Adaptive divergence in the Superb Fairy-wren (Malurus cyaneus): a mainland versus island comparison of morphology and foraging behaviour
Beth E. Schlotfeldt A and Sonia Kleindorfer A BA School of Biological Sciences, Flinders University, Bedford Park, SA 5042, Australia.
B Corresponding author. Email: sonia.kleindorfer@flinders.edu.au
Emu 106(4) 309-319 https://doi.org/10.1071/MU06004
Submitted: 27 January 2006 Accepted: 14 July 2006 Published: 16 November 2006
Abstract
Understanding patterns of adaptive divergence is a cornerstone for understanding the process of speciation. The theory of ecological speciation predicts that natural selection shapes adaptive divergence. In this observational study, we examined the first phase of ecological speciation, namely adaptive divergence in foraging behaviour and morphology across populations (island and mainland sites) of the sexually dimorphic Superb Fairy-wren (Malurus cyaneus) that have been separated for approximately 9000 years. Current island theory predicts larger body-size in island species, as well as more generalist foraging and occupation of a wider ecological niche that is favoured by large body-size. We examined the vegetation structure across replicate study areas on Kangaroo Island and the mainland Mount Lofty Ranges in South Australia and found significant differences in vegetation structure important for Superb Fairy-wrens (percentage bare earth, shrub size and shrub abundance). Compared with mainland birds, island birds of both sexes: (1) occupied a wider niche breadth; (2) were significantly larger in body-size; and (3) varied less in morphology. Between the sexes: (1) there were few inter-sexual foraging differences; (2) males had a larger body-size and bill-length at both island and mainland locations; and (3) females had larger bill-width and bill-depth at both locations. These findings support the hypothesis of adaptive divergence in this species, with evidence that vegetation structure is important in their foraging behaviour and affects their morphology across locations (niche breadth was potentially favoured by a different interspecific community). The lack of consistent sexual differences in foraging ecology suggests that the patterns of sexual dimorphism may be shaped by reproductive roles rather than vegetation structure in this species. The categorisation of the Kangaroo Island population as a separate subspecies (M. c. ashbyi) is supported by these findings.
Introduction
The theory of ecological speciation predicts that the first phase of speciation, adaptive divergence, is shaped by natural selection on phenotypes (Schluter 2000). Studies of island populations have been a source of insight into evolutionary processes, including adaptive divergence and speciation, in part owing to the geographical isolation of many islands from larger source populations as well as the occurrence of ecological differences between islands (MacArthur and Wilson 1967; Grant 1968; Schluter 2000). Some general patterns are evident in studies of island populations. In small passerine birds, for example, island populations tend to be larger in body-size (the ‘island rule’) compared with their mainland counterparts (Van Valen 1965; summary in Scott et al. 2003). The general explanation for larger intraspecific body-size in island birds has rested on two main arguments: (1) more generalist foraging, and (2) occupation of a wider ecological niche (Mayr 1970; Lack 1971; Abbott 1974b; Grant 1998), generally as a result of fewer interspecific competitors (Grant 1965; Lack 1971).
A niche refers to the set of resources that are regularly used by a particular species (Ridley 2004). Niche utilisation is now synonymous with the concept of peaks in adaptive landscapes (Keast 1970; reviewed in Schluter 2000). According to the adaptive landscape model, different phenotypes derive different fitness benefits from their locations on the adaptive landscape: phenotypes closest to the adaptive peak (i.e. phenotypes that exploit local resources most efficiently) will have higher fitness than intermediate phenotypes or phenotypes located further away from the peak (Schluter 2000). Sexual dimorphism in size is often related to differential niche utilisation (Selander 1966) as it may reduce competition between the sexes for limited resources (Selander 1966, 1972; Slatkin 1984), especially in species with biparental care of the young (Harvey and Bradbury 1991, in Owens and Hartley 1998). Differential niche utilisation between the sexes may permit a population to utilise a greater variety of food resources and therefore persist in a novel environment, potentially leading to population divergence (but see review in Price 1998).
Sexual size dimorphism is common among birds (Selander 1966; Whitehead and Tschirner 1992), with ample evidence that it is linked to sex-specific foraging differences (e.g. Darwin 1896; Selander 1966; Mayr 1972; Shine 1989; Suhonen and Kuitunen 1991; Hunt and McLean 1993). These differences are likely to be more apparent when food resources are scarce or intraspecific competition is high, or both, such as in depauperate island situations or during the non-breeding season (Selander 1966). For example, Noske (1986) found that female Varied Sittellas (Daphoenositta chrysoptera), which have shorter bills and wings than males, foraged higher and on smaller branches than males, particularly during the non-breeding season. High population densities on islands may intensify intraspecific competition, resulting in the ability to exploit vacant niches (in the absence of interspecific competitors) and associated sexual differences in phenotype (Selander 1966).
Sexual differences in body-size may be shaped by fitness benefits derived from male–male competition, such as for access to territories or mates, particularly in polygamous or promiscuous species (Darwin 1896; Amadon 1959; Selander 1966, 1972; Jehl and Murray 1986; Shine 1989). Sexual differences in size of bill may be allometric (i.e. the same order predicted from differences in body-size) (e.g. Whitehead and Tschirner 1992; Clegg et al. 2002; see also Fairbairn 1997 for review) but often this is not the case. For example, in the New Zealand Rifleman (Acanthisitta chloris) (Hunt and McLean 1993) and the extinct Huia (Heteralocha acutirostris) (Selander 1966) females of both species have a disproportionately longer bill. In general, bill-size may be shaped by fitness benefits derived from foraging technique (Selander 1966) and prey size (Hespenheide 1973; Grant 1998).
