall, directly affecting frogs, skinks and soil invertebrates and this may indirectly affect birds via their food supply (Williams et al. 2003). Additional threats such as habitat loss and fragmentation and competition from invasive species may accelerate species declines (Thomas et al. 2004).
Regional modelling studies, such as those for the highly regulated Macquarie River system and Macquarie Marshes (Johnson 1998; AGO 2002; Herron et al. 2002), suggest that future decreases in mean annual flow into the marshes are likely (reductions are expected to be 11–32%). This may result in a 20–40% reduction in area of the semi-permanent and ephemeral wetlands by the year 2030. Although most climate models project an increase in the incidence and intensity of extreme daily rainfall (Walsh et al. 2004), only major floods encroach onto the floodplains, owing to the highly regulated nature of the river systems (Beare and Heaney 2002). In addition, the timing of the rainfall events is critical, with wetlands more likely to flood when the rainfall coincides with high river levels, projected to occur less often under most scenarios (Beare and Heaney 2002). The implications for the many birds that use this area include less frequent breeding opportunities and possible declines or extinctions of local populations. Warmer temperatures, reduced rainfall, increased evaporation and decreased surface-water flows are expected over the entire Murray–Darling Basin, not just in the Macquarie River catchment (Beare and Heaney 2002). This would have large negative impacts on the many waterbirds that breed in this area (e.g. Kingsford and Norman 2002).
Birds are often seen as carriers of disease and when birds are driven by drought into urban parks, city residents may harbour safety concerns (Woodford 2003). Under global warming, increased frequency of droughts resulting in influxes of bird species to coastal regions is likely to result in an increase in conflicts between city-dwellers and birds, such as Galah (Cacatua roseicapilla), Sulphur-crested Cockatoo (C. galerita) and, in particular, ibis (Threskiornis sp., Woodford 2003). Other nomads moving in response to droughts may also affect humans. With the frequency and severity of drought expected to increase under many climate-change predictions, conflicts between farmers and some bird species, such as the Emu (Dromaius novaehollandiae) are likely to become increasingly common (e.g. see Beeby 2003b).
Increased drought frequency may also be expected to magnify or bring to completion sustained declines already evident for some species (e.g. the Paradise Parrot (Psephotus pulcherrimus) at Coomooboolaroo in central Queensland; Woinarski and Catterall 2004). Widespread droughts can also mark the onset of influxes of species, such as the Cockatiel (Nymphicus hollandicus), Budgerigar (Melopsittacus undulatus) and Yellow-throated Miner (Manorina flavigula) to central Queensland (Woinarski and Catterall 2004).
In a comprehensive survey of changes in forest avifauna in the south-west of Western Australia, Abbott (1999) speculates that increasing temperatures may allow greater penetration into the south-west of bird species currently restricted to eastern sectors of the forest. This would occur via increased competition for water by plant species, ultimately resulting in more open forests. Increased temperatures are also expected to increase fire frequency (Cary 2002), resulting in changes to forest structure. If rainfall also decreases, the distribution of Bassian bird species is expected to contract towards the higher rainfall regions in the lower south-west. Arnold (1988) investigated potential changes in the distribution of fauna in south-western Australia (a summary of the findings for bird species is shown in Table 2). As the south-west becomes drier during winter, Bassian species are expected to contract to the wetter south-west corner and Bassian–Eyrean species to spread into the south-west. The most noticeable impacts in the region are likely to be observed for the wetland species, with large reductions expected as coastal wetlands are lost. Other impacts expected are the loss of trees (and their associated hollows) and changes in the winter habitats of many trans-equatorial migratory wader species. The risk of a rapid loss of species that are found within narrow annual mean temperature ranges is high within the next 20–50 years (Pittock 2003 and references therein).
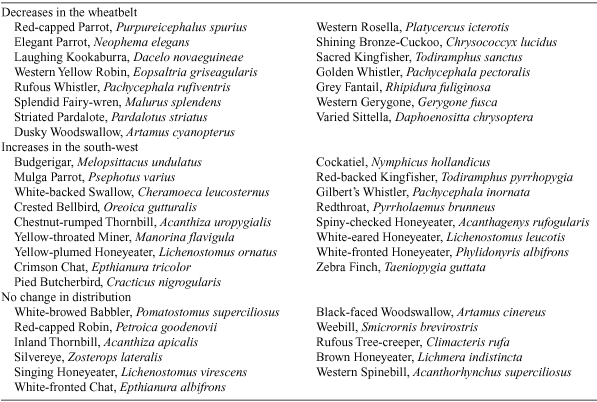
|
In south-eastern Australia, breeding success in Little Penguins (Eudyptula minor) has been linked with ocean temperatures in Bass Strait (Chambers 2004). Global warming is expected to increase ocean temperatures around Australia and may result in earlier laying (and in turn improved breeding success) for the Penguins of Phillip Island, Victoria (Chambers 2004).
Predicted reductions in extent and duration of snow cover in alpine regions (Whetton 1998) may have significant negative impacts on many alpine species (Green 1998). The expected combination of warming and reduced snow cover translates to a medium to high risk (over the next 50 years) of species losses within Australian alpine ecosystems (Pittock 2003). According to Pittock (2003) there is little that can be done in these regions in the way of landscape management and there is a correspondingly low rating of adaptability to climate change. Although a reduction in snow cover increases the area available to birds for feeding and breeding, potential losses of the plants and animals on which they feed may have a negative impact.
