Red Knots (Calidris canutus piersmai and C. c. rogersi) depend on a small threatened staging area in Bohai Bay, China
Danny I. Rogers A F H , Hong-Yan Yang B F , Chris J. Hassell C , Adrian N. Boyle C , Ken G. Rogers D , Bing Chen E , Zheng-Wang Zhang B and Theunis Piersma F GA Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, PO Box 137, Heidelberg, Vic. 3084, Australia.
B Key Laboratory of Ministry of Education for Biodiversity and Ecological Engineering, College of Life Science, Beijing Normal University, 100875 Beijing, China.
C Global Flyway Network, PO Box 3089, Broome, WA 6725, Australia.
D 340 Ninks Road, St Andrews, Vic. 3761, Australia.
E Room 2511, Building 1, 2 Nan-Fang-Zhuang, Fengtai District, Beijing 100079, China.
F Global Flyway Network, c/o Department of Marine Ecology, Royal Netherlands Institute of Sea Research (NIOZ), PO Box 59, 1790 AB Den Burg, Texel, The Netherlands.
G Animal Ecology Group, Centre for Ecological and Evolutionary Studies, University of Groningen, PO Box 14, 9750 AA Haren, The Netherlands.
H Corresponding author. Email: drogers@melbpc.org.au
Emu 110(4) 307-315 https://doi.org/10.1071/MU10024
Submitted: 8 April 2010 Accepted: 30 June 2010 Published: 9 November 2010
Abstract
We monitored numbers of Red Knots (Calidris canutus) staging in Bohai Bay, China (39°02′N, 118°15′E) on northward migration. Knots were identified to subspecies, and we systematically searched for colour-banded birds from the non-breeding grounds. We modelled migratory turnover, and revised estimates of flyway population using recently published counts from the non-breeding grounds. Two Russian-breeding subspecies occurred at our study site: C. c. rogersi (migrating to Chukotka), and C. c. piersmai (migrating to the New Siberian Islands); they co-occur on non-breeding grounds in Australia and New Zealand, but differ markedly in timing of migration. We conservatively estimate that our study site, comprising only 20 km of coastline, was used by over 45% of the combined world population of adult C. c. rogersi and C. c. piersmai – a conclusion supported by the independent data on frequency of resighting of colour-banded birds from north-western Australia and New Zealand. Much of this vital staging area is now being destroyed through construction of the Caofedian Industrial Zone and more westerly developments, which comprise only some of the many tidal flat ‘reclamation’ projects in the region. Preservation of the remaining tidal flats of Bohai Bay is essential to the conservation of Red Knots in the East Asian–Australasian Flyway.
Introduction
The Red Knot (Calidris canutus) is an iconic migrant, nesting in polar deserts of the high Arctic and carrying out very long-distance migrations to non-breeding grounds, mostly in the southern hemisphere (Piersma et al. 2005; Piersma 2007). It is highly specialised, foraging mainly on shellfish, with adaptations to this diet including a remote sense that enables detection of pore-water pressure differentials to locate hard, buried prey (Piersma et al. 1995, 1998) and a massively muscular gizzard for crushing bivalves which are swallowed whole (Piersma et al. 1993; van Gils et al. 2003, 2005a). It has a strictly coastal distribution outside the breeding grounds, being characteristically restricted to large tidal flat systems (Piersma 2007).
The migration systems of Red Knots that migrate through Europe and Africa have been studied intensively and are well known (Piersma and Davidson 1992; Nebel et al. 2000; Leyrer et al. 2009). In contrast, the migration system of Red Knots in the East Asian–Australasian Flyway (EAAF) is poorly known. Two subspecies occur in this flyway: C. c. rogersi, which nests in Chukotka, in far-eastern Siberia, and the recently described C. c. piersmai (Tomkovich 2001), which nests on the New Siberian Islands (Tomkovich 2001; Zöckler and O’Sullivan 2005). The non-breeding destination of these subspecies is imperfectly known, as they cannot be distinguished in non-breeding plumage. It is widely supposed that C. c. rogersi migrates predominantly to eastern Australia and New Zealand, whereas C. c. piersmai migrates predominantly to north-western Australia (e.g. Lindström et al. 1999; Piersma et al. 2005; Piersma 2007). However, few data were available when these ideas were initially proposed. Observations of both subspecies in breeding plumage in north-western Australia and New Zealand (Tomkovich and Riegen 2000; D. I. Rogers, C. J. Hassell and A. N. Boyle, pers. obs.), observations of birds colour-banded in north-western Australian in Chukotka (Minton 2007) and some documented movements of Red Knots between north-western Australia and New Zealand (Minton et al. 2005; Riegen et al. 2005; New Zealand Wader Study Group, unpubl. data; P. R. Battley, pers. comm.) suggest there may be more geographical overlap between the subspecies on the non-breeding grounds than previously thought.
Given the size of the non-breeding population of Red Knots in the EAAF, estimated at 220 000 individuals by Bamford et al. (2008), surprisingly few Red Knots have been found at staging sites in this Flyway. This situation was rectified to some extent by Barter et al. (2001), who counted 14 277 staging birds in the Tianjin Municipality in the west of Bohai Bay, China. Yet even after compiling all available count data for the Yellow Sea and extrapolating to estimate numbers in unsurveyed areas, Barter (2002) could account for only 66 300 birds (~30% of the supposed non-breeding population) on northward migration.
