Genetic affinities of newly sampled populations of Wandering and Black-browed Albatross
R. Alderman A C D , M. C. Double A , J. Valencia B and R. P. Gales CA Department of Botany and Zoology, Australian National University, Canberra, ACT 0200, Australia.
B Instituto Antártico Chileno, Luis Thayer Objeda 814, Provencia, Santiago, Chile.
C Marine Conservation Unit, Nature Conservation Branch, DPIWE, Box 44, Hobart, Tas. 7001, Australia.
D Corresponding author. Email: Rachael.Alderman@dpiwe.tas.gov.au
Emu 105(2) 169-179 https://doi.org/10.1071/MU04034
Submitted: 29 June 2004 Accepted: 7 April 2005 Published: 30 June 2005
Abstract
This study extends previous phylogeographic genetic studies of the Black-browed and Wandering Albatross species complexes through the addition of newly acquired genetic data from wandering-type albatrosses on Macquarie Island and Black-browed Albatrosses (Thalassarche melanophrys) on Macquarie Island and the Chilean islands of Diego de Almagro and Ildefonso. DNA sequencing of Domain I of the mitochondrial control region showed that the wandering-type albatrosses on Macquarie Island belong to the Diomedea exulans group and show close genetic affinity to populations on the Prince Edward and Crozet Islands. The populations of Black-browed Albatrosses on Diego de Almagro, Ildefonso and Macquarie Islands all fell into a distinct grouping that also included birds from Diego Ramirez, South Georgia and Kerguelen Islands. Both the Wandering and Black-browed species complexes show multiple distinct lineages, some with disjunct geographical distributions. We suggest that this is a consequence of prolonged isolation of populations during the Late Pleistocene followed by range expansion of D. exulans and T. melanophrys after glacial retreat from many subantarctic islands. Both species most likely dispersed from populations centred in the southern Indian Ocean.
Introduction
Banding studies suggest albatrosses exhibit strong natal philopatry and that gene flow between populations is very rare (Warham 1990). Thus, an allopatric model of speciation for albatrosses has been implicitly adopted in the literature, whereby restricted gene flow and variation in environmental conditions promotes divergence of geographically separate populations (e.g. Markow et al. 2002) and it is expected that many genetic, behavioural and morphological characteristics will be idiosyncratic to each breeding island (Clegg et al. 2002). This view of speciation among albatrosses heavily influenced the taxonomic revision proposed by Robertson and Nunn (1998) whereby the number of species in the family Diomedeidae was elevated from 14 to 24 to reflect the perception that ‘islands host terminal taxa’.
Several recent studies of albatrosses have investigated this view by examining the distribution of genetic variation among island populations. Some populations, from single or closely grouped islands, exhibited clear genetic differentiation with regard to other populations. For example, the detailed study by Burg and Croxall (2001) found that the Falkland Islands population of Black-browed Albatross (Thalassarche melanophrys) was clearly differentiated from all other populations of the same species. Also Abbott and Double (2003a) found that the three relatively proximate island populations of Shy Albatross (T. cauta) were genetically distinct. In contrast, many widely dispersed island populations do not show any genetic structuring. The Prince Edward and Crozet Islands populations of Wandering Albatrosses (D. exulans, Burg and Croxall 2004) are not genetically distinct despite being separated by more than 1000 km. Also, no structure was found among the widespread populations of Grey-headed Albatrosses (T. chrysostoma, Burg and Croxall 2001). These genetic studies have therefore revealed a complex picture whereby some islands host unique terminal taxa of albatrosses while others exhibit long-distance gene flow. These studies have also shown that many morphologically distinct species show clear but very low genetic divergence (Abbott and Double 2003a, 2003b; Double et al. 2003). This has inevitably promoted debate regarding the location of species boundaries among albatrosses (Penhallurick and Wink 2004).
The aim of this study was to extend the previous genetic studies of the Black-browed and Wandering Albatross species complexes (Burg and Croxall 2001, 2004) through the addition of newly acquired genetic data from Wandering Albatross on Macquarie Island and Black-browed Albatrosses on Macquarie, Diego de Almagro and Ildefonso Islands. We employ both standard population genetic approaches (Fst analysis) and nested clade analysis (NCA) to examine the geographical distribution of haplotypes derived from sequencing the Domain I region of the mitochondrial genome. By combining previously published DNA sequence data with data from newly sampled populations of Black-browed and Wandering Albatrosses, we hope to further our understanding of the population dynamics, reproductive isolation and evolutionary history of contemporary albatross populations. Analyses of both species have been presented together since both species have been exposed to similar evolutionary influences. The simultaneous comparison is informative as it reveals parallel or contrasting evolutionary patterns, which assist us to understand the possible mechanisms driving the process.