In western New South Wales species that have historically decreased in abundance were found to have certain characteristics in common: endemism, specific habitat preferences, small clutch-sizes and sedentary patterns of movement (Smith and Smith 1994). These traits are expected in birds with strong preferences for particular environmental conditions resulting in reduced abilities to cope with change (Smith and Smith 1994).
Observed changes
Terrestrial birds and waterbirds
In the Snowy Mountains, arrival times of migratory birds have advanced (in the 1980s and 1990s compared to the 1970s) (Green 2003). Seven out of the eleven species studied are now arriving at least 1 month earlier.
Several range shifts have been documented in recent literature. Reid (2003b) notes that species previously considered to be arid and semi-arid, such as the Galah, Little Corella (Cacatua Sanguinea) and Crested Pigeon (Ocyphaps lophotes) have been increasingly recorded in more temperate regions of Australia, particularly in the south and at higher altitudes, during the past 100 years. However, at least some of these range shifts can be attributed to sources other than climate change, such as changes in land-cover and land-use. Other species listed in Reid (2003b) as expanding their ranges southwards are the Figbird (Sphecotheres viridis), Channel-billed Cuckoo (Scythrops novaehollandiae), Common Koel (Eudynamys scolopacea), Australian Brush-turkey (Alectura lathami), Black-necked Stork (Ephippiorhynchus asiaticus) and the Pied Heron (Ardea picata). The extent to which these distributional changes can be attributed to changes in climate is currently unknown.
Baxter et al. (2001) have documented recent observations of the Black-necked Stork, in the Channel Country in north-eastern South Australia, well to the south of its customary range, and suggested that monsoon-flooding in Queensland and far-northern South Australia during the summer–autumn of 2001 may explain this unusual occurrence. Recent observations of the Magpie Goose (Anseranas semipalmata) in this region, a vagrant well outside its usual range, have also been noted (Baxter et al. 2001). While annual floods down the major rivers in the Channel Country in the summers of 1999–2001 were much larger than average annual flows and were linked to a La Niña phase of ENSO, they were not as great as the largest floods experienced in previous La Niña periods, such as 1974–76 and 1989–91. No records of Magpie Goose or Black-necked Stork were reported from north-eastern South Australia during these previous La Niña events. Baxter et al. (2001) concluded that the intrusion of these species into the area is highly unusual and may not have occurred in the recent past. However, vagrant birds often occur in unusual habitats, and well outside their normal range, making the underlying causes of these occurrences uncertain.
Olsen et al. (2003) comment that the southern range extensions of the Noisy Pitta (Pitta versicolor) and Pacific Baza (Aviceda subcristata) are consistent with climate change. They also note that high altitude species, including the Rufous Scrub-bird (Atrichornis rufescens), Gang-gang Cockatoo (Callocephalon fimbriatum) and Flame Robin (Petroica phoenicea), were ‘over-represented among the species showing a decrease in reporting rate’ and may provide early indications of climate change. Southern range expansions of some species, including several fruit-eating pigeons, may also be linked to climate change (Olsen et al. 2003).
In eastern Australia, the southward expansion of the range of the Beach Stone-curlew (Esacus neglectus) – a northern species – broadly corresponds to the southern contraction of the Hooded Plover (Thinornis rubricollis) – a southern species (Garnett and Crowley 2000). These types of changes are predicted under global warming.
White-throated Nightjars (Eurostopodus mystacalis) and Little Bronze-Cuckoos (Chrysococcyx minutillus), once thought to be strictly summer visitors to south-eastern Queensland, are now over-wintering (Roberts 2003). Other changes observed in the region are Forest Kingfishers (Todiramphus macleayii) breeding twice a year (compared with once historically) and Noisy Pittas, which normally feed in the warmer lowland regions during the winter, now spending the entire year in the mountain forests (Roberts 2003). All these changes may be linked to a warming climate.
Productivity in the Blue-breasted Fairy-wren (Malurus pulcherrimus), in south-western Australia, is significantly lower during dry years and concerns exist over the negative effects that climate change may have on this species (Rowley and Russell 2002). Reduced mean winter rainfall, and corresponding increases in the number of dry or drought years, can lead to a reduction in survival rates of native plants and decreased seed production, negatively affecting granivores, such as some parrots (Mawson and Long 1995). The observed rainfall reduction in the south-west of Western Australia, together with other factors such as altered fire regimes, land clearing, salinity changes, and grazing by introduced herbivores, is likely to be linked to changes in distribution and local population declines in the Regent Parrot (Polytelis anthopeplus) and the inland subspecies of the Western Rosella (Platycercus icterotis xanthogenys) (Mawson and Long 1995).
Severe droughts, combined with high stocking rates and rabbit plagues, are thought to be responsible for the dramatic decline and range contraction of the eastern subspecies of Thick-billed Grasswren (Amytornis textilis) (National Parks and Wildlife Service (NPWS) 2002).