A potential explanation for the low numbers of staging Red Knots counted on northward migration was suggested by the discovery that Red Knots from north-western Australia are late migrants (Battley et al. 2005), with many birds not leaving north-western Australia until May. The peak in numbers at their staging grounds could, therefore, be in mid- to late May, later in the year than the completion of many previous shorebird surveys in the Yellow Sea. Climate records from the breeding grounds, and comparison of arrival times at the breeding grounds of other subspecies of Red Knot would suggest that C. c. rogersi arrives on the breeding grounds close to the start of June, and C. c. piersmai close to mid-June. Accordingly, another implication of the late migratory departures of Red Knots from north-western Australia is that they have little time for staging and refuelling in Asia before moving on to the breeding grounds. Consequently they are likely to require staging grounds with high quality of prey to migrate successfully (Battley et al. 2005).
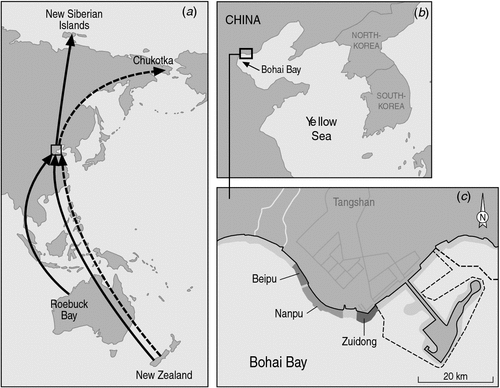
Here we examine the migration of Red Knots through Bohai Bay, Yellow Sea, China (39°02′N, 118°15′E), in greater detail than has been managed previously. We counted numbers of Red Knots in our study area repeatedly throughout the northward migration of 2009. Pre-breeding moult was well advanced or complete by this time, so we were able to use plumage characters to assess the relative proportions of C. c. rogersi and C. c. piersmai. We compared these results against an independent dataset from the same site, comprising resightings of individually colour-banded Red Knots from non-breeding grounds in New Zealand and north-western Australia. We aimed to: (1) resolve the passage times of C. c. rogersi and C. c. piersmai; (2) determine how many individuals of each subspecies staged in our study area; and (3) assess the importance of the region to the Flyway population of Red Knot. The final objective is especially urgent, as Bohai Bay is bordered by one of the most densely populated regions in the world, and its tidal flats are rapidly being lost to coastal realignment projects. The largest of these so far, to develop the Caofedien Industrial Zone, is in progress on the margins of our study area, with more reclamations just west of this area in 2010.
Methods
Fieldwork was carried out in northern Bohai Bay, China (Fig. 1). The study site comprised ~20 km of coastline, dominated by tidal flats that were 1–3 km wide on the lowest tides and submerged at high tide. The upper margins of the tidal flats were bordered by sea-walls (mainly incremental reclamations for the development of saltworks and aquaculture). Counts were carried out by H.-Y. Yang and B. Chen when the tide had ebbed 0.5–1 km from the sea-walls, when all Red Knots had moved to the flats from high-tide roosts, yet were still close enough to the sea-wall to be counted. The survey area was broken into three discrete blocks (Fig. 1), separated by channels that could not be crossed on foot. It was only possible to count the shorebirds in one block per high tide, except early and late in the season when fewer birds were present and quicker counts were possible. Adjacent blocks were therefore counted on consecutive days, and data were summed from adjacent blocks to give an overall count of Red Knots for the region in intervals of 3 days.
|
Birds were identified as subspecies rogersi or piersmai on the basis of the colour and pattern of breeding plumage. Birds identified as C. c. piersmai had deep brick-red underparts and reddish napes. The mantle and scapulars were black, boldly marked by rufous fringes and panels within each feather; many scapulars had narrow white tips, but these were inconspicuous and did not dominate the appearance. Birds identified as C. c. rogersi had paler, peachier underparts, and whitish napes. They had more extensive silvery variegation on the upperparts caused by a combination of broader grey-white tips to individual feathers, the presence of many scapulars with grey-white panels within the feather, and only a pale rufous tinge to other markings within the scapulars. These characters can be used to distinguish subspecies piersmai and rogersi on the breeding grounds in June–July (Tomkovich 1992, 2001), indicating that they should not be confused because of plumage wear when staging on northward migration. In China we found distinguishing these plumage extremes was usually straightforward provided birds were not obscured by other birds in the flocks. Some individuals were difficult to identify early in the migration season before they had developed extensive diagnostic breeding plumage, and were not identifiable to subspecies. It was not practical to scan all flocks closely enough to carry out separate counts of C. c. piersmai and C. c. rogersi. Instead we carried out scans to assess the relative proportions of C. c. piersmai and C. c. rogersi in subsets of birds. These scans were carried out by two separate field teams: H.-Y. Yang and B. Chen, and C. J. Hassell and A. N. Boyle. Within particular days, scans were consistent between observers and between sites, and data from different observers are pooled here. Subspecies scans were not practicable every day. Accordingly, daily subspecific proportions of C. c. piersmai were gently smoothed (SYSTAT 11, Systat 2005: NONLIN Smooth and Plot Feature with polynomial smoothing, Gaussian kernel, nearest neighbour proportion = 0.25) and values for missing days interpolated. The smoothed values were used in subsequent analyses. Standard errors associated with these proportions assumed a binomial distribution. No data were collected on subspecies representation in the first two counts. The plot of known C. c. piersmai proportion increased almost linearly for the next four counts. A linear regression (r = 0.988) result for the four data points from Days 116 to 130 was extrapolated and gave first C. c. piersmai arrivals on Day 99, i.e. after the first count, and a proportion of 0.218 C. c. piersmai for Day 130 with a standard error of the estimate of 0.022. We calculated the number of C. c. piersmai and C. c. rogersi present on each shorebird survey day as the product of the total number of Red Knots and the proportion of each subspecies within Red Knot flocks at that time.