In this study, we examine evidence for the first phase of ecological speciation, namely adaptive divergence under conditions of differential resource use, in a sexually dimorphic passerine, the Superb Fairy-wren (Malurus cyaneus). We compare two populations on Kangaroo Island, and three populations on the mainland, in the Mount Lofty Ranges. Superb Fairy-wrens are a good model system to test theories of sexual dimorphism and the island rule because they are sexually dimorphic in size and colouration (so are easy to sex) and are common at both study locations. Schodde (1982) highlighted that although male and female Superb Fairy-wrens have slightly different bills and rictal bristles, there is a need for studies on sexual foraging differences and food resource partitioning within this species.
The Superb Fairy-wren on Kangaroo Island is currently recognised as a subspecies (M. c. ashbyi) (Schodde and Mason 1999) but its subspecific status has been debated (see Rowley and Russell 1997; Schodde and Mason 1999). Superb Fairy-wrens are considered poor fliers and dispersers (Rowley 1965; Schodde 1982; Rowley and Russell 1997) and have probably been resident on Kangaroo Island since it was separated from the mainland by rising sea levels approximately 9000 years ago (Schodde 1982). In this observational study, we compare the foraging ecology and morphology of the species to examine evidence for adaptive divergence in the island and mainland forms. The first phase of adaptive divergence is often driven by behavioural use of novel resources (discussed in Kleindorfer et al. 2006), with subsequent selection favouring particular phenotypes in particular environments. We examine whether island Superb Fairy-wren populations have begun to diverge by comparing resource availability (vegetation structure) and resource partitioning across location and sex in relation to morphology.
We test the following predictions to explain variation in foraging behaviour and morphology between island and mainland populations and between sexes: (1) vegetation structure will differ significantly between island and mainland, and will correlate with foraging behaviour; (2) island birds will have a wider foraging niche than their mainland counterparts; (3) island birds will have larger body- and bill-size and vary more in their morphology; (4) the sexes will occupy different foraging niches; and (5) males will be larger than females in body- and bill-size at both locations.
Materials and methods
Study species
The Superb Fairy-wren is a small Australian passerine of the family Maluridae (subfamily Malurinae) (Schodde and Mason 1999). This species inhabits woodlands and heaths and often forages in clearings (Paton et al. 2002), although it is common in shrubby habitats, and found in coastline, island and inland areas of eastern and south-eastern Australia (Rowley and Russell 1997; Higgins et al. 2001). Superb Fairy-wrens prefer areas of mid- to low elevation, favouring low open forests associated with rivers, creeks and gullies, and dense shrub cover (for cover and nesting sites) interspersed with open ground for foraging (Neave et al. 1996). They are insectivorous ground-foraging birds, and tend to forage socially in small family groups comprising a dominant male (generally in breeding plumage) and female, and several male helpers (generally in non-breeding plumage) (Schodde 1982; Rowley and Russell 1997). They forage on the ground, among leaf-litter, on logs and fallen branches, and sometimes from lower branches of understorey shrubs, generally within 2 m of the ground (Schodde 1982; Rowley and Russell 1997; Paton et al. 2002).
Superb Fairy-wrens weigh ~10 g, are sexually dichromatic and dimorphic, and breed cooperatively (Rowley 1965; Rowley and Russell 1997). They have short rounded wings and strong legs, but are not strong flyers and tend to be sedentary (Rowley 1965; Schodde 1982; Schodde and Mason 1999). Males have finer and more slender bills than females, and in breeding plumage have metallic sky-blue cap, mantle and erectile ear-tufts, with a black collar, stripe running from lores through eyes to collar, and black rest of upperbody, and a whitish belly. Adult females have a dark grey-brown back and off-white underside, a rufous loral stripe and eye-ring, and red-brown bill. Males in non-breeding plumage are similar in plumage colouration to females, but have a black eye-ring, loral stripe and bill (Schodde 1982). The sexes are thus easily distinguished, but care is required when determining the sex of immature birds (see Rowley and Russell 1997; Higgins et al. 2001).
Study sites
Kangaroo Island is a large island (4500 km2) only 14 km from mainland South Australia (SA) and that has undergone a period of separation and isolation of ~8900 years (Abbott 1973, 1974b; Schodde 1982; Belperio and Flint 1999). This has led to avifaunal differences between the island and mainland owing to limited movements across the oceanic barrier, especially by birds with poor dispersal abilities, resulting in subspecies that have evolved slight morphological differences (MacArthur and Wilson 1967; Abbott 1974b; Paton et al. 2002). Several passerine species on Kangaroo Island differ sufficiently from the mainland SA populations to be treated as subspecies (see Ford 1979; Robinson and Armstrong 1999). In their review of Australian passerines, Schodde and Mason (1999) used the presence of one genetically based morphological trait difference as their criterion for classification into a subspecies. In the case of the Superb Fairy-wren, the Kangaroo Island form has a longer tarsus and females have darker plumage colouration than the adjacent mainland form.
In this study, morphological data were collected from 2003 to 2005 at five reserves in two locations (island and mainland) in southern SA: two reserves on Kangaroo Island (hereafter KI) – Flinders Chase National Park (35°56′S, 136°44′E) and Pelican Lagoon (35°48′S, 137°47′E); and three reserves in the Mount Lofty Ranges (hereafter MLR) – Sandy Creek Conservation Park (34°36′S, 138°51′E), Scott Creek Conservation Park (35°05′S, 138°40′E) and Scott Conservation Park (35°24′S, 138°44′E). Foraging data were collected during the non-breeding and breeding seasons (April–August and September–November respectively) of 2003 to 2005 in these same reserves, whereas vegetational data were collected only in 2005, and from four reserves (Flinders Chase National Park, Pelican Lagoon, Sandy Creek Conservation Park and Scott Creek Conservation Park).
