Do bird species richness and community structure vary with mistletoe flowering and fruiting in Western Australia?
Kathryn R. Napier A D , Suzanne H. Mather B , Todd J. McWhorter C and Patricia A. Fleming AA School of Veterinary and Life Sciences, Murdoch University, Murdoch, WA 6150, Australia.
B 3 Hardy Road, Nedlands, WA 6009, Australia.
C School of Animal and Veterinary Sciences, University of Adelaide, Roseworthy Campus, SA 5371, Australia.
D Corresponding author. Email: k.napier@murdoch.edu.au
Emu 114(1) 13-22 https://doi.org/10.1071/MU12098
Submitted: 3 November 2011 Accepted: 25 March 2013 Published: 25 June 2013
Abstract
Worldwide, mistletoes act as a keystone resource, providing food (nectar, fruit and foliage) and structural (nesting sites) resources to hundreds of fauna species. In Australia, loranthaceous mistletoes depend on birds for pollination and dispersal, and provide important nectar and fruit resources to a large number of nectarivorous and frugivorous species of bird. We investigated whether avian species richness and community structure varies with flowering and fruiting of two common mistletoe species (Loranthaceae : Wireleaf Mistletoe, Amyema preissii; and Box Mistletoe, A. miquelii), conducting monthly surveys of both birds and mistletoes over 1 year at five sites in south-western Western Australia (WA). Flowering and fruiting periods were distinct and differed both among sites and between mistletoe species. Nectar and ripe fruit were available for up to 5 months (Box Mistletoe) or 6–7 months (Wireleaf Mistletoe) at individual sites, but were available every month of the year across all sites. The presence of fruiting, but not flowering, mistletoe was associated with changes in bird community structure. Mistletoebirds (Dicaeum hirundinaceum) were significantly more likely to be recorded during months when ripe mistletoe fruit was present and the overall bird species richness was higher for these survey months. Mistletoes provide important resources, but further investigation is required to assess whether they act as a keystone resource in south-western WA.
Introduction
Mistletoes are a polyphyletic group of shrubby, aerial, hemiparasitic flowering plants, with more than 1500 species and found on all continents except Antarctica and in a wide range of habitats (Kuijt 1969; Calder 1983; Watson 2001). The family Loranthaceae is native to Australia (~85% of which are endemic), with 75 species of showy mistletoes currently recognised (Barlow 1984, 1992; Watson 2011). Watson (2001) first proposed that mistletoes act as a keystone resource (sensu Power et al. 1996) in forests and woodlands worldwide, owing to the pervasive effects they have on these habitats through the provision of nutritional and nesting resources (confirmed by Watson and Herring 2012). Peres (2000) identified four criteria used to define a keystone plant: reliability and abundance of resources, degree of consumer specificity and temporal redundancy. Kotliar (2000) further proposed that keystone species should perform functions not otherwise carried out. In this study, we address whether two species of loranthaceous mistletoes in south-western Western Australia (WA) meet these keystone criteria.
In addition to providing a source of nutritious food (e.g. Keast 1958; Reid 1987; Turner 1991; Brown et al. 1997), many species of mistletoe also have extended flowering and fruiting phenologies that minimise competition with other plant species but which are also important for sustaining populations of their avian pollinators and dispersers. Australian mistletoes continually draw upon the water and nutrient resources of their host and can thus flower and set fruit during dry seasons when little other nectar or fruit is available in the landscape (Paton and Ford 1977; Reid 1986; Watson 2001). In addition to flowering and fruiting at different times to most other plants, discontinuous ripening (both within a species and among species within communities) extends flowering and fruiting periods (Reid 1986; Hawksworth and Wiens 1996; Watson 2001). For example, most temperate southern Australian mistletoes flower during summer, in contrast to the largely spring and autumn flowering of most ornithophilous plant species present in the same regions (Reid 1986; Watson 2011). In more arid areas, where mistletoes are a critical food source for nectarivorous species, some species of mistletoe flower during winter, others during summer, and others flower year-round (Reid 1986; Watson 2011). Mistletoes also provide the most reliable (and sometimes the only) source of fruit for their avian dispersers. Further, their semi-succulent leaves have a high concentration of nutrients and are consumed by herbivores (Watson 2001). Their dense, multi-branched structure means that many species of mistletoe provide important nesting and foraging sites for animals in an otherwise open canopy (see review by Watson 2001). In Australia, avian species from more than 60 families (across 16 orders) and species from several mammalian families have been recorded nesting in mistletoes, and 66% (of 330 species) of Australian arboreal nesting bird species have been recorded using mistletoes as nesting sites (Cooney et al. 2006). Many insectivorous species (e.g. thornbills, Acanthiza spp.; whistlers, Pachycephala spp.) also use clumps of mistletoe as a foraging substrate, as mistletoes often have abundant and distinctive insect assemblages (Turner 1991; Watson 2001; Start 2011; Watson 2011), although Burns et al. (2011) found no difference in insect assemblages between mistletoe clumps and surrounding vegetation. Mistletoes therefore reliably provide important food and shelter resources for hundreds of fauna species, providing resources and ecosystem services out of proportion to their abundance and contribution to biomass (Davidson et al. 1989; Watson 2001, 2002; Mathiasen et al. 2008; Watson and Herring 2012).