From 10 to 29 May 2009, C. J. Hassell and A. N. Boyle conducted intensive searches for individually colour-banded birds from banding projects in New Zealand, and Roebuck Bay in north-western Australia (Fig. 1). Birds with unique engraved leg-flags were also recorded, though it was often impossible to approach closely enough to read the engravings. Birds marked only with plain leg-flags (yellow if initially captured in north-western Australia, orange if initially captured in south-eastern Australia, white if initially captured in NZ) were also recorded systematically. Within each day it was possible to ensure that leg-flagged individuals were not double-counted, but as the birds with single leg-flags could not be individually distinguished, some individuals might have been sighted repeatedly on different days. Many colour-marked birds were undoubtedly overlooked, as they mingled in large dense flocks and it was never possible to examine every leg of every individual in a single day. Whenever possible, resighted birds were identified to subspecies on the basis of plumage characters. Comparing these identifications against the known non-breeding origin of the resighted birds provided insights into the location of the non-breeding grounds of the two subspecies. It also provided some insight into the repeatability of subspecific identifications made on plumage characters: of 45 individuals that were seen more than once, only one was assigned to different subspecies on different occasions.
We modelled our count data using the approach proposed by Thompson (1993) to estimate passage times and number of birds migrating through the staging area. Date of arrival and departure of each subspecies were assumed to be normally distributed. Iterative modelling enabled us to estimate the number of individuals that staged in the study site and the dates of arrival and departure (and associated standard errors) that best explained the changes in numbers of birds counted through the study period. Realistic starting values for the calibrations were obtained by an iterative branch-and-bound procedure. In this, at each iteration, the ranges bounding the ‘best’ values of each parameter were successively reduced. Final parameters and standard errors were estimated using the NONLIN procedure in SYSTAT 11 (Systat 2005) with a sum-of-squares loss function.
Attempts to model turnover by calculation of apparent daily survival probability of colour-banded individuals (Schaub et al. 2004; Verkuil et al. 2010) were unsuccessful, because too large a proportion of the colour-banded birds observed were sighted only once. Instead, we estimated the number of colour-banded birds that were present but undetected by examining the distribution of the number of times that individually marked birds were resighted. Another measure of the proportion of the flyway population occurring in the study site was a direct comparison of the number of birds present compared with estimates of the non-breeding population. Estimates of shorebird populations in non-breeding grounds of the EAAF (Barter 1992, 2002; Watkins 1993; Bamford et al. 2008) were based to some extent on maximum historical counts, many of which are out of date. We updated the estimates on the basis of published counts of major non-breeding sites in Australia and New Zealand over the last five years, when possible using average non-breeding counts (between November and February) from 2004–05 to 2008–09 as an index of current population size.
Results
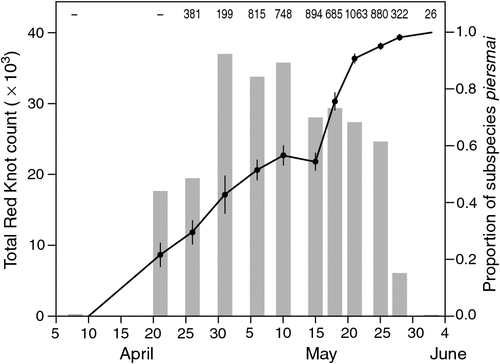
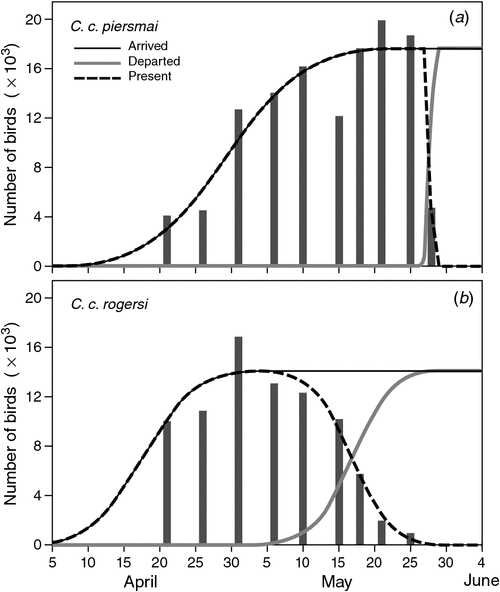
Red Knots were absent from the study area from January to March 2009; the first migrating birds were counted on 8 April. Numbers built up rapidly through April and peaked in early May, with single counts of 36 890 birds being made on 1 May, and 35 610 on 10 May; numbers remained high until the last week of May but thereafter declined rapidly, with only 26 birds present on 2 June (Fig. 2). The proportion of birds identified as C. c. piersmai on plumage characters increased steadily from 26 April to the end of May. The proportion of C. c. rogersi declined correspondingly, and by the end of the study period, only C. c. piersmai was present (Fig. 2).