The vegetation in both study locations (KI, MLR) was mainly open eucalypt woodland. On KI, the vegetation of Flinders Chase was predominantly open Eucalyptus woodland with an understorey of tea-tree (Melaleuca spp.) and Kangaroo Thorn (Acacia paradoxa), and a grass-layer of native lily tussocks (Orthosanthus multiflorus). The vegetation of Pelican Lagoon was a combination of shrubland and woodland dominated by mallee Eucalyptus, Kangaroo Thorn and sheoaks (Allocasuarina sp.) with an understorey of hakea (Hakea spp.) and Common Correa (Correa reflexa) shrubs, and a grass-layer of native lily tussocks (O. multiflorus).
In MLR, vegetation in Sandy Creek Conservation Park consisted of two distinct types, one comprising open Eucalyptus woodland with a herb layer of invasive Bridal Creeper (Asparagus asparagoides), and the other comprising predominantly Native Pine (Callitris preissii). Other dominant plant species were Silver Banksia (Banksia marginata) and Drooping Sheoak (Casuarina stricta), with the dominant understorey species Flame Heath (Astroloma conostephoioides), Honey Fringe-myrtle (Calytrix tetragona), Grevillea lavandulaceae, grass-trees (Xanthorrhoea semiplana) and tea-trees (Leptospermum sp.) (Rix 1976). Scott Creek Conservation Park was dominated by low open Eucalyptus woodlands with an understorey of Golden Wattle (Acacia pycnantha), tea-tree (Leptospermum spp.) and heath, and areas of open grassland and dense clumps of Blackberry (Rubus fruticosus). The vegetation of Scott Conservation Park was predominantly open Eucalyptus woodland with and understorey of Kangaroo Thorn and Golden Wattle.
Vegetation characteristics
Vegetation was sampled in plots located within three representative areas where Superb Fairy-wrens were regularly observed foraging. Plots were 70 × 160 m, with 20 25-m2 quadrats per plot. Sampling quadrats were located along four transects that were separated by 50 m. Along each transect, the sampling quadrats were separated by 15 m, with five replicates per transect. Each quadrat was 5 × 5 m and was centred on the transect line. Plots were sampled during September when plants were flowering, which allowed easier identification of plants. For each quadrat we recorded: (1) number of trees; (2) number of shrubs; (3) number of shrub species; (4) average height of understorey (i.e. grasses, herbs, and ferns), measured as the mean of five randomly sampled understorey plants; (5) percentage ground cover and bare earth (visual estimation); (6) percentage canopy cover (visual estimation); and (7) shrub attributes (species, height, and width). Shrubs were considered to be woody plants, generally <3 m tall and multi-branched, that were >0.2 m tall and 0.2 m wide; smaller plants were considered herbaceous understorey, and larger plants were considered trees (adapted from Heard and Channon 1997). Data were pooled for reserves within locations (KI, MLR) for statistical analysis.
Vegetation variables were analysed using Principal Components Analysis (PCA) with Varimax rotation using the SPSS® statistical package Version 11.5 (SPSS Inc., Chicago, Illinois).
Foraging observations
Data were collected in each reserve during periods of peak foraging activity (0600–0900 hours and 1500–1800 hours). Walking transects incorporated a variety of habitat types in each reserve (e.g. open grassland, open woodland, edge and riparian habitats) and usually followed fire access tracks or walking paths. Foraging data were collected during the non-breeding and breeding seasons from Scott Creek Conservation Park over 18 days; Sandy Creek Conservation Park over 24 days; and Scott Conservation Park over 5 days; Flinders Chase was visited once in the non-breeding season and once in the breeding season, for a total of 12 days; and Pelican Lagoon was visited every 2 months, over a total of 24 days. Only one foraging observation per individual bird per day was made to avoid statistical non-independence of the data. Transects were walked slowly until a bird was located, then observed until it had made a foraging attempt or disappeared from view. Opportunistic foraging observations were also made, especially at island sites, whereby birds were recorded foraging near the banding station or located by sound and followed until located by sight. A foraging observation consisted of descriptive information about the sex of the bird (adult birds were either male or female, and males classified as either breeding or non-breeding), foraging substrate, foraging strata, foraging method, dietary item (when known), and foraging height.
Foraging substrate was recorded as: (1) bark, (2) leaves, (3) flowers, (4) ground, or (5) air. These were later reclassified for statistical analyses into the variables: (1) bark, (2) foliage (leaves and flowers), (3) ground, and (4) air. Foraging strata were classified as: (1) ground, (2) tree, (3) shrub, or (4) air. Foraging method was classified as: (1) pick (removal of prey item from the ground surface), (2) glean (removal of prey item from the surface of foliage and bark substrates), (3) sally (removal of prey item from the air), or (4) probe (removal of prey item from below the surface, involving partial insertion of the bill into the substrate) (adapted from Ford 1989); the latter two techniques (sally and probe) were reclassified as ‘other’ for statistical analyses as there were too few cases of each type. Foraging height was measured as a continuous variable, but analysed in relation to category (corresponding to habitat strata): (1) ground (0 m), (2) 0.01–1 m, (3) 1.1–3 m, and (4) 3.1–6 m.