Methods
Nomenclature
For the purposes of this paper we have adopted the taxonomic nomenclature described by Croxall and Gales (1998), which is largely based on the taxonomic hypothesis of Robertson and Nunn (1998). This nomenclature is likely to change (Burg and Croxall 2004) but at present is considered a reasonable working hypothesis and has been the taxonomic basis of recent albatross genetic research (Burg and Croxall 2001, 2004; Abbott and Double 2003a, 2003b). We use the terms ‘black-browed-type’ and ‘wandering-type’ albatrosses to refer collectively to the groups of albatross species that, before the recent taxonomic revision, were classed as single polytypic species D. melanophrys and D. exulans respectively (see below, also Croxall and Gales 1998; Robertson and Nunn 1998).
Species, samples and sampling locations
Wandering-type albatrosses
The taxonomic revision by Robertson and Nunn (1998) proposed that the original Wandering Albatross was in fact composed of five species (nomenclature based on Croxall and Gales 1998): Wandering Albatross (Diomedea exulans), Amsterdam Albatross (D. amsterdamensis), Tristan Albatross (D. dabbenena), Gibson’s Albatross (D. gibsoni) and Antipodean Albatross (D. antipodensis). This revision was largely based on mitochondrial cytochrome b sequence data (Robertson and Nunn 1998) but also considered morphological and behavioural differences among putative taxa, including body size, breeding phenology and plumage maturation (e.g. Cuthbert et al. 2003; Marchant and Higgins 1990; Prince et al. 1997).
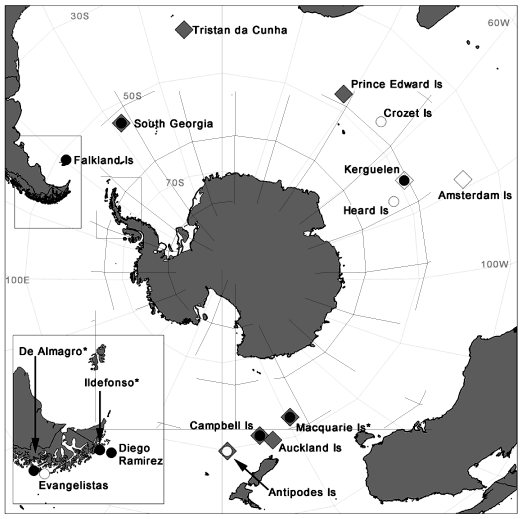
Morphology and behaviour suggest that the wandering-type albatrosses on Macquarie Island (55°30′S, 158°56′E, see Fig. 1) are more closely related to D. exulans of the southern Indian and Atlantic Oceans than D. antipodensis and D. gibsoni found in the New Zealand subantarctic region (Holdaway et al. 2001). The first records of wandering-type albatrosses breeding on Macquarie Island came from visits by sealers in the early 1800s but the records provide no estimate of breeding numbers (de la Mare and Kerry 1994; Tickell 2000). Remains of albatross bones found on the island suggest a larger population before human disturbance (Gillham 1967) but by 1911, the population had dwindled to one or two nests (Falla 1937). The population then increased steadily to 31 active nests in 1968 only to decline to two nests in 1985 (de la Mare and Kerry 1994). Currently the population is stable, with 19 pairs breeding annually (Terauds 2002). DNA analysis was conducted on nine blood samples taken in 2000 and 2001 from nestling wandering-type albatrosses in the south-western ‘Petrel Peak’ (54°46′S, 158°47′E) and north-western coast ‘Featherbed’ (54°32′S, 158°52′E) breeding locations. Samples were taken from the tarsal vein of chicks that were close to fledging.
|
Black-browed-type albatrosses
Robertson and Nunn (1998) proposed that the black-browed-type albatrosses comprise two species: the ‘true’ Black-browed Albatrosses (Thalassarche melanophrys) and the Campbell Albatross (T. impavida), which breeds only on Campbell Island. The morphometrics of these taxa differ significantly (Waugh et al. 1999); also T. impavida develop light-coloured irises as they mature in contrast to the consistently dark-brown irises of T. melanophrys (Tickell 2000). Small numbers of T. melanophrys also breed alongside T. impavida on Campbell Island and the two species are known to interbreed (Burg and Croxall 2001; Moore et al. 2001).
Black-browed Albatrosses were first recorded on Macquarie Island in 1949, despite intensive fieldwork on the island before this (Copson 1988). This has led to speculation that sealers exterminated colonies on Macquarie Island and recolonisation has occurred since (Copson 1988). A colony on Bishop and Clerk Islets, 37 km south of Macquarie Island, was discovered in 1965 (Mackenzie 1968) and other small colonies of Black-browed Albatrosses have been discovered on Campbell Island, Antipodes Islands and the Snares Islands in 1975, 1978 and 1984 respectively (Moore et al. 2001). These discoveries led to suggestions that Black-browed Albatrosses have recently colonised the Pacific (Moore et al. 2001). However, it is not known if contemporary Macquarie Island populations are the result of long-distance colonisation, or are remnants of larger populations decimated by sealers, or if they have always been small (Copson 1988). Currently, ~40 pairs of Black-browed Albatrosses nest at South West Point (54°76′S, 158°78′E) on Macquarie Island, and ~100 pairs nest on the Bishop and Clerk Islets (Garnett and Crowley 2000; Terauds 2002). In 2001, blood samples were taken from twelve nestling Black-browed Albatrosses at the South West Point colony.