|
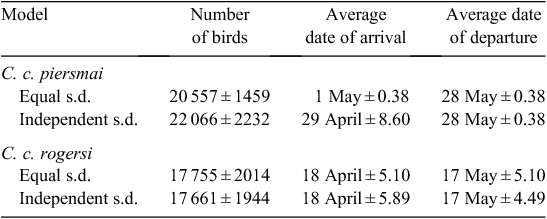
Using plumage characters to assess the proportion of C. c. piersmai and C. c. rogersi present over time, we modelled the passage times of both subspecies. They migrated on different schedules (Table 1; Fig. 3), with C. c. rogersi arriving earlier (average date of arrival, 21 April) and departing earlier (average date of departure, 17 May). Arrival and departure dates of C. c. rogersi showed similar scatter, with little difference between the results of a model that assumed that standard deviations of these dates were the same, and another model which calculated standard deviations of arrival and departure dates independently. C. c. piersmai arrived later (average date of arrival, 29 April) and departed later (average date of departure, 28 May) with departures being concentrated in a much smaller period than the period of arrivals. As a result, late-arriving C. c. piersmai have less time to stage than early arriving individuals, unlike C. c. rogersi in which our data are consistent with all individuals having a stopover duration of ~29 days. Average stopover duration of C. c. piersmai was similar, but the models imply that 16.3% of C. c. piersmai stage for 21 days or less, and that 3.3% stage in Bohai Bay for 14 days or less.
|
|
The passage-schedule models indicated that the Bohai Bay study area was used by a total of 39 760 Red Knots on northward migration: 17 660 C. c. rogersi and 22 100 C. c. piersmai. This constitutes 18.1% of the Flyway population estimate of 220 000 of Bamford et al. (2008). However, this estimate is out of date, and reappraising the Flyway population estimate to include the most recent published data (Table 2), it appears that the Flyway population is only ~105 000 birds, 37.9% of which staged in our study site in the Bohai Bay. As non-breeding counts from Australia and New Zealand include first-year birds that are not old enough to migrate north (Rogers et al. 2006), the proportion of migrating adult Red Knots using Bohai Bay is higher still. The average proportion of first-year Red Knots in north-western Australia from the austral summers of 1998–99 to 2008–09 was 17.0% (Minton et al. 2009). Assuming a similar proportion of first-year birds occurs elsewhere in the non-breeding range, the migrating population can be calculated as 87 150 adult Red Knots in the Flyway, 45.6% of which staged in our small study area.
The banding origin of birds resighted in the Bohai Bay area and identified to subspecies at the time of observation (Table 3) revealed substantial overlap in non-breeding grounds between C. c. piersmai and C. c. rogersi. Searches for colour-banded birds were made between 10 and 29 May, a period in which our models indicate that numbers of C. c. piersmai peaked (>90% of the staging population present on all days between 11 and 24 May). However, C. c. rogersi had already begun to depart: 20% of C. c. rogersi had already migrated from the study area by the beginning of the resighting period on 10 May and <1% remained by the end of the month. The average daily proportion of C. c. rogersi present during the colour-band resighting period was 30.3%. If we use this factor to account for departed birds we can calculate the relative proportions of C. c. rogersi and C. c. piersmai from several non-breeding populations for which we resighted many colour-banded or leg-flagged birds (Table 2): subspecies C. c. piersmai constituted 83.1% of the migrants from north-western Australia, 35.2% of the migrants from Victoria and 24.6% of the migrants from New Zealand. There was no relationship between date and the proportion of north-western Australian birds present among colour-marked birds (r = 0.287, n = 18). In contrast there was a clear relationship between date and the proportion of C. c. piersmai identified on plumage (Fig. 2 and above). Together, these results suggest that the timing of northward migration of each subspecies through Bohai Bay is driven more by migratory destination than by migratory origin.
Discussion
The study site in Bohai Bay – a 20-km stretch of coastline – proved to be extraordinarily important to Red Knots, supporting over 45% of the Flyway population of Red Knots during northward migration. Outside Bohai Bay, no other staging sites of comparable importance to Red Knot have been found in the Yellow Sea (Barter 2002). However, historical data suggest that our study site is probably not the only area within Bohai Bay with staging Red Knots. Barter et al. (2003) found over 14 000 Red Knots staging near Tianjin in 2002, some 40 km to the west; over 2400 Red Knots were found in eastern Zuidong just east of our study site; and patchy count data suggest that Red Knots also occur in the coastal areas between Tianjin and our study site (H.-Y. Yang, unpubl. data). These counts may already be out of date, so overall numbers of Red Knots staging in Bohai Bay remain unclear, but it is clear that this fairly small area is the key staging area on northward migration for well over half of the Red Knots in the East Asian–Australasian Flyway. Exactly why they should be so localised during this time of year has yet to be resolved. The Bohai Bay mudflats discovered in this study may provide exceptionally high quality prey, a condition required by Red Knots in general (van Gils et al. 2005b) and especially the late-migrating migrants from north-western Australia (Battley et al. 2005).