Foraging niche breadth was determined for males and females in island and mainland populations using Shannon’s formula:
H′ = −Σ (pi ln pi)
where pi is the proportion of observations in foraging category i (Ford et al. 1986; Scott et al. 2003), i.e. pi = ni / N where ni is the number of observations of each foraging category i and N is the total number of foraging observations of all foraging categories (Adamík et al. 2003). Niche breadth was calculated for all foraging variables.
Morphological data
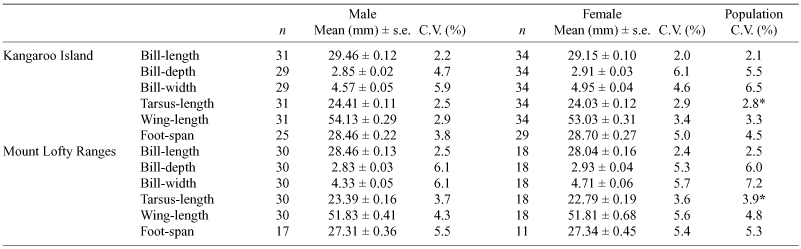
Superb Fairy-wrens at each study site were captured using mist-nets placed in areas of vegetation where they regularly foraged. At the time of capture, we made standard morphological measurements of bill-length (measured from the back of the head to the tip of the bill), bill-width (measured just behind the nares), bill-depth (measured just behind the nares), tarsus (tarsometatarsus) length and diameter, wing-length (measured using the flattened wing from the wrist joint to tip of the longest primary feather), foot-span with and without claws (from tip of hallux to tip of middle front toe, measured from an imprint of the bird’s right foot pressed into flattened plasticine so that the toes were as straight as possible), and mass. Each bird was individually marked with an Australian Bird and Bat Banding Scheme alloy identification band. Birds were weighed using an electronic scale (to the nearest 0.1 g). The sex of captured individuals was determined using plumage colouration. Recaptured individuals were not included in the analyses to avoid statistical bias. In total, we banded and collected morphological data from 113 adult birds: 31 males and 34 females on KI, and 30 males and 18 females from MLR.
Both morphological and foraging data were pooled for reserves within locations to determine differences between the mainland and island, as sample sizes were too small to permit meaningful comparisons between reserves.
Factor analysis of morphological variables
Morphological variables (excluding foot-span, which was analysed separately owing to small sample size) were reduced using PCA with Varimax rotation using SPSS® statistical package Version 11.5.
Results
Vegetation characteristics
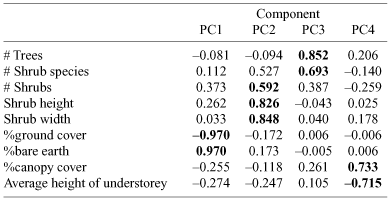
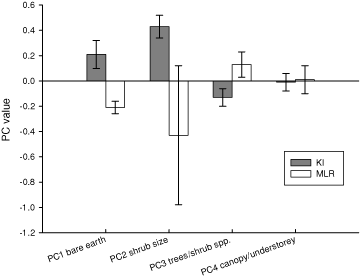
Four components were retained for analyses as they all had an eigenvalue greater than one and cumulatively explained 78.6% of the variance. The four derived vegetation variables were: PC1 (Bare Earth), PC2 (Shrub-size), PC3 (Trees and Shrubs), and PC4 (Canopy and Understorey) (factor loadings shown in Table 1). The derived vegetation variables were analysed in relation to location using ANOVA. Bare earth (PC1) and size and abundance of shrubs (PC2) varied significantly across location (F1,239 = 10.54, P = 0.001, and F1,239 = 53.72, P < 0.001 respectively); KI study sites had more bare earth (i.e. less ground cover) and more and larger shrubs than the mainland (Fig. 1). There was a non-significant trend for fewer trees and species of shrubs on KI than MLR (F1,239 = 10.54, P = 0.051), and no significant differences in canopy cover and height of understorey between island and mainland (F1,239 = 0.01, P = 0.932) (Fig. 1).
|
|
Island–mainland foraging behaviour
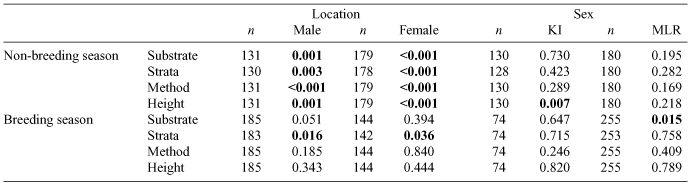
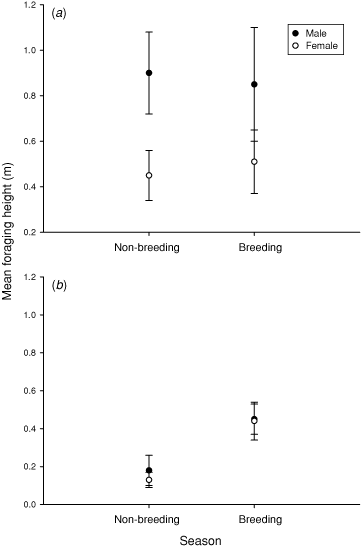
In a comparison of KI versus MLR birds, there were significant differences within each sex for all foraging variables examined, but only during the non-breeding season (Tables 2, 3, Fig. 2). During the non-breeding season, both KI males and females gleaned significantly more on bark and foliage in shrubs and trees, and at greater heights, and picked significantly less on the ground than MLR birds (Table 3, Fig. 2). During the breeding season, males and females differed significantly in foraging strata across location: KI birds foraged more in shrubs and trees and less on the ground than did MLR birds.