Black-browed Albatrosses breed at four sites off the Chilean coast: Diego Ramirez and Diego de Almagro Islands, Ildefonso and the Evangelistas Islets (Fig. 1). Genetic data from nesting Black-browed Albatrosses sampled at Diego Ramirez were previously reported by Burg and Croxall (2001). In 2002, blood samples were taken from ten incubating adult Black-browed Albatrosses at the Cabo Gruta colony (51°24′S, 75°13′W) on Diego de Almagro. This colony contains ~6000 of the 15 900 pairs dispersed between six colonies on this island (Lawton et al. 2003). Isla Ildefonso is home to ~47 000 breeding pairs of Black-browed Albatrosses (Robertson et al. 2003) and in 2002, blood samples were taken from ten adult albatrosses nesting in the Isla Camp colony, which comprises ~29 000 breeding pairs. The Evangelistas Islets contain ~4000 pairs of Black-browed Albatross, and were only discovered as a breeding site in 2003 (Arata et al. 2003). No samples were available from this breeding location.
Sequences
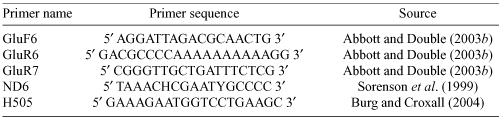
DNA was extracted from blood samples using an ammonium acetate salting-out procedure (Nicholls et al. 2000). Domain I of the mitochondrial control region was amplified from black-browed-type albatrosses using the GluF6 and GluR6 primers (Table 1; Abbott and Double 2003b) and from wandering-type albatrosses using primers ND6 and H505 (Table 1; Burg and Croxall 2004). Polymerase chain reactions (PCR) were performed using the expand high fidelity PCR system (Boehringer Mannheim, Mannheim, Germany). Each 15 µL reaction contained 50–100 ng of genomic DNA, 1.5 µL dNTPs, 3 pmol of each primer, and 0.3 µL of expand high fidelity enzyme mix in 1 × PCR buffer. The PCR cycling parameters were: 94°C for 2 min; 30 cycles of 5 s at 94°C, 30 s at 55°C and 2.5 min at 72°C, with one final cycle at 72°C for 5 min. Following amplification, PCR products were run on a 1% agarose gel and then purified using the concert DNA rapid gel extraction system (Life Technologies, Gaithersburg, Maryland, USA). Between 2 and 5 µL of purified PCR product was sequenced using the BigDye sequencing system (Applied Biosystems, Foster City, California, USA) and 1.6 pmol of primer in a 10-µL reaction volume. The nested primer GluR7 (Table 1) was used to sequence a 187 base pair section of the control region in the black-browed-type albatross and primer H505 was used to sequence a 210 base pair section of Domain I for the wandering-type albatrosses. The sequencing products were run on an ABI Prism 377 automated sequencer and sequence data were subsequently imported into sequence analysis software (Sequencher, Gene Codes Corporation, Ann Arbor, Michigan, USA).
|
Published sequences
All published DNA sequences for Domain I of the mitochondrial control region of wandering-type and black-browed-type albatrosses were downloaded from the genetic database GenBank (Benson et al. 2004). These sequences were first described in detail by Burg and Croxall (2001, 2004). Ambiguous bases within these sequences were coded as missing data for the purpose of this analysis. For simplicity, the species classification of potential hybrid T. melanophrys and T. impavida albatrosses on Campbell Island were based on their morphology rather than on haplotype (Burg and Croxall 2001; Moore et al. 2001).
Population genetic and nested clade analysis
The software Arlequin (Schneider et al. 2000) was used for descriptive and population genetic analysis of sequence data. The genetic structure between island populations of albatrosses was investigated using an analysis of molecular variance approach (AMOVA, Excoffier et al. 1992). The statistical significance of the pairwise Fst estimates for each pairwise comparison was assessed from 1023 random permutations of haplotypes among populations. A principal components analysis (PCA) was performed to display visually the genetic relationship between the individuals samples using GenAlex software (Peakall and Smouse 2001).