Seabirds
Changes in ocean temperatures and climatic conditions along the coast of Western Australia are suggested as the main reason for the observed range expansions of seabirds such as the Red-tailed Tropicbird (Phaethon rubricauda), Bridled Tern (Sterna anaethetus), and Roseate Tern (Sterna dougallii) (Dunlop and Wooller 1986; Dunlop 2001). These range shifts suggest that tropical species are extending their ranges southward while southern, high-latitude, cool-water species are extending their breeding seasons. Dunlop and Wooller (1990) raise the concern that increasingly warm Leeuwin Currents, combined with tropical seabird range expansions, may compromise the marine habitats of some of the southern cool-water species.
These changes to the climate and ocean are also thought to explain the anomalous (double nesting or protracted) breeding seasons of Little Penguin, Pied Cormorant (Phalacrocorax varius), Silver Gull (Larus novaehollandiae), Crested Tern (Sterna bergii) and Roseate Tern (Dunlop and Wooller 1986; Dunlop 2001).
More frequent and stronger ENSO events, and warmer SST in Bass Strait, may have influenced the observed increases in Australasian Gannets (Morus serrator) in southern Australian waters (Bunce et al. 2002). Another potential response to climate change is the increased distances travelled by Short-tailed Shearwaters (Puffinus tenuirostris) to obtain food for their chicks (Olsen et al. 2003).
Abnormally high SST on the Great Barrier Reef during summer in 2002 were accompanied by reduced provisioning, decreased growth rates and reproductive failure in the Wedge-tailed Shearwater (Puffinus pacificus), indicating that adults were unable to compensate for changes in prey availability (Smithers et al. 2003). On Heard Island, dramatic increases in the number of Black-browed Albatross (Diomedea melanophris), a trebling over the last 50 years, has been linked to changes in both climate and improved fishing practices around the island (Kirkwood and Mitchell 1992; Bergstrom 2003). Numbers of King Penguins (Aptenodytes patagonicus) on Heard Island have also increased, possibly in response to changes in atmospheric and oceanographic circulation (Budd 2000). In the southern Indian Ocean, air temperatures have been steadily increasing since the 1960s and, at the same time (but lagging by 2–9 years), the populations of most seabirds in the region has decreased (Weimerskirch et al. 2003).
On the western Antarctic Peninsula (WAP), mid-winter warming and the associated reduction in sea-ice cover, combined with increased early spring snow, is thought to be responsible for recent declines in Adelie Penguin (Pygoscelis adeliae) numbers (Kaiser 1997). In the Ross Sea the growth of colonies of Adelie Penguins is ‘inversely proportional to sea-ice extent during winter with a five-year time lag’ (Ainley 2002) and related to the Southern Oscillation (Wilson et al. 2001). In contrast, warming trends in the WAP have lead to increased numbers and a southward expansion of Chinstrap Penguins (Pygoscelis antarctica), a species that prefers open water (Fraser et al. 1992; Smith et al. 1999; Woehler et al. 2001). The Adelie Penguin has been designated the status of an environmental indicator, owing to the large amount of research that has been carried out on this species and the belief that it is a sensitive indicator of ecosystem change (Ainley 2002). Warm ocean temperatures and reduced sea-ice extent have also been associated with increased mortality in Emperor Penguins (Aptenodytes forsteri), whereas hatching success was lower when winter sea-ice was extended (Barbraud and Weimerskirch 2001).
Climate change impacts and conservation policy in Australia
Climate change should be considered as a major threat to avifauna in Australia. Some societies, such as the Ecological Society of Australia (ESA), now include position statements on climate change on their websites. In particular, the ESA (http://www.ecolsoc.org.au) encourages scientific research into: identifying species and habitats most under threat from projected climate changes; predicting changes in the ‘structure, suitability and distribution of habitats of native and introduced species’; links between habitat changes and species population changes (including interactions) and ‘improving understanding of mechanisms and capabilities of dispersal and migration in a wide range of species and the interaction between landscape fragmentation and the mobility and dynamics of plant and animal populations’.
Of the 17 Commonwealth recovery plans currently available for Australian birds (Table 3), only three explicitly list climate change as a threat (those for the Malleefowl, Abbott’s Booby (Papasula abbotti) and for some colonies of albatross and giant-petrel). Severe or extended droughts are listed as threats for a further three species (Lord Howe Woodhen (Gallirallus sylvestris), Helmeted Honeyeater, and Thick-billed Grasswren) and flood and fire frequency for the Helmeted Honeyeater. In addition, Table 4 lists species identified as being threatened by climate or climate-change processes in the Action Plan for Australian Birds 2000 (Garnett and Crowley 2000).