|
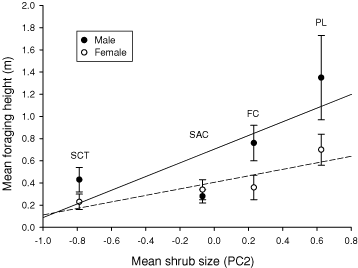
There was a trend for a positive correlation between mean foraging height and mean shrub size and availability (PC2) in both sexes (Fig. 3). Males appeared to show a stronger positive association between foraging height and shrub-size than females (Fig. 3), although sample sizes were too small to test this pattern statistically.
|
Sexual differences in foraging behaviour
Overall, there were few differences between sexes in foraging behaviour (Table 2). On KI during the non-breeding season, foraging height differed significantly between males and females, with males foraging higher than females (Table 2, Fig. 2). In MLR during the breeding season, foraging substrate differed significantly between males and females, with males foraging more on bark and less on foliage than females (Tables 2, 3).
On KI and MLR there were significant differences in substrate use between males in breeding or non-breeding plumage (Fisher’s exact test: P = 0.001 and P = 0.049 respectively), with non-breeding plumaged males foraging significantly more on the ground than on bark and foliage. Strata use also varied significantly in KI males but not MLR males (Fisher’s exact test: P = 0.010 and P = 0.207 respectively), with breeding plumaged males using shrubs and trees significantly more than non-breeding plumaged males on KI. Foraging method and foraging height showed a similar trend (Fisher’s exact test: KI: P < 0.001 and P = 0.005 respectively; MLR: P = 0.076 and 0.134 respectively), with males in breeding plumage on KI gleaning significantly more than males in non-breeding plumage, and at greater heights.
Niche breadth across locations
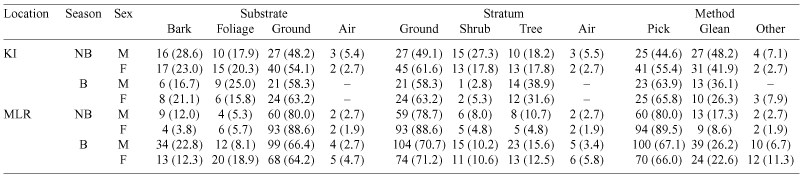
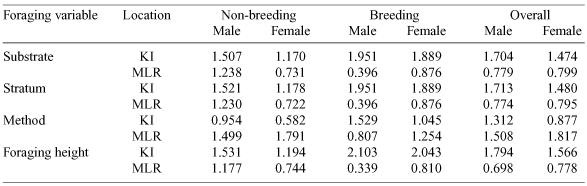
Niche breadth (calculated for substrate) differed the most across seasons in MLR birds, with males decreasing their foraging niche (from 1.238 to 0.396) and females increasing their foraging niche (0.731 to 0.876) in the breeding season. However, when season was not considered, MLR males and females had almost the same niche breadth (Table 4). KI birds increased their niche breadths from the non-breeding to breeding season (males: 1.507 to 1.951; females: 1.170 to 1.889). Overall, males had a wider foraging niche than females (Table 4). Niche breadth (substrate) for KI birds was comparable to that found by Ford et al. (1986) in eucalypt woodlands in Armidale, northern New South Wales. Niche breadth calculated for foraging stratum and foraging height yielded similar results (Table 4).
|
Overall, foraging method yielded similar niche breadths in MLR males and females, which were higher than KI birds (with KI males having a wider niche breadth than females). During the non-breeding season, MLR birds had much wider niches than KI birds, but this pattern was reversed during the breeding season. KI and MLR females had similar niche breadths during the breeding season, which decreased in the non-breeding season (Table 4).
Morphology: effects of location and sex
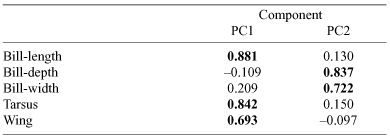
Two components were retained for analyses as they both had an eigenvalue of greater than one and cumulatively explained 65.8% of the variance. One component (PC1, – body-size) loaded heavily for body-size variables (bill, tarsus, wing-length) and the other (PC2, – bill-size) loaded heavily for bill-size and shape variables (bill-depth, bill-width), independent of overall body-size (Table 5).
|
Derived body- and bill-size variables (PC1, PC2) were examined in relation to location (KI, MLR) and sex using univariate ANOVAs (see also Table 6). Body-size was significantly larger in KI than MLR birds (F1,110 = 84.84, P < 0.001), and males were significantly larger than females at both locations (F1,110 = 12.99, P < 0.001). There was no significant interaction effect of location and sex (F1,110 = 0.00, P = 0.983). Bill-size was significantly larger in KI than MLR birds (F1,110 = 4.86, P = 0.030), and larger in females than males (F1,110 = 31.27, P < 0.001), with no significant interaction effect of location and sex (F1,110 = 0.07, P = 0.791). Thus, body-size and bill-size were significantly larger in KI birds than MLR birds, with evidence of sexual dimorphism in both variables independent of location. Levene’s test revealed no significant differences in variance of PC1 (body-size) and PC2 (bill-size) between locations in males (P = 0.415 and P = 0.074 respectively) or females (P = 0.280 and P = 0.536 respectively) or between the sexes on KI (P = 0.920 and P = 0.167) or MLR (P = 0.750 and P = 0.347). Likewise, there were no significant differences (P > 0.1 in all cases) in heterogeneity of individual morphological characters (e.g. bill-length, wing-length) (Table 6) between locations within each sex or between the sexes on KI and MLR.
|
Foot-span was examined separately, and was significantly different between locations (F1,81 = 16.05, P < 0.001). KI birds had a larger foot-span, with no difference between the sexes (F1,81 = 0.09, P = 0.762) (Table 6) and no significant interaction effect of sex and location (F1,81 = 0.05, P = 0.821).