In fact our estimate of the proportion of the Flyway population of Red Knots staging in our study area is conservative, and the true value must be higher. Our count models, essentially the non-linear regressions, necessarily pass through the data points. However, given that some birds leave before all have arrived, the numbers present will always be lower than actual number that use the staging area. In addition, our revised estimate of the Flyway population is likely to be an underestimate. It was considerably lower than previous published estimates (Table 2). In part this was because former estimates of numbers occurring on Eighty Mile Beach, north-western Australia (based on extrapolation from aerial surveys), were inflated, but also because of population decline, with numbers of Red Knots in adequately surveyed areas of eastern Australia and New Zealand now considerably lower than they were in the 1980s. Although our revised estimate is a closer approximation of the current population of Red Knots in the EAAF, we emphasise that it needs to be refined further, as some important non-breeding sites (notably the Gulf of Carpentaria) have not been surveyed for many years.
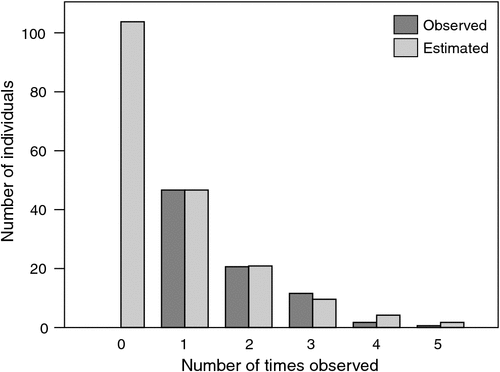
An independent check of the proportion of the Flyway population of Red Knot staging in Bohai Bay can be made on the basis of frequency of resightings of birds colour-banded in the non-breeding grounds. Of the 93 individually colour-banded birds resighted and identified to subspecies in Bohai Bay, 83 were C. c. piersmai (71 from Roebuck Bay, 12 from New Zealand). It was clear from the distribution of the number of resightings that many more must have been present but undetected. Assuming that the frequency of resightings had an exponential distribution (Fig. 4), we estimated that a further 104 colour-banded C. c. piersmai were present but overlooked, bringing the total number of individually colour-banded C. c. piersmai in the staging area to 187 (~160 from north-western Australia, ~27 from New Zealand). At the time of the study, 361 Red Knots had been individually colour-banded in north-western Australia (C. J. Hassell, T. Piersma and D. I. Rogers, unpubl. data). We can calculate that ~241 of these were alive during the resighting period in our study area (assuming average adult annual survival of 0.84; Brochard et al. 2002) and that ~200 of these living birds were C. c. piersmai (assuming 83.1% of the north-western Australian population was C. c. piersmai; see above). Following the same approach, we calculate that of the 370 Red Knots individually colour-banded in New Zealand, ~175 were still alive, and ~43 of these living birds were C. c. piersmai (assuming 24.6% of the New Zealand population was C. c. piersmai; see above). Using these figures we can roughly estimate that 80.0% of C. c. piersmai colour-banded in north-western Australia, and 62.8% of C. c. piersmai colour-banded in New Zealand, staged in the study area.
|
Only ten individually colour-banded C. c. rogersi were found during the study period, However, it is likely that we only found 45% of the birds present (assuming the same detection rate as for C. c. piersmai, see above). Moreover, our staging models indicate that the average daily proportion present of C. c. rogersi during the colour-band resighting period was 30.3%, many birds having already migrated north before we began to search for colour-bands. Correcting for these effects, we estimate 73 individually colour-banded C. c. rogersi staged in our study site (22 from north-western Australia, 51 from New Zealand). Using the approach described above for C. c. piersmai, we estimate that at the time of the study there were 203 individually colour-banded C. c. rogersi in the Flyway, 41 from north-western Australia (53.6% of which staged in our study area) and 132 from New Zealand (38.6% of which staged in our study area). These estimates are clearly crude, but they suggest that there is a tendency for Bohai Bay to be used more by staging Red Knots from north-western Australia than from New Zealand. In addition, the colour-band resighting data are consistent with the independent count data in indicating that Bohai Bay is used by a very large proportion of the Flyway population of Red Knots.
Separate estimates of the population size of C. c. rogersi and C. c. piersmai have never been made before because of the difficulty of distinguishing the subspecies on the non-breeding grounds (Delany and Scott 2006; Bamford et al. 2008). Applying our subspecies ratios to the non-breeding populations of north-western Australian, south-eastern Australia and New Zealand, and assuming that proportion of C. c. piersmai in the poorly known population in the Gulf of Carpentaria is intermediate between that seen in the populations of north-western Australia, we can tentatively make the first estimates of populations of the subspecies. The world population of C. c. piersmai is likely to be between 48 736 and 60 068 birds, and that of C. c. rogersi between 50 669 and 62 000 birds.
There were clear differences in the migration schedules of the two subspecies. C. c. piersmai was a late migrant, as was previously predicted on the basis of observations of departures from north-western Australia (Battley et al. 2005). The average arrival date for C. c. piersmai (29 April) corresponded closely enough in time to departure dates observed in north-western Australia (Battley et al. 2005) to suggest this subspecies makes a direct flight to Bohai Bay. In contrast, the average arrival date for C. c. rogersi was later (18 April) than observed departures from New Zealand by the end of March or start of April (P. F. Battley, pers. comm.), suggesting this subspecies uses an unknown staging area or areas elsewhere before arrival in Bohai Bay.