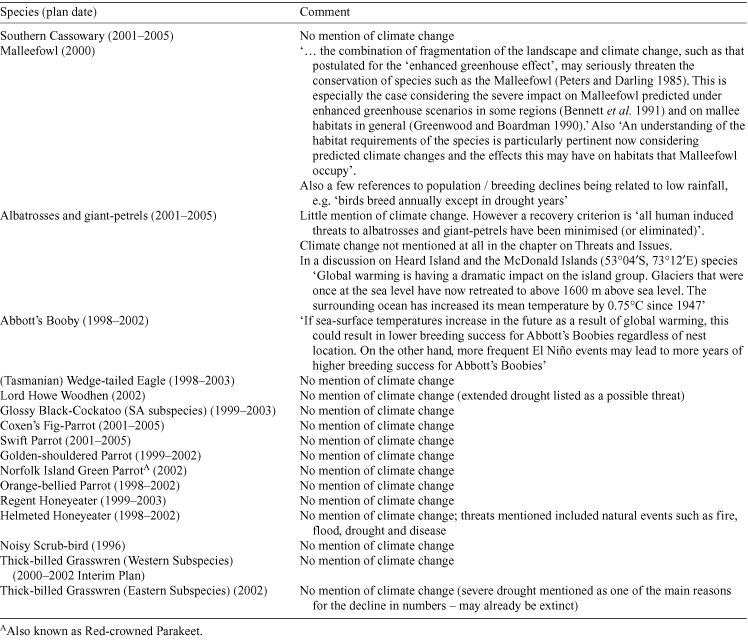
|
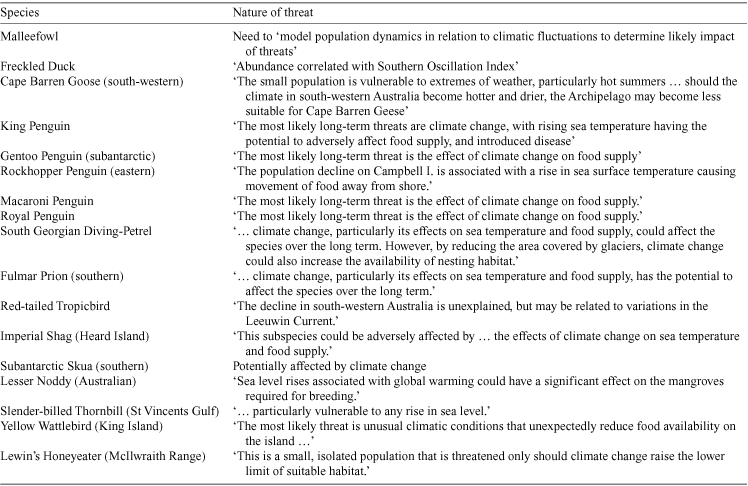
|
Priorities and gaps in knowledge
There are a large number of knowledge gaps associated with climate change and its potential impact on Australian avifauna. Some of the key questions and gaps are listed below (see also Dunlop and Howden 2003; Howden et al. 2003):
-
How are Australian bird species already responding to climate change?
-
What are the expected changes and how fast will species respond? We need to understand the factors that determine species resilience and adaptive capacity, including the role of habitat fragmentation, quantity and quality. We also need to determine for which regions, and for which species, reductions in pressures on biodiversity other than climate change would increase resilience to climate change.
-
How important is climate as a driver of change? We need to investigate this question at the level of species, ecosystems and regions, particularly those that are expected to be most vulnerable to climate change.
-
Which processes and phases of species’ life cycles are most likely to be affected by climate change? We need to understand which factors influence the distribution and abundance of species, particularly those affecting survivorship, and other parameters that are important in the population dynamics of species.
-
How will the impact of climate change on biodiversity affect economic and societal values relevant to birds and bird conservation? For example, projected increases in drought frequency are likely to result in higher concentrations of birds in coastal and irrigated regions. Conflicts may result between landholders and some species, such as cockatoos and Emu, and with conservationists (e.g. if control measures are introduced).
-
How should existing biodiversity conservation actions be improved to account for climate change? We need to develop policies that are robust to the uncertainties and to the long response times of climate impacts and adaptations. We also need to consider the practicality of controlling the spread of native or naturalised species that may become invasive under climate change.
-
What characteristics define locations that could become climate-change refuges and how can we manage shifting mosaics of ecosystems and species? Can potential barriers to movement and adaptation be identified and reduced?
-
What are the risks associated with translocating species and other interventionist management actions?
Managing the effects of climate change on birds
From a conservation viewpoint, a key gap in our knowledge is how the effects of climate change on birds can be managed to maintain diversity of wild living populations. At present we do not have sufficient information to set priorities for the management of climate-change impacts (Howden et al. 2003). However, a number of general management strategies are available:
-
Promoting adaptation among birds. Some species may be able to adapt ‘naturally’ to climate change, through shifts in range, or micro-evolutionary adaptation in situ (Howden et al. 2003). Facilitation of such adaptation may occur through management efforts that enhance the potential for range shifts (e.g. by promoting landscape connectivity), or by maintaining genetic diversity within existing populations (e.g. by reducing other threats that limit population size). Some initiatives along these lines are currently underway in Australia, including initiatives that emphasise the importance of connecting landscapes across latitudinal and altitudinal zones, such as the Gondwana Links program in Western Australia (www.gondwanalink.org) and NatureLinks in South Australia (www.environment.sa.gov.au/biodiversity/naturelinks.html). Studies of the adaptive capacity of different species could also be conducted in captivity or in controlled translocation experiments.
-
Promoting resilience among birds. Many bird species are negatively affected by anthropogenic influences, which apparently act in a cumulative or interactive fashion. Alleviation of some of these threats will enable bird populations to withstand greater climatic variation than would have otherwise been possible. Some effort towards alleviation of threats to birds is occurring in some parts of Australia, but this effort is not yet part of an overall strategy to optimise benefits to avian biodiversity, and population studies are needed to determine how sensitive populations are to different threatening processes, including climate change.
-
Intensive management of climate-sensitive species. The most probable mechanism through which species management will occur is through existing threatened species management frameworks, such as recovery plans. Examples of possible management actions include translocating species, identifying climate-change ‘refuge’ sites, linking sites across regions, or more intensive management of some key habitats (Howden et al. 2003). Some of these interventions are controversial and under-developed (see Weston 2002).