Discussion
Vegetation in relation to foraging
Island and mainland study sites differed significantly in their vegetation structure, with KI (island) study plots having a greater number of shrubs, larger shrubs, and more bare earth compared to the mainland (MLR). Island birds foraged differently in all foraging aspects compared to MLR birds during the non-breeding season (gleaning more bark and foliage in shrubs and trees than mainland birds, which mainly picked on the ground), but differed only in use of strata during the breeding season. Island birds foraged significantly more in shrubs and trees than mainland birds across the year, a potential result of the greater size of shrubs and increased availability of shrubs on the island (Table 3, Figs 2, 3).
Overall, island birds had a wider foraging niche breadth than mainland birds (except in foraging method), and island birds increased their niche breadth in the breeding season, whereas mainland males decreased their foraging niche and mainland females increased their foraging niche. These foraging differences are more likely to correspond to levels of food resources, which were not measured in this study, rather than to vegetation structure.
Foraging in relation to morphology – island versus mainland
Island birds of both sexes foraged significantly differently (and had wider foraging niches) compared to their mainland counterparts. Foraging differences may be correlated with morphological shifts, as island birds were more generalist foragers with a wider foraging niche, larger body-size (bill, tarsus, wing-length) and foot-span, and a trend towards larger bill-size, confirming trends found in previous island studies (e.g. Grant 1965).
Niche shifts, such as increasing niche breadth in foraging strata and substrate, are considered an evolutionary adaptation to changes in food availability (Abbott et al. 1977, in Abbott 1980; Blondel 1985; Grant 1998; Kleindorfer et al. 2006), suggesting that food abundance is greater on KI than in MLR. In this study we did not measure size or abundance of prey, so are unable to compare niche shifts in relation to food availability. However, changes in body- and bill-size are usually correlated with diet (e.g. Abbott 1977), with evidence that large-bodied individuals can consume a broader diet (Cody 1974, in Blondel 1985).
Our results support the predictions of Grant (1965, 1998) that increased body-size among island birds may reflect different use of trophic structures (e.g. bill and tarsus) and adaptation to local environmental conditions (such as vegetation structure) by the process of natural selection. For example, increased tarsal length may allow more efficient foraging on a wider variety of substrates (Grant 1965, 1971), and longer bills may allow the intake of a greater range or size of food items (Grant 1965; Abbott 1974a, 1980), an adaptation to generalist foraging in the absence or reduction of interspecific competition (Clegg and Owens 2002), or an adaptation to increased size of food items (Abbott 1977). In this study we found that island fairy-wrens had significantly longer tarsi and foraged on a wider variety of substrates than their mainland counterparts. Future research will examine size and abundance of prey as well as the utility of phenotypic traits across locations to determine empirically whether morphology correlates with diet in this species.
Clearly, other factors contribute to patterns of morphological shifts, including levels of intraspecific competition (which may be greater on islands owing to denser populations), energy constraints, and physiological optimisation (Clegg and Owens 2002). Body-size may increase in relation to thermoregulatory requirements following release from predation and competition pressures (Brown and Maurer 1976, in Clegg et al. 2002), or confer a fitness advantage in high-density populations (MacArthur et al. 1972, in Clegg et al. 2002). Robinson-Wolrath and Owens (2003) found that Silvereyes (Zosterops lateralis chlorocephalus) on Heron Island, Queensland, conformed to the ‘dominance hypothesis’, whereby high population density resulted in higher intraspecific competition and therefore selection for success in agonistic interactions. Under this model, higher fitness and social dominance in Silvereyes were associated with larger body-size. Information on group sizes of Superb Fairy-wrens among island and mainland populations is needed to test this hypothesis (e.g. Rathburn and Montgomerie 2003), but it is already known that dominance hierarchies are important to male Superb Fairy-wrens for territoriality and position in the breeding pair (Rowley 1965; Blackmore 2002; Cockburn et al. 2003).
Data from the present study were insufficient to test explicitly the mechanism underlying these patterns, but the results support the argument for adaptive divergence (in foraging behaviour) in relation to vegetation structure and occupation of a wider foraging niche breadth (and associated larger body-size) under conditions of reduced interspecific competition on islands. On Kangaroo Island there are no treecreepers (Climacteridae; neither Brown (Climacteris picumnus) nor White-throated (Cormobates leucophaeus)) or Varied Sitellas (Daphoenositta chrysoptera), which commonly forage for insects on bark of trees on the mainland (Keast 1968; Abbott 1974b; Paton et al. 2002). This may explain the higher use of bark and trees by Superb Fairy-wrens on the island (and hence their wider foraging niche breadth) found in this study. The increase in foraging niche breadth of island birds in the breeding season suggests differential niche utilisation in relation to high intraspecific competition (e.g. Selander 1966), and may be a result of the depauperate flora and fauna species of the island (Ford 1989). However, greater use of higher strata on the island during the breeding season may also be associated with parental vigilance behaviour, despite the lack of predators on the island.
This study showed slightly reduced morphological variation in island birds compared with the mainland, though the differences were not significant. Other studies have found significantly reduced variation in morphology of island compared with mainland bird populations (e.g. Grant 1976; Keast 1976; Abbott 1977; but see Van Valen 1965 and Abbott 1974b). This could be interpreted as a result of lower habitat and food diversity on the island (Grant 1971) and may indicate that lower competitor diversity may not play an important role in shifts in morphological variation in island populations (Abbott 1973). Our data suggest comparable habitat diversity on the island compared to the mainland, although food diversity was not examined.