On average C. c. piersmai arrived 11 days later than C. c. rogersi, staged for a similar period and, most strikingly, had later departure dates that were far more compressed in time. One possible interpretation is that the timing of breeding varies more in C. c. rogersi than in C. c. piersmai because it has a more extensive breeding range (from at least 63°N to 69°N; Tomkovich 1992; Zöckler and O’Sullivan 2005). Alternatively, the difference may be explained by the differing terrains the subspecies fly over en route to their breeding grounds. For C. c. piersmai, the great circle route to the New Siberian Islands involves a long overland flight before reaching the shores of the Eastern Siberian Sea, which are frozen in late May and early June; they should therefore schedule their departures tightly in order to arrive on the breeding grounds when the thaw begins. Even when doing this they may encounter late snowfalls that force them to survive on their own body stores on reaching the breeding grounds, as has been demonstrated for subspecies islandica of the Red Knot in high arctic Canada (Morrison et al. 2005). In contrast, the great circle route from Bohai Bay to Chukotka takes C. c. rogersi parallel to the north-western shores of the Sea of Okhotsk (Fig. 1), where numerous estuaries, tidal flats and coastal lagoons may provide stopover opportunities for Red Knots should they encounter adverse wind conditions or heavy snow cover. Although there are few data, satellite imagery suggests there are ice-free areas along these shores in late May (e.g. http://www.natice.noaa.gov, accessed 22 September 2010) and there are observations of Red Knots from these shores consistent with such an interpretation (Gerasimov and Huettmann 2006). If C. c. rogersi does undertake further staging on northward migration, and does have more flexibility in arrival at the breeding grounds at a time when weather conditions are suitable, this could also explain another apparent paradox in our data: C. c. rogersi has a similar stopover period in Bohai Bay to C. c. piersmai (average 29 days), despite being slightly more distant from the breeding grounds (it is ~4700 km to from Bohai Bay to Chukotka, cf. ~4200 km to the New Siberian Islands).
The apparent dependence of Red Knots in this Flyway on Bohai Bay makes the conservation of C. c. rogersi and C. c. piersmai a matter of grave concern. In other flyways, subspecies rufa and islandica of Red Knots have suffered serious declines in population through degradation of staging areas (Baker et al. 2004; van Gils et al. 2006; Kraan et al. 2009). Bohai Bay lies in one of the most densely populated regions in the world, and it is undergoing rapid economic development, which includes conversion of tidal flats to land along much of the coastline. One of the biggest of these developments is at Caofeidian in northern Bohai Bay, a huge port and industrial development that had already destroyed approximately 110 km2 of tidal flats and shallow water area by the end of 2008 (SOAPRC 2009), including the former Red Knot site at Zuidong East; this project is now expanding into our study area. The Tianjin sites to the west are being lost to an even larger development project, the Tianjin New Area, which has already resulted in the loss of 80 km2 of tidal flats to the end of 2008 (SOAPRC 2009). Other development projects of similar scale are in progress elsewhere in Bohai Bay and at present there seems to be no legal impediment to further projects being planned. Protection of the remaining tidal flats of Bohai Bay is likely to be essential to the continued survival of Red Knots in the East Asian–Australasian Flyway.
Acknowledgements
This project was supported by the National Basic Research Program of China (Grant No. 2006CB403305). We thank BirdLife-Netherlands for the substantial financial contribution to the Global Flyway Network which enabled the individual marking of good numbers of Red Knots in north-western Australia and searches for them in Bohai Bay. We appreciate support of our field research from Ming-Lu Wu, Section Chief of Forestry Department, Hebei Province, China. The Australasian Wader Studies Group, Ornithological Society of New Zealand, and the Shorebirds 2020 project of Birds Australia allowed us to examine their count data. We are grateful to Si-Hai Yan for his field assistance; Brett Sandercock, Yvonne Verkuil and other participants of the Global Flyway Network demography workshop in September 2009 (hosted by NIOZ, Texel) for their feedback; Heather Gibbs and Phil Battley for summaries of data on numbers of birds colour-banded on the non-breeding grounds; Dick Visser for preparation of the figures; and Adrian Riegen, Ian Southey, Rob Schuckard, David Melville, Rob Clemens, Clive Minton and two anonymous referees for comments and discussions on the current world population of Red Knot.
References
Baker, A. J., Gonzalez, P. M., Piersma, T., Niles, L. J., de Lima Serrano do Nascimeto, I., Atkinson, P. W., Clark, N. A., Minton, C. D. T., Peck, M., and Aarts, G. (2004). Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proceedings of the Royal Society of London. Series B. Biological Sciences 271, 875–882.| Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay.Crossref | GoogleScholarGoogle Scholar |
Bamford, M., Watkins, D., Bancroft, W., Tischler, G., and Wahl, J. (2008). Migratory shorebirds of the East Asian – Australasian Flyway; population estimates and internationally important sites. Wetlands International – Oceania, Canberra.
Barter, M. (1992). Distribution, abundance, migration and moult of the Red Knot Calidris canutus rogersi. Wader Study Group Bulletin 64, 64–70.
Barter, M. (2002). Shorebirds of the Yellow Sea: importance, threats and conservation status. Wetlands International, International Global Series report 9; and Wader Study Group, Global International Wader Studies report 12; Canberra.
Barter, M. A., Li, Z. W., and Xu, J. L. (2001). Shorebird numbers on the Tianjin Municipality coast in May 2000. Stilt 39, 2–9.
Barter, M., Riegen, A., and Xu, Q. (2003). Shorebird numbers in Bohai Wan during northward migration. Stilt 44, 3–8.