-
Ensuring mitigation measures do not compromise bird conservation. An indirect consequence of climate change is the drive to decrease CO2 emissions through the application of new power-generating technologies. These developments can impinge upon bird habitat, and in the case of windfarms, there is significant debate over the impact of wind turbines on birds, including threatened species such as the Orange-bellied Parrot (Starks 2000; Weston 2003b; Mairs 2004). Similarly, measures to reduce coastal erosion, which may be exacerbated by rises in sea level, has the potential to damage habitat of coastal dwelling species such as the Hooded Plover (Weston 2003a). Although reductions in greenhouse gas emissions are clearly desirable, planning of such developments should fully account for bird conservation.
-
Monitoring. One way to increase our knowledge of how to manage birds in the face of climate change is to design and implement long-term monitoring systems, which can flag emerging problems and be used to assess the success of existing conservation efforts (see Olsen et al. 2003). This is dealt with in detail below.
Monitoring climate change and its impacts
Effective environmental monitoring relies on choosing suitable indicator species and measuring appropriate parameters. For long-term monitoring, it is also important to build on past monitoring to increase the time series available for analysis. This section discusses the choice of indicators and summarises available data sets that may have application to the study and management of birds under climate change.
Selecting climate-change indicators and parameters
Two of the main types of indicator organisms are ‘detectors’ and ‘sentinels’ (Hughes 2003b). A detector species occurs naturally in an area of interest and may display measurable responses to changes in the environment. Species known to be sensitive to environmental changes that are introduced to the environment, as early-warning devices, are known as sentinels. Detectors are expected to be the most useful indicators for monitoring climate-change impacts (Hughes 2003b).
A number of filters have been suggested to aid the selection of a climate-change indicator (CCI) species (Hughes 2003b). The most important is that the species is sensitive to climate, or strongly suspected to be so (in distribution, physiology or life cycles), and less affected by other changes in the environment (such as land-use changes, or predator control). The availability of historical data for use as a baseline is also desirable, although long-term monitoring studies on Australian species are uncommon (see below). Climate-related distribution changes are more likely to be detected earlier at range boundaries, so monitoring locations should be carefully placed. Mobile species are probably able to move to more favourable climatic zones faster than more sedentary species with low rates of dispersal and thus are expected to be more useful as early climate-change detectors. It is an advantage if the CCI species is representative of a whole functional group so that results can be more readily generalised to other species. Sparks and Smithers (2002) suggest a number of additional criteria, particularly relevant to phenological studies, that are aimed at increasing widespread community involvement. These include selecting fairly common species that occur in both urban and rural environments. Finally, the ideal CCI species needs to be easily recognisable, easy to observe, and should be cost-effective to monitor on a repeatable basis.
According to Sparks and Menzel (2002) ‘phenology is the most responsive aspect of nature to warming and the simplest to observe. While it is anticipated that global warming will affect species distributions, population sizes and community composition, … changes in these will be much harder and more expensive to detect. The timing of events is a very simple concept to understand and is popular with the general public and with the media’, and has the potential to increase community awareness and involvement. Sparks and Menzel (2002) recommend that at least 20 years of data be used to detect trends in phenological time series with regard to climate signals. The start and end dates are also important, as particular decades tend to be warmer, or cooler, than others (for example the 1990s was a particularly warm period and the inclusion of data from this decade increases the likelihood of detecting a trend). The website ‘Biowatch’ hosted by Macquarie University (www.bio.mq.edu.au/ecology/biowatch) is now capable of receiving phenological data from the community and readers of this review are encouraged to contribute (B. Rice, personal communication). Detailed observations of bird breeding can also be submitted to Birds Australia’s Nest Record Scheme (www.birdsaustralia.com.au).
An interesting study is suggested by Serventy (1977) and involves the mapping over time of the hybrid zone of black-backed and white-backed forms of Australian Magpies (Gymnorhina t. tibicen and G. t. hypoleuca) in south-eastern Australia. Does this zone shift north and south according to climatic changes or is the ‘drier-country tibicen’ invading the area of the ‘humid-country hypoleuca’, as was suggested around the time of Serventy’s paper (Condon (personal communication) in Burton and Martin 1976; Serventy 1977)?
Available data sets
Table 5 lists some Australian avian data sets of potential use for monitoring the effects of climate change. It is highly likely that other data sets exist, but are not yet widely known, such as those collected by amateur naturalists. Longer-term data sets allow an examination of changes in parameters over time, such as laying dates, migration dates, or abundance measures. Some of these changes may then be attributable to changes in climate. Shorter data sets of sufficient quality, scope and repeatability, could be used as baselines for future monitoring. Other data sets can provide information on interactions between birds and environmental parameters, for example relationships between waterbird counts and rainfall.