Another hypothesis to explain reduced morphological variation on islands focuses on foraging specialisation. According to this hypothesis, island populations comprise individual specialists that vary less morphologically than dietary generalists (Hespenheide 1973; Wiens and Rotenberry 1980; Scott et al. 2003). Tidemann (2004) examined gut contents and found that Superb Fairy-wrens in mainland New South Wales were specialists with a foraging niche overlapping that of the coexisting White-winged Fairy-wren (Malurus leucopterus), but had a generalist niche breadth and flexibility in feeding habits. Support for this pattern in our study comes from the finding that island birds had a smaller (specialist) niche breadth in foraging method, which was used across a wider range of foraging strata and substrates than the mainland birds (e.g. Petit et al. 1990, in Carrascal et al. 1994).
Sexual differences in foraging behaviour and morphology
We found consistent non-allometric sexual differences in bill-size (depth and width of bill), with females having a wider and deeper bill but a smaller body-size (including bill-length) than males at both locations. In general, there were few foraging differences between the sexes: island males foraged higher than females during the non-breeding season, and mainland males foraged more on bark and less on foliage than females during the breeding season. The higher foraging height of island males may be explained by the availability of more and larger shrubs on the island in combination with male vigilance and territorial behaviour.
Despite being sexually dimorphic in body- and bill-size, there were few foraging differences between males and females in this study, suggesting that it does not contribute to adaptive divergence on the island. A number of other studies in mainland and island sites in Australia have found no inter-sexual differences in foraging ecology in Superb Fairy-wrens (e.g. Abbott 1973; Nias 1987; Recher and Holmes 2000). Rowley and Russell (1997) proposed that the lack of sexual differences in foraging ecology is explained by the fact that fairy-wrens forage on a wide variety of insect prey (primarily ants, which are abundant and common) and are foraging opportunists. Nonetheless, prey and habitat partitioning between the sexes remains a possibility. For example, males and females may select prey of different size (e.g. Selander 1966; Grant 1968) or partition resources on a microhabitat scale, e.g. spatial partitioning, with females foraging close to the nest and males foraging near territorial boundaries during the breeding season (Robins 1971; Holmes 1986).
Notably, there were no morphological differences between males in breeding or non-breeding plumage, other than in plumage colouration (S. Kleindorfer, unpublished data) but there were significant foraging differences between males in breeding and non-breeding plumages; non-breeding plumaged males tended to forage more on the ground and close to the female while bright breeding plumaged males tended to glean more on bark and foliage in shrubs and trees at greater heights (B. Schlotfeldt, personal observation). These foraging differences occurred exclusively on the island where there are fewer predators than the mainland (Paton et al. 2002), so male differential foraging presumably is not a mechanism to avoid detection and subsequent predation, but may relate to social dominance (e.g. Schneider 1984; Radford and du Plessis 2003) or vigilance by the dominant male and density of shrubs (with fewer and smaller shrubs on the mainland meaning greater visibility from the ground, hence less movement to shrubs and trees). Activity by males in breeding plumage may account for the two sex-related foraging differences found in this study, suggesting that sexual size-dimorphism is not related to foraging behaviour in this species.
A number of other hypotheses have been suggested to account for sexual dimorphism in body-size (see review in Webster 1992). Owens and Hartley (1998) found that sexual size-dimorphism in birds was correlated with reproductive and social behaviour (being positively associated with levels of social polygamy and negatively associated with parental care provisioning), and obvious sexual dichromatism was associated with high levels of extra-pair paternity (with sexual selection for showiness in males and natural selection for cryptic plumage coloration in females). Dunn et al. (2001) also found that sexual dimorphism in body-size and plumage was correlated with mating system, with greater dimorphism in polygynous and lek or promiscuous bird species. Owens and Hartley (1998) noted that Superb Fairy-wrens were similar in body-size but highly sexually dichromatic, suggesting biparental care of the young and high levels of extra-pair paternity. In fact, both sexes (and males in both breeding and non-breeding plumage) contribute to defence of nests, but the female is predominantly responsible for provisioning of the young (Rowley 1965; Nias 1987), many of which tend to be offspring from extra-pair matings (Mulder et al. 1994).
There is ample evidence that males may increase their body-size to increase their success in intrasexual competitions and thereby increase mating opportunities (Darwin 1896; Downhower 1976; Jehl and Murray 1986; Shine 1989; Andersson 1994; see review in Fairbairn 1997), which may be true as the Superb Fairy-wren is territorial (Rowley 1965) and highly promiscuous (Mulder et al. 1994). There may be selective pressure for smaller body-size in female Superb Fairy-wrens to enable them to fit inside the nest (e.g. Shine 1989; Figuerola and Green 2000), which is often small and well concealed, presumably to minimise the risk of predation or parasitism by cuckoos (Nias 1986). The increased size of the bill (depth, width) found in females may provide benefits in nest-building and nestling care (Shine 1989), as females are the nest-builders (Rowley 1965) and primary carers of nestlings (Nias 1987), the needs of which change as they grow (Rowley 1965).