A nested clade analysis (NCA) is a network-based phylogeographic method that aims to disentangle historical population processes from contemporary gene flow using a simple statistical analysis of the genetic and geographical relationship among haplotypes (Templeton 1998). This approach has become widespread for the analysis of intraspecific data (e.g. Turner et al. 2000; Schultheis et al. 2002) and data from closely related species (e.g. Abbott and Double 2003b; Burg and Croxall 2004). Sequence data were converted into a statistical parsimony network with a 95% confidence limit using the software TCS v1.13 (Clement et al. 2000). Clades within this network were identified using the TCS software and following the guidelines described by Templeton and Sing (1993) and Templeton (2001). Briefly, the nesting procedure moved in from the tips of the network enclosing haplotypes connected by one mutational step into a ‘one-step clade’. This was repeated until all haplotypes within the network were enclosed in ‘one-step clades’. The next level of nesting used the ‘one-step clades’ as units to enclose in a ‘two-step clade’. This was repeated until reaching a clade level at which the next nesting level would result in the entire cladogram being enclosed in a single clade (Templeton 1998). Statistical analysis of the geographical data overlaid upon the nested cladogram was performed using GeoDis V2 software (Posada et al. 2000). Rhumb line distances (maintaining a constant angle with respect to latitude) were calculated between each sampling location. The GeoDis software tests for non-random geographical distribution of haplotypes by: (a) performing clade-by-clade contingency analyses of haplotypes and sampled locations as categorical variables; and (b) a nested geographical distance analysis. This second analysis calculates: (a) the geographical spread within a clade (Dc); and (b) the geographical spread of a clade in relation to other clades nested in the same higher level category (Dn) (Posada et al. 2000). The significance of these statistical tests was calculated by random permutation that simulates the null hypothesis of random geographical distribution for all clades within a nesting category (Posada et al. 2000). Inference keys by Templeton et al. (1995) and Templeton (2004) were used to help interpret the population genetic or evolutionary implications of significant haplotype associations. These keys principally discriminate between three biological processes: restricted gene flow; range expansion and colonisation events; and fragmentation or splitting events. Recently, authors have argued that NCA may be misleading because it does not estimate the reliability of the inference (Creer et al. 2001; Knowles and Maddison 2002) and have highlighted the need to treat any predictions with caution and to look for independent lines of supporting evidence (cf. Templeton 2004).
Results
Wandering-type albatrosses
A total of 88 newly generated and published sequences was available from eight island populations of wandering-type albatrosses (Table 2). These sequences defined 50 haplotypes, including 17 that were shared between individuals. Of these, 11 haplotypes were exclusively shared among individuals from the same island population. The nine samples from Macquarie Island defined six haplotypes, none of which was shared with any other population.

|
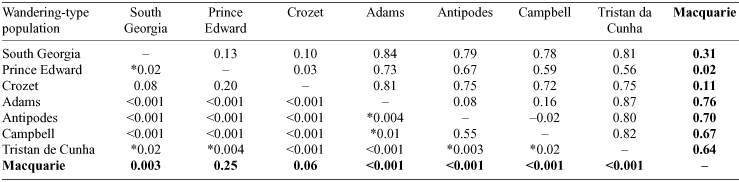
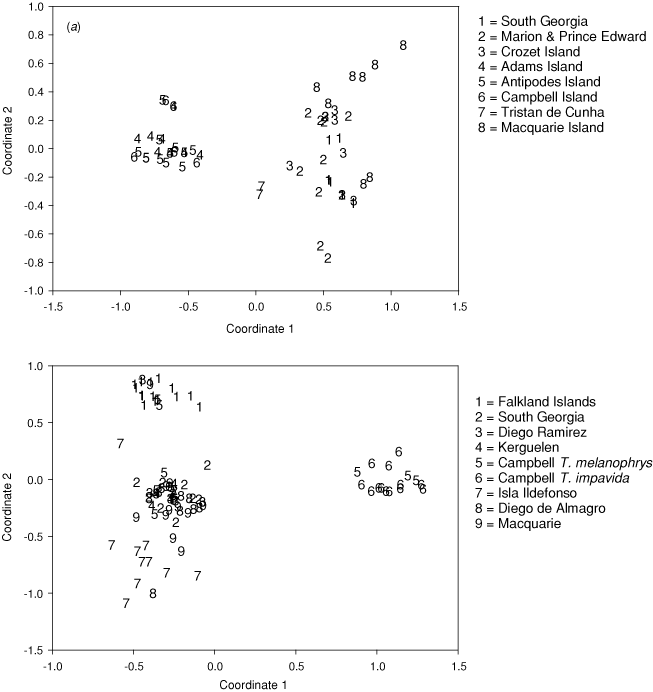
Population genetic analyses yielded pairwise Fst values (Table 3) that ranged between –0.02 (interpreted as zero) between Antipodes Island and Campbell Island, and 0.87 between D. dabbenena on Tristan de Cunha and D. gibsoni on Adams Island. Twelve of the 28 pairwise comparisons did not show statistical significance (P > 0.05) after Bonferroni correction. The pairwise Fst values and PCA (Fig. 2a) identified three population groups: (1) D. exulans from South Georgia, Prince Edward, Crozet and Macquarie Islands; (2) D. antipodensis and D. gibsoni from Adams, Antipodes and Campbell Islands; and (3) D. dabbenena on Tristan da Cunha.