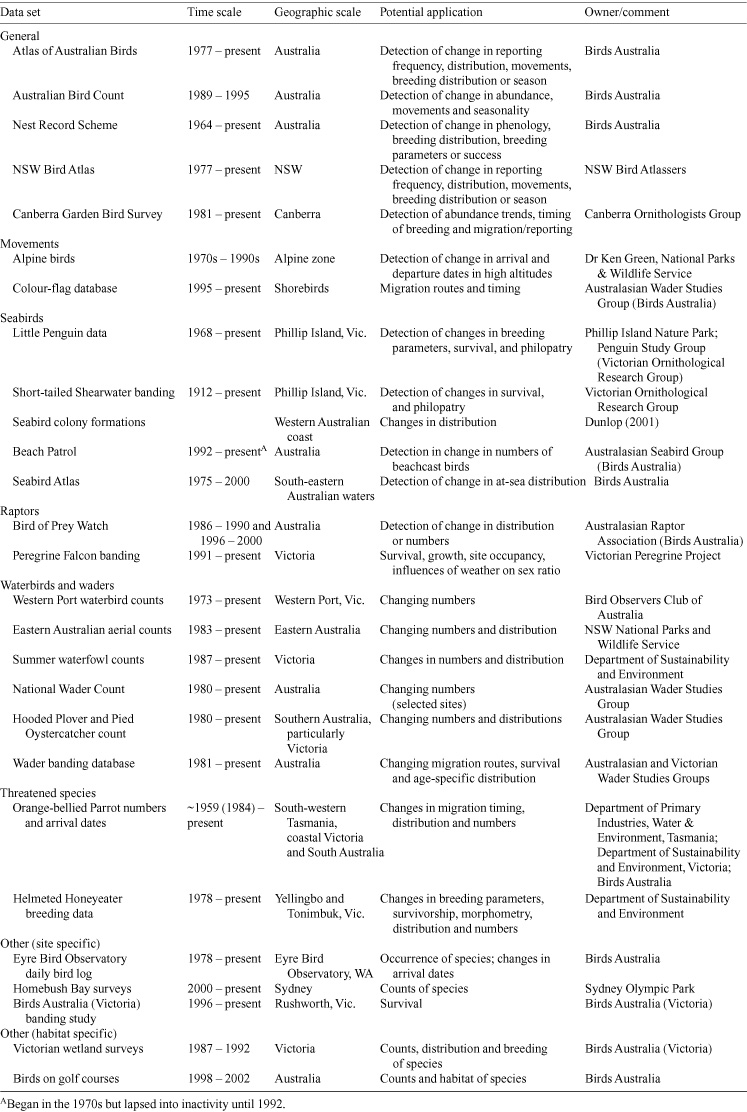
|
Long-term data sets on Australian birds are few, and are often limited in scope to a particular species or site (see Table 5). In late 2003, Olsen et al. (2003) compiled a list of long-term data sets on Australian birds that were still extant. Of the long-term monitoring programs identified, most were initiated last decade, with only a few spanning 30 or 40 years (see Fig. 1). These programs had run for 5 to 41 years.
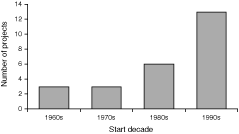
|
In addition, monitoring effort is sometimes not uniform over the life of the monitoring period. This is illustrated in Fig. 2, which shows the number of records submitted to the Birds Australia Nest Record Scheme from 1964 to 1998.
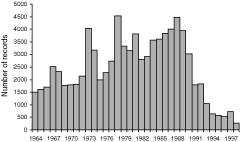
|
Recommendations for data collection and analysis
-
Although not originally collected for the purpose, there are a number of long-term data sets, documented in Table 5, that are yet to be analysed in relation to climate-change impacts. Additional data sets are likely to be identified that may also provide useful links between climate and avian systems. It is hoped that increasing awareness of the importance of avian data for climate-change impact work will result in even more data becoming available, that the community-at-large may become increasingly involved in data collection, and that there will be a new concentration of research in this area. The availability and collection of these data will also depend upon the availability of funding for long-term monitoring.
-
There is a need to identify and evaluate currently available long-term avian data sets available in Australia (Hughes 2003b). This will involve a survey of scientists and amateurs to locate data sets not currently listed. Further information can be collected from the scientific literature and relevant experts on species for which we do not have historical data, to determine their future usefulness for detection of the effects of climate change. Importantly, we need to have a national monitoring program for climate-change indicators. A useful model for such a program is the set of 34 climatic, environmental and socio-economic indicators selected in the UK (Cannell et al. 1999).
-
The use of phenological data sets is recommended for species with long-term monitoring programs. For migratory birds, changes in phenology, such as arrival and laying dates, will most likely provide the clearest links between climate change and avian ecology, as these parameters are influenced less by changes in management practices than measures of abundance.
-
Historical and current distributional data can be used in modelling studies designed to predict future species ranges under climate change, if direct links can be found between the species and climate parameters. Such modelling should be robust to problems identified in some previous modelling attempts. For example, modelling of distributions should not be restricted to socio-political subsets of species distributions (see above). In addition, modelling of potential shifts in species distribution in relation to the distribution of current reserve networks is needed to assess how well the current system will help conserve species in the future. Several studies from elsewhere in the world may provide a model for such analysis (e.g. Rutherford et al. 1999; Burns et al. 2003).
Implications for policy
‘Loss of terrestrial climatic habitat caused by anthropogenic emissions of greenhouse gases’ was listed as a key threatening process under the Australian Environmental Protection and Biodiversity Conservation Act 1999 (Commonwealth) in April 2001 (http://www.deh.gov.au/biodiversity/threatened/ktp). It is important to note that the scope of a Threat Abatement Plan under this Act is for adaptation to climate change, rather than working towards reducing greenhouse gas emissions, reducing climate change or reducing climatic habitat loss (owing to the global nature of the threat). For affected species and communities listed under the Act, the Act suggests that adaptive responses to climate change should be facilitated by a ‘habitat conservation planning regime’.