In summary, Superb Fairy-wrens on Kangaroo Island conformed to the ‘island rule’ (Van Valen 1965), being larger in body-size and having a wider foraging niche than their adjacent mainland counterparts. Findings from this study provide evidence for adaptive divergence in the island Superb Fairy-wren, driven by changes in foraging ecology across location, supporting their current subspecific classification on Kangaroo Island. Foraging patterns between the sexes did not contribute to adaptive divergence on the island or support sexual dimorphism theory in this species, suggesting that reproductive roles may exert stronger selection pressures on sex-specific morphology. Future work will provide greater insight into the complex relationships of ecomorphology, sexual dimorphism, and adaptive divergence in this species.
Acknowledgments
This study was funded by the Conservation Council of South Australia and the South Australian Department for Environment and Heritage, with awards to S. Kleindorfer. Sincere thanks to Peggy Rismiller and Mike McKelvey at Pelican Lagoon Research Station for use of the research facility and stimulating discussion, staff at the Flinders Chase National Park for logistical support, and Diane Colombelli-Négrel, Sarah Lambert, and Margot Oorebeek for field assistance.
Abbott, I. (1973). Birds of Bass Strait. Evolution and ecology of the avifaunas of some Bass Strait islands, and comparisons with those of Tasmania and Victoria. Proceedings of the Royal Society of Victoria 85, 197–223.
Adamík, P. , Kornan, M. , and Vojtek, J. (2003). The effect of habitat structure on guild patterns and the foraging strategies of insectivorous birds in forests. Biologia 58, 275–285.
Carrascal, L. M. , Moreno, E. , and Valido, A. (1994). Morphological evolution and changes in foraging behaviour of island and mainland populations of Blue Tit (Parus caeruleus) – a test of convergence and ecomorphological hypotheses. Evolutionary Ecology 8, 25–35.
| Crossref | GoogleScholarGoogle Scholar |
Downhower, J. F. (1976). Darwin’s finches and the evolution of sexual dimorphism in body size. Nature 263, 558–563.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Ford, H. A. , Noske, S. , and Bridges, I. (1986). Foraging of birds in eucalypt woodlands in north-eastern New South Wales. Emu 86, 168–179.
Hespenheide, H. A. (1973). Ecological inferences from morphological data. Annual Review of Ecology and Systematics 4, 213–229.
| Crossref | GoogleScholarGoogle Scholar |
Holmes, R. T. (1986). Foraging patterns of forest birds: male–female differences. Wilson Bulletin 98, 196–213.
Keast, A. (1968). Competitive interactions and the evolution of ecological niches as illustrated by the Australian honeyeater genus Melithreptus (Meliphagidae). Evolution 22, 762–784.
| Crossref | GoogleScholarGoogle Scholar |
Kleindorfer, S. , Chapman, T. , Winkler, H. , and Sulloway, F. J. (2006). Adaptive divergence in contiguous populations of Darwin’s small ground finch (Geospiza fuliginosa). Evolutionary Ecology Research 8, 357–372.
Mulder, R. A. , Dunn, P. O. , Cockburn, A. , Lazenby-Cohen, K. A. , and Howell, M. J. (1994). Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proceedings of the Royal Society of London. Series B. Biological Sciences 255, 223–229.
Noske, R. A. (1986). Intersexual niche segregation among three bark-foraging birds of eucalypt forests. Australian Journal of Ecology 11, 255–267.
| Crossref | GoogleScholarGoogle Scholar |
Price, T. (1998). Sexual selection and natural selection in bird speciation. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences 353, 251–260.
| Crossref | GoogleScholarGoogle Scholar |
Rix, C. E. (1976). The birds of Sandy Creek Conservation Park. Australian Bird Watcher 6, 209–222.
Robinson-Wolrath, S. I. , and Owens, I. P. F. (2003). Large size in an island-dwelling bird: intraspecific competition and the Dominance Hypothesis. Journal of Evolutionary Biology 16, 1106–1114.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Schneider, K. J. (1984). Dominance, predation, and optimal foraging in White-throated Sparrow flocks. Ecology 65(6), 1820–1827.
| Crossref | GoogleScholarGoogle Scholar |
Scott, S. N. , Clegg, S. M. , Blomberg, S. P. , Kikkawa, J. , and Owens, I. P. F. (2003). Morphological shifts in island-dwelling birds: the roles of generalist foraging and niche expansion. Evolution 57, 2147–2156.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Shine, R. (1989). Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quarterly Review of Biology 64, 419–461.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Slatkin, M. (1984). Ecological causes of sexual dimorphism. Evolution 38, 622–630.
| Crossref | GoogleScholarGoogle Scholar |

Suhonen, J. , and Kuitunen, M. (1991). Intersexual foraging niche differentiation within the breeding pair in the Common Treecreeper Certhia familiaris. Ornis Scandinavica 22, 313–318.

Tidemann, S. C. (2004). Use of space, foraging behaviour and strategies of survival among three coexisting species of fairy-wrens (Malurus). Emu 104, 31–36.
| Crossref | GoogleScholarGoogle Scholar |

Van Valen, L. (1965). Morphological variation and width of ecological niche. American Naturalist 99, 377–390.
| Crossref | GoogleScholarGoogle Scholar |

Webster, M. S. (1992). Sexual dimorphism, mating system and body size in New World Blackbirds (Icterinae). Evolution 46, 1621–1641.
| Crossref | GoogleScholarGoogle Scholar |

Whitehead, P. J. , and Tschirner, K. (1992). Sex and age related variation in foraging strategies of Magpie Geese Anseranas semipalmata. Emu 92, 28–32.

Wiens, J. A. , and Rotenberry, J. T. (1980). Patterns of morphology and ecology in grassland and shrub-steppe bird populations. Ecological Monographs 50, 287–308.
| Crossref | GoogleScholarGoogle Scholar |