|
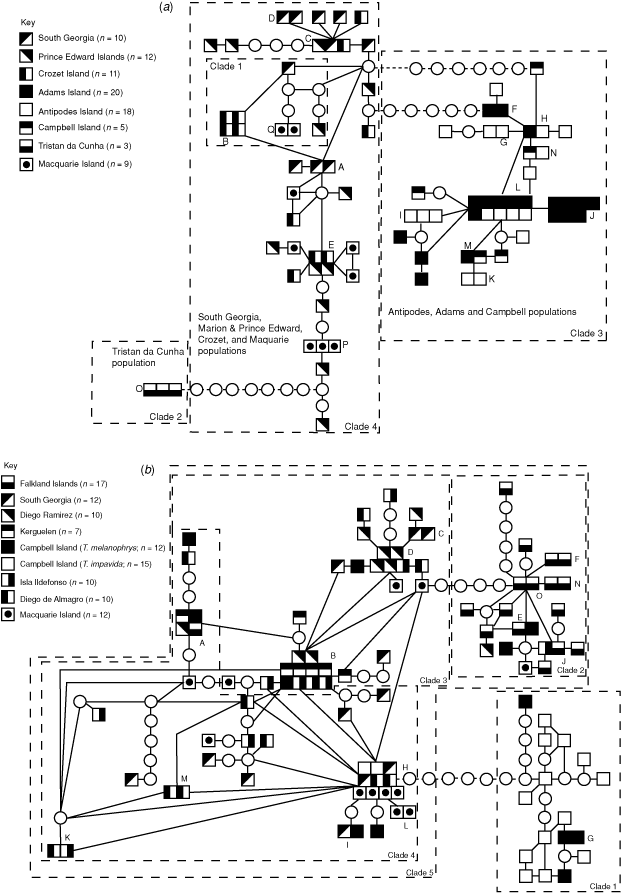
The three wandering-type groups identified above were also apparent in the construction of the parsimony network. The three groups could not form a single network within the limits of statistical parsimony (six mutational steps, Fig. 3a). Nested clade analysis (NCA) was therefore initially applied to the three networks separately and then finally the three networks were joined via most similar haplotypes to allow a final analysis of the distribution of haplotypes among the entire sample network (Fig. 3a). The South Georgia, Prince Edward, Crozet and Macquarie group was nested into a four-step clade (Clade 4) but the haplotypes within were distributed randomly. The two other discrete clades (Clades 2 and 3) were enclosed in fewer steps (one and three respectively) and were coded as four-step clades to describe the entire cladogram for statistical analysis. The null hypothesis of no geographical association of haplotypes was rejected by the nested contingency analysis for only two lower clades: one two-step clade (Clade 1: χ2 = 16; P < 0.05) and one three-step clade (Clade 3: χ2 = 12.2; P < 0.05). The NCA inference key did not predict an evolutionary scenario for the haplotype distribution in Clade 1 but suggested restricted gene flow with isolation by distance to explain the distribution of haplotypes within Clade 3. The entire network showed a highly non-random distribution of haplotypes (χ2 = 84; P < 0.001) and the discrete geographical distribution of individuals within Clades 1–3 implicates allopatric fragmentation as the likely phylogeographic scenario.
|
Black-browed-type albatrosses
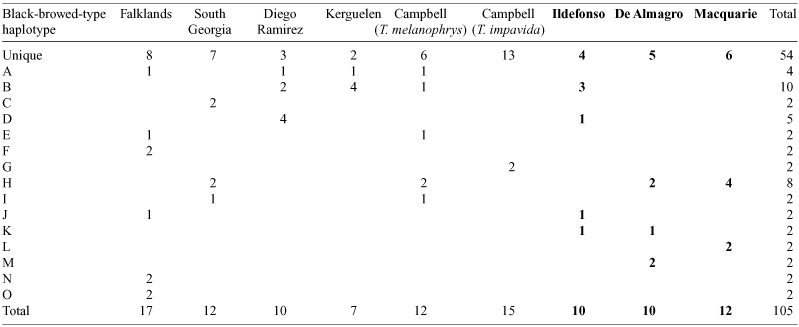
A total of 105 newly generated and previously published sequences was available from nine island populations of black-browed-type albatrosses (Table 4). These sequences defined 69 haplotypes, including 15 that were shared between individuals and of these, eight haplotypes were shared between individuals from different island populations. The twelve samples from Macquarie Island defined eight haplotypes, six of which were not shared with any other sampled individual. Of the remaining two shared haplotypes, one was unique to Macquarie and the other was also found on South Georgia, Diego de Almagro and Campbell Island. Besides this haplotype, Diego de Almagro yielded a further seven haplotypes: five unique, one shared only by individuals on this island, and one shared with Ildefonso. Ildefonso additionally shared a haplotype with Diego Ramirez, one with the Falkland Islands and a further haplotype was shared by individuals sampled from Diego Ramirez, Kerguelen and Campbell Islands (Table 4).