Battley, P. F., Rogers, D. I., van Gils, J. A., Piersma, T., Hassell, C. J., Boyle, A., and Yang, H.-Y. (2005). How do Red Knots leave northwest Australia in May and reach the breeding grounds in June? Predictions of stopover times, fuelling rates and prey quality in the Yellow Sea. Journal of Avian Biology 36, 494–500.
| How do Red Knots leave northwest Australia in May and reach the breeding grounds in June? Predictions of stopover times, fuelling rates and prey quality in the Yellow Sea.Crossref | GoogleScholarGoogle Scholar |
Brochard, C., Spaans, B., Prop, J., and Piersma, T. (2002). Use of individual colour-ringing to estimate annual survival in male and female Red Knot Calidris canutus islandica: a progress report for 1998–2001. Wader Study Group Bulletin 99, 54–56.
Chatto, R. (2003). The distribution and status of shorebirds around the coast and coastal wetlands of the Northern Territory. Technical Report 73, Parks and Wildlife Commission of the Northern Territory.
Delany, S., and Scott, D. (2006). ‘Waterbird Population Estimates.’ 4th edition. (Wetlands International: Wageningen, The Netherlands.)
Gerasimov, Y. N., and Huettmann, F. (2006). Shorebirds in the Sea of Okhotsk: status and overview. Stilt 50, 15–22.
Kraan, C., van Gils, J. A., Spaans, B., Dekinga, A., Bijleveld, A. I., van Roomen, M., Kleefstra, R., and Piersma, T. (2009). Landscape-scale experiment demonstrates that Wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds. Journal of Animal Ecology 78, 1259–1268.
| Landscape-scale experiment demonstrates that Wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds.Crossref | GoogleScholarGoogle Scholar | 19490380PubMed |
Leyrer, J., Pruiksma, S., and Piersma, T. (2009). On 4 June 2008 Siberian Red Knots at Elbe Mouth kissed the canonical evening migration departure rule goodbye. Ardea 97, 71–79.
Lindström, Å., Minton, C. D. T., and Bensch, S. (1999). First recovery of a Red Knot Calidris canutus involving the breeding population on the New Siberian Islands. Wader Study Group Bulletin 89, 33–35.
Minton, C. (2007). Increased links between Australia and NZ with Siberia. Tattler 4, 11.
Minton, C. D. T., Wahl, J., Jessop, R., Collins, P., and Gibbs, H. (2005). Migration routes of waders which spend the non-breeding season in Australia. Stilt 50, 135–157.
Minton, C. D. T., Jessop, R., and Hassell, C. J. (2009). Wader breeding success in the 2008 Arctic summer, based on juvenile ratios of birds which spend the non-breeding season in Australia. Arctic Birds. Newsletter of International Breeding Conditions Survey 11, 58–62.
Morrison, R. I. G., Davidson, N. C., and Piersma, T. (2005). Transformations at high latitudes: why do red knots bring body stores to the breeding grounds? Condor 107, 449–457.
| Transformations at high latitudes: why do red knots bring body stores to the breeding grounds?Crossref | GoogleScholarGoogle Scholar |
Nebel, S., Piersma, T., van Gils, J., Dekinga, A., and Spaans, B. (2000). Length of stopover, fuel storage and a sex-bias in the occurrence of Red Knots Calidris c. canutus and C. c. islandica in the Wadden Sea during southward migration. Ardea 88, 165–176.
Piersma, T. (2007). Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. Journal of Ornithology 148, 45–59.
| Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide.Crossref | GoogleScholarGoogle Scholar |
Piersma, T., and Davidson, N. C. (1992). The migrations and annual cycles of five subspecies of knots in perspective. In ‘The Migration of Knots. Wader Study Group Bulletin Vol. 64, Supplement’. (Eds R. Piersma and N. C. Davidson.) pp. 187–197.
Piersma, T., Koolhaas, A., and Dekinga, A. (1993). Interactions between stomach structure and diet choice in shorebirds. Auk 110, 552–564.
Piersma, T., van Gils, J., de Goeij, P., and van der Meer, J. (1995). Holling’s functional response model as a tool to link the food-finding mechanism of a probing shorebird with its spatial distribution. Journal of Animal Ecology 64, 493–504.
| Holling’s functional response model as a tool to link the food-finding mechanism of a probing shorebird with its spatial distribution.Crossref | GoogleScholarGoogle Scholar |
Piersma, T., van Aelst, R., Kurk, K., Berkhoudt, H., and Maas, L. R. M. (1998). A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proceedings of the Royal Society of London. Series B. Biological Sciences 265, 1377–1383.
| A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics?Crossref | GoogleScholarGoogle Scholar |
Piersma, T., Rogers, D. I., Gonzalez, P., Zwarts, L., Niles, L. J., de Lima Serrano do Nascimeto, I., Minton, C. D. T., and Baker, A. J. (2005). Fuel storage rates before northward flights in Red Knots worldwide. In ‘Birds of Two Worlds: The Ecology and Evolution of Migratory Birds’. (Eds R. Greenberg and P. Marra.) pp. 262–274. (Johns Hopkins University Press: Baltimore.)
Riegen, A. C., Minton, C. D. T., Jessop, R., and Collins, P. (2005). Movements of Red Knot between Australia and New Zealand. In ‘Status and Conservation of Shorebirds in the East Asian – Australasian Flyway. Proceedings of the Australasian Shorebirds Conference 13–15 December 2003, Canberra, Australia’. Wetlands International, Wetlands International Global Series report 18, and Wader Study Group, International Wader Studies report 17. (Ed. P. Straw.) pp. 175–182. (Wetlands International and the Wader Study Group: Sydney.)