The Threatened Species Scientific Committee, set up under the Act, concluded that ‘along with the issues of emissions reduction, the adaptation requirements of species and communities likely to be affected by climate change should be given greater priority’ (http://www.deh.gov.au/biodiversity/threatened/ktp/greenhouse.html).
In the context of what has been documented in this paper, it is important that those involved in the development of recovery plans should consider the potential of climate change to act upon the target species and its community. Direct or indirect effects of climate change will affect most species, though other non-climate related threats, such as habitat loss as a result of land-clearing, may need to be given greater priority in the short-term. Another way of approaching this is to develop climate-change action plans for threatened species whose recovery plans currently do not adequately deal with climate-change impacts.
In 2003 the National Resource Management Ministerial Council in Australia launched the National Biodiversity and Climate Change Action Plan 2004–2007 (NBCCAP). The NBCCAP builds on Commonwealth and State and Territory government commitments and objectives outlined in various biodiversity and greenhouse strategies. The NBCCAP will help coordinate the activities of different jurisdictions (Commonwealth, State, Territory) in dealing with the impact of climate change on biodiversity. The actions in the NBCCAP will be integrated into the development of broader biodiversity policy and programs across all of levels of government.
Conclusions
Climate-change signals, particularly those associated with increased temperatures, have already been observed both globally and in Australia. Under enhanced greenhouse conditions, annual average temperatures are projected to further increase in the Australian region and may be associated with rainfall changes, though for rainfall there is a higher level of uncertainty associated with the projections.
Relative to the northern hemisphere, little is known about the current and potential effects of climate change on Southern Hemisphere birds, although the impacts will be significant. Here we have reviewed both observed and predicted effects of climate change on birds, with particular reference to Australian species. Potential impacts include changes in geographic range, migration patterns, morphology, physiology, abundance, phenology and community composition. The evidence presented shows that some of these changes are already happening, both overseas and in Australia.
The findings of this review, with regard to avifauna, are consistent with those of Pittock (2003) in his comprehensive Australian guide to climate change and its potential impacts: ‘the sensitivities of many Australian plant and animal species and ecosystems to climate change, as well as the potential threats to biodiversity, are still unknown’ and ‘knowledge is required for assessment of potential impacts and for development of conservation strategies’. The current paucity of documented climate change impacts from the Australian region is illustrated further in the latest IPCC report (IPCC 2001a: table TS-16 and fig. TS-11). The lack of documented impacts is unlikely to be because Australian species are unaffected by climate change, but rather the result of the scarcity of long-term monitoring data on Australian avifauna, the lack of collaboration between climate modellers and ecologists, and the lack of resources for a centralised depository or national monitoring program for climate change indicators, such as avian data sets.
This paper highlights the need for further research into the interrelationships between climate and avian communities. Without this knowledge we are unable to make environmentally, and economically, sound policies to deal with climate-change adaptation and mitigation, particularly if we wish to avoid significant damage to the ecology, economy and society of Australia. In the absence of clear information on the potential impact of climate change, adaptive management and monitoring will be key elements in dealing with the issue of climate change. Promoting adaptation and resilience is an important approach that should begin as soon as possible.
Acknowledgments
We would like to acknowledge the assistance given by the Commonwealth Bureau of Meteorology library staff, particularly Laurie Long, and by John Peter of Birds Australia. We would also like to thank Drs Rob Colman and Scott Power of BMRC and Linda Beaumont of Macquarie University for constructive reviews of this paper and the insightful comments from two anonymous reviewers.
Abbott, I. (1999). The avifauna of the forests of south-west Western Australia: changes in species composition, distribution, and abundance following anthropogenic disturbance. CALM Science Suppl. 5, 1–176.
Ahas, R. (1999). Long-term phyto-, ornitho- and ichthyophenological time-series analyses in Estonia. International Journal of Biometeorology 42, 119–123.
| Crossref | GoogleScholarGoogle Scholar |
Allan, R. J. , and D’Arrigo, R. D. (1999). ‘Persistent’ ENSO sequences: how unusual was the 1990–1995 El Niño? The Holocene 9, 101–118.
| Crossref | GoogleScholarGoogle Scholar |
Baker, R. H. A. , Sansford, C. E. , Jarvis, C. H. , Cannon, R. J. C. , MacLeod, A. , and Walters, K. F. A. (2000). The role of climatic mapping in predicting the potential geographical distribution of non-indigenous pests under current and future climates. Agriculture Ecosystems & Environment 82, 57–71.
| Crossref | GoogleScholarGoogle Scholar |
Ballard, G. , Geupel, G. R. , Nur, N. , and Gardali, T. (2003). Long-term declines and decadal patterns in population trends of songbirds in western North America, 1979–1999. Condor 105, 737–755.
Baxter, C. I. , Reid, J. R. W. , and Jaensch, R. P. (2001). First South Australian records of the Black-necked Stork, Ephippiohynchus asiaticus and occurrence of vagrants in south-western Queensland. South Australian Ornithologist 33, 164–169.