|
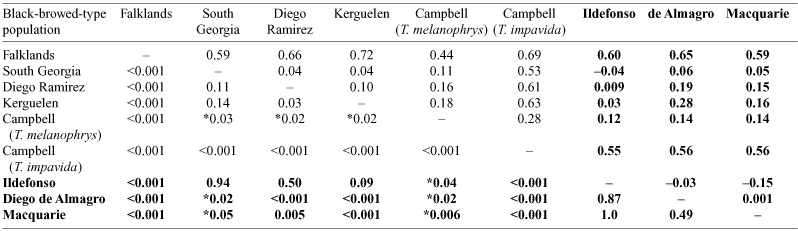
Population genetic analyses yielded pairwise Fst values (Table 5) that ranged between –0.15 (interpreted as zero) between Macquarie Island and Ildefonso, and 0.72 between Kerguelen and the Falkland Islands. After Bonferroni correction, 20 of 36 comparisons were significant (P < 0.05). Pairwise Fst values and the PCA (Fig. 2b) identified three population groups: (1) T. melanophrys from South Georgia, Kerguelen, Diego Ramirez, Diego de Almagro, Ildefonso and Macquarie Islands, including some T. impavida phenotypes; (2) T. melanophrys from the Falkland Islands; and (3) T. impavida from Campbell Island, including some T. melanophrys phenotypes. Very low pairwise Fst values were recorded among the Diego De Almagro, Ildefonso and Macquarie Island populations as well as between Ildefonso and South Georgia, and between Ildefonso and Diego Ramirez. These results appear to reflect the greater diversity of haplotypes found on Ildefonso (cf. Fig. 3b).
The T. impavida grouping on Campbell Island could not be connected to the rest of the network (Fig. 3b) within the limits of statistical parsimony. Therefore the NCA was initially applied to the two networks separately, which were then linked for the analysis of the entire network (Fig. 3b). The T. impavida group was contained within a three-step cladogram (Clade 1) while the rest of the black-browed-type albatrosses, which contained some T. impavida phenotypes, were contained within a five-step cladogram (Clade 5). To analyse the entire cladogram, the T. impavida clade was later recoded as a five-step clade. The null hypothesis of no geographical association of haplotypes was rejected by the nested contingency analysis for only the set of three-step nested clades within Clade 3 (χ2 = 12; P = 0.05; Fig. 2b). This largely reflects the absence of Kerguelen samples among the haplotypes around Haplotype D, whereas they are common in the three-step clade centred on Haplotype B. Clade 5, which contains all but the T. impavida albatross grouping, showed highly significant geographical association of similar haplotypes (χ2 = 84; P < 0.001) generated by the distinct separation of the Falkland Islands group (Fig. 3b). Similarly the entire cladogram showed highly significant geographical association of haplotypes (χ2 = 58; P < 0.001) driven by the clustering of similar haplotypes within the Falkland Island and T. impavida populations.
The inference key suggested restricted gene flow between Kerguelen and the other Black-browed populations as the explanation of the haplotype distribution within Clade 3. Past fragmentation or long-distance colonisation, or both, was inferred for the distribution of haplotypes in Clade 5. Clearly, the Falkland Island Black-browed Albatross forms a genetically distinct group but long-distance colonisation events have spread the Falkland’s haplotypes to populations as distant as Macquarie and Campbell Islands. Finally the entire cladogram implied allopatric fragmentation to explain the distribution of haplotypes between the Campbell Island T. impavida (Clade 1) and the remaining T. melanophrys (Clade 5) haplotypes.
Discussion
Wandering-type albatrosses
The wandering-type albatrosses on Macquarie Island clearly belong to the D. exulans group and show close genetic affinity to those on Prince Edward and Crozet Islands. A South Georgia–Crozet–Prince Edward grouping was previously identified by Burg and Croxall (2004), who found no differentiation between these islands even when using microsatellite genetic markers that are more suited to detect contemporary population structuring. Gene flow within this group has also been revealed through banding studies. Over a 25-year period 1.4% of the 4364 chicks banded on the Prince Edward or the Crozet Islands were exchanged between the two island groups (Cooper and Weimerskirch 2003). Also, through modelling of the dispersal characteristics of D. exulans chicks banded on the Crozet Islands, Inchausti and Weimerskirch (2002) predicted that the juvenile dispersal rates were such that the population dynamics of the colonies throughout the species’ range were interconnected. However, the Macquarie Island population of D. exulans is so far from the larger colonies in the Indian Ocean that the model predicts an extremely low probability of immigration (P < 0.0001 per juvenile). Therefore, although migration will affect genetic structure it is unlikely to impact on local population dynamics (Inchausti and Weimerskirch 2002). The genetic distances between all haplotypes within this clade are nonetheless small, implying a relatively recent common ancestor for the whole clade (see below).
As described by Burg and Croxall (2004), the wandering-type albatrosses form three distinct groups: the one described above, those breeding in New Zealand (D. antipodensis and D. gibsoni) and those breeding on Tristan da Cunha (D. dabbenena). Burg and Croxall (2004) suggested that D. antipodensis and D. gibsoni should not be regarded as distinct species because they share mitochondrial haplotypes, although little gene flow occurs between these two island populations despite their geographical proximity.