Rogers, D. I., Minton, C. D. T., Boyle, A. N., Hassell, C. J., and Silcocks, A. (2006). Growing up slowly by the sea-side: age of first northwards migration of shorebirds from Australian non-breeding grounds. In ‘Hidden Costs: Challenges faced by Migratory Shorebirds living on Intertidal Flats’, Ch. 10. (Ed. D. I. Rogers.) Ph.D. Thesis, Charles Sturt University.
Rogers, D. I., Hassell, C. J., Oldland, J., Clemens, R., Boyle, A., and Rogers, K. G. (2009). Monitoring Yellow Sea Migrants in Australia (MYSMA): north-western Australian shorebird surveys and workshops, December 2008. Report to Department of Environment, Water, Heritage and the Arts, and the Western Australian Department of Environment and Conservation. Australasian Wader Studies Group, Melbourne.
Sagar, P. M., Shankar, U., and Brown, N. (1999). Distribution and number of waders in New Zealand. Notornis 46, 1–43.
Schaub, M., Gimenez, O., Schmidt, B. R., and Pradel, R. (2004). Estimating survival and temporary emigration in the multistate capture–recapture framework. Ecology 85, 2107–2113.
| Estimating survival and temporary emigration in the multistate capture–recapture framework.Crossref | GoogleScholarGoogle Scholar |
SOAPRC (State Oceanic Administration People’s Republic of China) (2009). Communique on marine environment quality of Bohai Sea 2008. Beihai Branch of State Oceanic Administration of China (BBSOAC), Qingdao, China. Available at http://www.coi.gov.cn/hygb/dfhjzl/2008/bh/ [verified 22 September 2010, in Chinese].
Southey, I. (2009). Numbers of waders in New Zealand 1994–2003. Department of Conservation, Research and Development Series No. 308, Wellington, New Zealand.
Systat (2005). ‘Statistics.’ (Systat Software Inc.: Richmond, CA.)
Thompson, J. J. (1993). Modelling the local abundance of shorebirds staging on migration. Theoretical Population Biology 44, 299–315.
| Modelling the local abundance of shorebirds staging on migration.Crossref | GoogleScholarGoogle Scholar |
Tomkovich, P. S. (1992). An analysis of the geographical variability in Knots Calidris canutus based on museum skins. Wader Study Group Bulletin 64, 17–23.
Tomkovich, P. S. (2001). A new subspecies of Red Knot Calidris canutus from the New Siberian Islands. Bulletin of the British Ornithologists’ Club 121, 257–263.
Tomkovich, P. S., and Riegen, A. (2000). Mixing of Red Knot populations in Australia: some thoughts. Stilt 37, 25–27.
van Gils, J. A., Piersma, T., Dekinga, A., and Dietz, M. W. (2003). Cost-benefit analysis of mollusc-eating in a shorebird. II. Optimizing gizzard vsize in the face of seasonal demands. Journal of Experimental Biology 206, 3369–3380.
| Cost-benefit analysis of mollusc-eating in a shorebird. II. Optimizing gizzard vsize in the face of seasonal demands.Crossref | GoogleScholarGoogle Scholar | 12939369PubMed |
van Gils, J. A., de Rooij, S. R., van der Meer, J., Dekinga, A., Piersma, T., and Drent, R. (2005a). Digestive bottleneck affects foraging decisions in red knots (Calidris canutus). I. Prey choice. Journal of Animal Ecology 74, 105–119.
| Digestive bottleneck affects foraging decisions in red knots (Calidris canutus). I. Prey choice.Crossref | GoogleScholarGoogle Scholar |
van Gils, J. A., Battley, P. F., Piersma, T., and Drent, R. (2005b). Reinterpretation of gizzard sizes of red knots world-wide emphasises overriding importance of prey quality at migratory stopover sites. Proceedings of the Royal Society of London. Series B. Biological Sciences 272, 2609–2618.
| Reinterpretation of gizzard sizes of red knots world-wide emphasises overriding importance of prey quality at migratory stopover sites.Crossref | GoogleScholarGoogle Scholar |
van Gils, J. A., Piersma, T., Dekinga, A., Spaans, B., and Kraan, C. (2006). Shellfish dredging pushes a flexible avian top predator out of a marine protected area. PLoS Biology 4, e376.
| Shellfish dredging pushes a flexible avian top predator out of a marine protected area.Crossref | GoogleScholarGoogle Scholar | 17105350PubMed |
Verkuil, Y. I., Wijmenga, J. J., Hooijmeijer, J. C. E. W., and Piersma, T. (2010). Spring migration of ruffs Philomachus pugnax in Fryslân: estimates of staging duration using resighting data. Ardea 98, 21–33.
Watkins, D. (1993). A National Plan for Shorebird conservation in Australia. RAOU Report 90, Australian Wader Studies Group, Royal Australasian Ornithologists Union and World Wide Fund for Nature, Melbourne.
Wilson, J. R. (2000). South Australia wader surveys January 2000 and February 2000. Australian Wader Studies Group Report, Melbourne.
Zöckler, C., and O’Sullivan, J. (2005). New Zealand Red Knot breeding in Meinopylgino,Chukotka, NE Russia. Wader Study Group Bulletin 108, 76.