Boersma, P. D. (1998). Population trends of the Galapagos Penguin: impacts of El Nino and La Nina. Condor 100, 245–253.
Butler, C. J. (2003). The disproportionate effect of global warming on the arrival dates of short-distance migratory birds in North America. Ibis 145, 484–495.
| Crossref | GoogleScholarGoogle Scholar |
Chambers, L. E. (2004). Delayed breeding in Little Penguin: evidence of climate change. Australian Meteorological Magazine 53(1), 13–19.
Cunningham, D. M. , and Moors, P. J. (1994). The decline of Rockhopper Penguins Eudyptes chrysocome at Campbell Island, Southern Ocean and the influence of rising sea temperatures. Emu 94, 27–36.
Dunlop, N. (2001). Sea-change and fisheries: a bird’s-eye view. Western Fisheries Magazine Spring, 11–14.
Gaston, A. J. , Hipfner, J. M. , and Campbell, D. (2002). Heat and mosquitoes cause breeding failures and adult mortality in an Arctic-nesting seabird. Ibis 144, 185–191.
| Crossref | GoogleScholarGoogle Scholar |
Haylock, M. , and Nicholls, N. (2000). Trends in extreme rainfall indices for an updated high quality data set for Australia, 1910–1998. International Journal of Climatology 20, 1533–1541.
| Crossref | GoogleScholarGoogle Scholar |
Herron, N. , Davis, R. , and Jones, R. (2002). The effects of large-scale afforestation and climate change on water allocation in the Macquarie River catchment, NSW, Australia. Journal of Environmental Management 65, 369–381.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Hilbert, D. W. , Bradford, M. , Parker, T. , and Westcott, D. A. (2004). Golden bowerbird (Prionodura newtonia) habitat in past, present and future climates: predicted extinction of a vertebrate in tropical highlands due to global warming. Biological Conservation 116, 367–377.
| Crossref | GoogleScholarGoogle Scholar |
Hubalek, Z. (2003). Spring migration of birds in relation to North Atlantic Oscillation. Folia Zoologica 52, 287–298.
Huppop, O. , and Huppop, K. (2003). North Atlantic Oscillation and timing of spring migration in birds. Proceedings of the Royal Society of London. Series B. Biological Sciences 270, 233–240.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Inouye, D. W. , Barr, B. , Armitage, K. B. , and Inouye, B. D. (2000). Climate change is affecting altitudinal migrants and hibernating species. Proceedings of the National Academy of Sciences, USA 97, 1630–1633.
| Crossref | GoogleScholarGoogle Scholar |
Jarvinen, A. (1994). Global warming and egg size in birds. Ecography 17, 108–110.
Jones, J. , Doran, P. J. , and Holmes, R. T. (2003). Climate and food synchronize regional forest bird abundances. Ecology 84, 3024–3032.
Kingsford, R. T. , and Norman, F. I. (2002). Australian waterbirds – products of the continent’s ecology. Emu 102, 47–69.
| Crossref | GoogleScholarGoogle Scholar |
Olsen, P. , Weston, M. , Cunningham, R. , and Silcocks, A. (2003). The State of Australia’s Birds 2003. Wingspan 13(4),Supplement
Plummer, N. , Salinger, M. J. , Nicholls, N. , Suppiah, R. , Hennessy, K. J. , Leighton, R. M. , Trewin, B. C. , Page, C. M. , and Lough, J. M. (1999). Changes in climatic extremes over the Australian region and New Zealand during the twentieth century. Climatic Change 42, 183–202.
| Crossref | GoogleScholarGoogle Scholar |
Root, T. L. , Price, J. T. , Hall, K. R. , Schneider, S. H. , Rosenzweig, C. , and Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature 421, 57–60.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Smith, R. C. , Ainley, D. , Baker, K. , Domack, E. , and Emslie, S. , et al. (1999). Marine ecosystem sensitivity to climate change. Bioscience 49(5), 393–404.
Taylor, R. H. , and Wilson, P. R. (1990). Recent increase and southern expansion of Adelie Penguin populations in the Ross Sea, Antarctica, related to climatic warming. New Zealand Journal of Ecology 14, 25–29.
Veit, R. R. , Pyle, P. , and McGowan, J. A. (1996). Ocean warming and long-term change in pelagic bird abundance within the California current system. Marine Ecology Progress Series 139, 11–18.
Weston, M. (2003b). Windfarms. Wingspan 13(1), 6–7.
Whittaker, J. B. (1999). Impacts and responses at population level of herbivorous insects to elevated CO2. European Journal of Entomology 96, 149–156.
Wilson, P. R. , Ainley, D. G. , Nur, N. , Jacobs, S. S. , Barton, K. J. , Ballard, G. , and Comiso, J. C. (2001). Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic Circumpolar Current. Marine Ecology Progress Series 213, 301–309.
Woinarski, J. C. Z. , and Catterall, C. P. (2004). Historical changes in bird fauna at Coomooboolaroo, northeastern Australia, from the early years of pastoral settlement (1873) to 1999. Biological Conservation 116, 379–401.
| Crossref | GoogleScholarGoogle Scholar |
Yom-Tov, Y. (2001). Global warming and body mass decline in Israeli passerine birds. Proceedings of the Royal Society of London. Series B. Biological Sciences 268, 947–952.
| Crossref | GoogleScholarGoogle Scholar | PubMed |