It is important to remember, however, that the picture of genetic relationships among all extant breeding sites of wandering-type albatrosses is still incomplete. Currently no genetic data are available for the colony of ~1000 breeding pairs on the Kerguelen Islands (Tickell 2000), but given the proximity of the Crozet and Prince Edward Islands populations, it is suspected that there will be relatively frequent migration between Kerguelen and these islands (Inchausti and Weimerskirch 2002). Finally, little is known about the genetic affinities of the Amsterdam Albatross (D. amsterdamensis). This wandering-type albatross breeds on Amsterdam Island in the Indian Ocean and was described as a separate species in 1983 (Roux et al. 1983). However, in character with the rest of the wandering-type albatross complex, DNA sequencing of the cytochrome b gene showed very low sequence differentiation from the other wandering-type species (Nunn and Stanley 1998). It would be extremely interesting to generate genetic data for this species that could be included in a global analysis such as that presented here.
Black-browed-type albatrosses
The genetic analysis of black-browed-type mitochondrial haplotypes revealed three distinct groups: T. impavida on Campbell Island, T. melanophrys from the Falkland Islands, and all other sampled populations of T. melanophrys (Burg and Croxall 2001). The newly sampled populations on Diego de Almagro, Ildefonso and Macquarie Islands all fell into the latter grouping, which also included birds from Diego Ramirez, South Georgia and Kerguelen Islands. However, the population genetic analyses implied that the Macquarie Island T. melanophrys show the greatest affinity to birds breeding on Ildefonso and Diego de Almagro whereas low genetic differentiation was apparent between Kerguelen, South Georgia and Diego Ramirez. Ildefonso populations showed high genetic diversity and therefore only showed differentiation with the distinct Falkland Island T. melanophrys and Campbell Island T. impavida. As yet no genetic data are available from the 1000 pairs of Black-browed Albatross on the Crozet Islands, the 700 pairs on the Heard and MacDonald Island group, or the 100 pairs on the Antipodes Islands.
The NCA and PCA both revealed intermixing of divergent haplotypes. The Falkland Islands T. melanophrys haplotypes were also found in low frequencies on Macquarie, Diego Ramirez and Campbell Islands. T. impavida haplotypes occurred on Macquarie Island and T. melanophrys were found amongst the T. impavida clade. Hybridisation between T. impavida and T. melanophrys on Campbell Island is known to occur, although whether hybrid offspring are viable has not yet been established (Moore et al. 2001). Historical survey data suggest that several Black-browed populations in the New Zealand region have been established recently, including Campbell Island, Antipodes Islands and the Snares Islands (Moore et al. 2001). The movement of the Falklands’ haplotypes, in combination with the apparently recent invasion of T. melanophrys into the New Zealand region and the hybridisation of T. impavida and T. melanophrys on Campbell Island, imply past fragmentation of these groups, followed by secondary contact through recent range expansion.
The genetically close, yet distinct, lineages within both the black-browed-type and wandering-type complexes imply that both have been influenced by allopatric fragmentation. In the black-browed-type albatrosses this geographical fragmentation has recently been compromised by the establishment of T. melanophrys colonies in the South Pacific and the secondary contact of T. melanophrys and T. impavida on Campbell Island (Moore et al. 2001). The historical allopatric fragmentation is likely to have been generated by long-distance dispersal followed by restricted gene flow, which may in turn have been driven by glacial cycles. Speciation processes in birds are thought to depend more on the divergent evolution of ecological and behavioural characteristics than by the accumulation of genetic differences (Grant and Grant 1997). Glacial cycles may be expected to exert strong ecological selective forces, which are believed to be important in driving the accumulation of differences (Schluter 2001). The divergence of behavioural, ecological and morphological traits (e.g. Lequette and Jouventin 1991; Prince et al. 1997; Nicholls et al. 2002) during separation may be sufficient to maintain effective isolation during secondary contact. However, in the case of T. impavida and T. melanophrys it appears likely that the period of separation was not prolonged enough for reproductive isolation to occur (cf. Liebers and Helbig 2002).
The glacial cycles and frequent sea-level changes of the Pleistocene period will have greatly influenced the distribution of albatrosses and will have driven many colonies to extinction (Olson and Hearty 2003). The state of many subantarctic islands during the last ice age is largely unknown but many islands that currently host albatross populations are thought to have been extensively glaciated. These include South Georgia (Sugden and Clapperton 1977) and the Kerguelen Islands (Hall 2002). Also many of the Chilean offshore islands, including Diego de Almagro and Ildefonso, would have been covered by the Andean ice-cap (Tickell 2000). In contrast, the Prince Edward Islands (Hall 2002), Falkland Islands and Macquarie Island (Bergstrom and Chown 1999) are thought not to have been heavily glaciated, if at all. Given their latitude, it is reasonable to add Amsterdam Island and Tristan da Cunha to this list (Denton and Hughes 1981). We therefore think it reasonable to speculate that islands hosting island-specific lineages may plausibly be considered to have escaped heavy glaciation during the Pleistocene. We further speculate that those islands, and also any areas that were exposed in their environs, could have acted as glacial refugia when the environment on other subantarctic islands became unsuitable for nesting (Olson and Hearty 2003). A corollary of our argument is that at least some areas on the New Zealand subantarctic islands remained ice-free during the Late Pleistocene and this would be consistent with the analysis of McGlone et al. (1997) of the environment of some of those islands. Given the likely availability of suitable nesting sites during glacial maxima and the contemporary star-like haplotype pattern (cf. Moum and Arnason 2001; Liebers and Helbig 2002) of major clades in both D. exulans and T. melanophrys it would further be plausible that the Prince Edward Islands or Crozet Islands or both were refugia for both species, and that both subsequently expanded throughout the southern Atlantic and Indian Ocean islands following glacial retreat from South Georgia, Kerguelen and the Chilean islands.
In summary, the newly sampled populations of D. exulans and T. melanophrys examined here are closely related to contemporary populations in the southern Indian Ocean and were probably founded by long-distance dispersal from this region. Such rare, long-distance dispersal events were obviously restricted during the Late Pleistocene, leading to distinct genetic lineages on many islands. This study also demonstrates that geographically distant colonies may not necessarily be genetically differentiated and that both D. exulans and T. melanophrys have undergone relatively recent (since the last glacial maximum) expansion of range.
Acknowledgments
We are extremely grateful to Roger Kirkwood, Kieran Lawton, Graham Robertson and Barbara Weinecke for sampling the Black-browed Albatrosses on Diego de Almagro and Ildefonso, and to Aleks Terauds for assistance on Macquarie Island. This work could not have been conducted without the cooperation of Chile’s Instituto Antártico or the Australian Antarctic Division. We also thank Andrew Cockburn for supplying laboratory facilities and financially supporting M. C. Double, and Barry Baker for his invaluable help in facilitating this project. Australia’s Department of Environment and Heritage and the Antarctic Scientific Advisory Committee funded genetic and field research in Australia.
Abbott, C. , and Double, M. C. (2003a). Genetic structure, conservation genetics, and evidence of speciation by range expansion in shy and white-capped albatrosses. Molecular Ecology 12, 2953–2962.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Benson, D. A. , Karsch-Mizrachi, I. , Lipman, D. J. , Ostell, J. , and Wheeler, D. L. (2004). GenBank: update. Nucleic Acids Research 32, D23–D26.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Cuthbert, R. J. , Phillips, R. A. , and Ryan, P. G. (2003). Separating the Tristan Albatross and the Wandering Albatross using morphometric measurements. Waterbirds 26, 338–344.
Double, M. C. , Gales, R. , Reid, T. , Brothers, N. , and Abbott, C. L. (2003). Morphometric comparison of Australian Shy and New Zealand White-capped Albatrosses. Emu 103, 287–294.
| Crossref | GoogleScholarGoogle Scholar |
Grant, P. R. , and Grant, B. R. (1997). Genetics and the origin of bird species. Proceedings of the National Academy of Sciences of the United States of America 94, 7768–7775.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Markow, T. A. , Castrezana, S. , and Pfeiler, E. (2002). Flies across the water: genetic differentiation and reproductive isolation in allopatric desert Drosophila. Evolution 56, 546–552.
| PubMed |
Penhallurick, J. , and Wink, M. (2004). Analysis of the taxonomy and nomenclature of the Procellariiformes based on complete nucleotide sequences of the mitochondrial cytochrome b gene. Emu 104, 125–147.
| Crossref | GoogleScholarGoogle Scholar |
Roux, J.-P. , Jouventin, P. , Mougin, J.-L. , Stahl, J.-C. , and Weimerskirch, H. (1983). Un nouvell albatros Diomedea amsterdamensis n. sp. decouverte sur I’Ile Amsterdam (37°50′S, 77°35′E). Oiseau Revue Francaise d’Ornithologie 53, 1–11.
Schultheis, A. S. , Weigt, L. A. , and Hendricks, A. C. (2002). Gene flow, dispersal, and nested clade analysis among populations of the stonefly Peltoperla tarteri in the southern Appalachians. Molecular Ecology 11, 317–327.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Turner, T. F. , Trexler, J. C. , Harris, J. L. , and Haynes, J. L. (2000). Nested cladistic analysis indicates population fragmentation shapes genetic diversity in a freshwater mussel. Genetics 154, 777–785.
| PubMed |
Waugh, S. M. , Prince, P. A. , and Weimerskirch, H. (1999). Geographical variation in morphometry of Black-browed and Grey-headed Albatrosses from four sites. Polar Biology 22, 189–194.
| Crossref | GoogleScholarGoogle Scholar |