Loranthaceous mistletoes provide birds with important nectar and fruit resources (Kuijt 1969; Calder 1983; Davidar 1985; Reid 1986; Ladley et al. 1997; Robertson et al. 1999), with at least 50 bird species, including several species of honeyeater, recorded consuming mistletoe nectar or fruit in Australia (Keast 1958; Reid 1987; Turner 1991; Brown et al. 1997). With two exceptions, the root-parasitic Western Australian Christmas Tree (Nuytsia floribunda) and Atkinsonia (Atkinsonia ligustrina) (Hawkeswood 1981; Watson 2011), all Australian loranthaceous mistletoes are pollinated by birds. These mistletoes tend to have brightly coloured, odourless flowers, with abundant, sugar-rich nectar (up to 60% total sugar content, primarily glucose and fructose sugars) (Reid 1986; Stiles and Freeman 1993; Baker et al. 1998), characteristics typically associated with ornithophilous pollination. Although they may not be dependent on mistletoe nectar as a primary source of food, a wide range of avian species pollinate these mistletoes, including several species of honeyeaters (Reid 1986; Watson 2001, 2011). The degree of consumer specificity between mistletoes and their pollinators has led several authors to suggest that long-term negative consequences for both interacting organisms, and possibly the entire ecosystem, may ensue if this balance were disrupted (Reid et al. 1995; Watson 2001).
Birds are also responsible for seed dispersal for nearly all Australian loranthaceous mistletoes (with the exception of the Western Australian Christmas Tree, which is wind dispersed, Watson 2011). Mature mistletoe fruits are often brightly coloured, fairly large and sweet, and are a food source for obligate and opportunistic bird species worldwide (see review by Watson 2001). The composition of the fleshy fruit pulp surrounded by a layer of viscin varies among species, but most mistletoe fruits contain a high proportion of carbohydrates, lipids and protein (López de Buen and Ornelas 2001; Watson 2001; Barea 2008).
In addition to supporting generalist feeders, Australian mistletoes also support two mistletoe-fruit specialists. The Mistletoebird (Dicaeum hirundinaceum) is found throughout mainland Australia (Keast 1958; Barrett et al. 2003) and is locally mobile, its presence corresponding with the availability of fruiting mistletoe (Rawsthorne et al. 2012). The rare Painted Honeyeater (Grantiella picta), classified as vulnerable C1 (BirdLife International 2012), is found across inland eastern Australia (Reid 1986), and can be considered the original Australian mistletoe bird as the Mistletoebird did not colonise Australia until possibly as recently as the Holocene (c. 12 000 years before present, Reid 1991). The diversification and radiation of mistletoes across Australia has therefore depended upon species such as the Painted Honeyeater and its ancestors (Watson 2011).
Despite their importance as a food resource, there have been few studies examining how mistletoes may influence changes in Australian avian communities (see studies by Turner 1991; Watson 2002; Watson et al. 2011; Watson and Herring 2012). Additionally, we have very little information on the fruiting and flowering phenology of mistletoes in south-western WA. The aim of this study was to investigate if avian species richness and community structure varies with flowering and fruiting of two species of Loranthaceae mistletoe common in south-western WA, the Wireleaf Mistletoe (Amyema preissii) and Box (or Stalked) Mistletoe (A. miquelii). This study examined how flowering and fruiting phenology compared with year-round species richness. We predicted that: (1) there would be a greater number of bird species present (i.e. greater species richness) at our study sites when mistletoe flowers or fruit were available; (2) avian community structure at our study sites would reflect variability in available resources; and (3) Mistletoebirds would only be present in an area when ripe mistletoe fruit was available. We then discuss the findings of this study in relation to the keystone criteria identified by Peres (2000) and Kotliar (2000).
Methods
Study sites
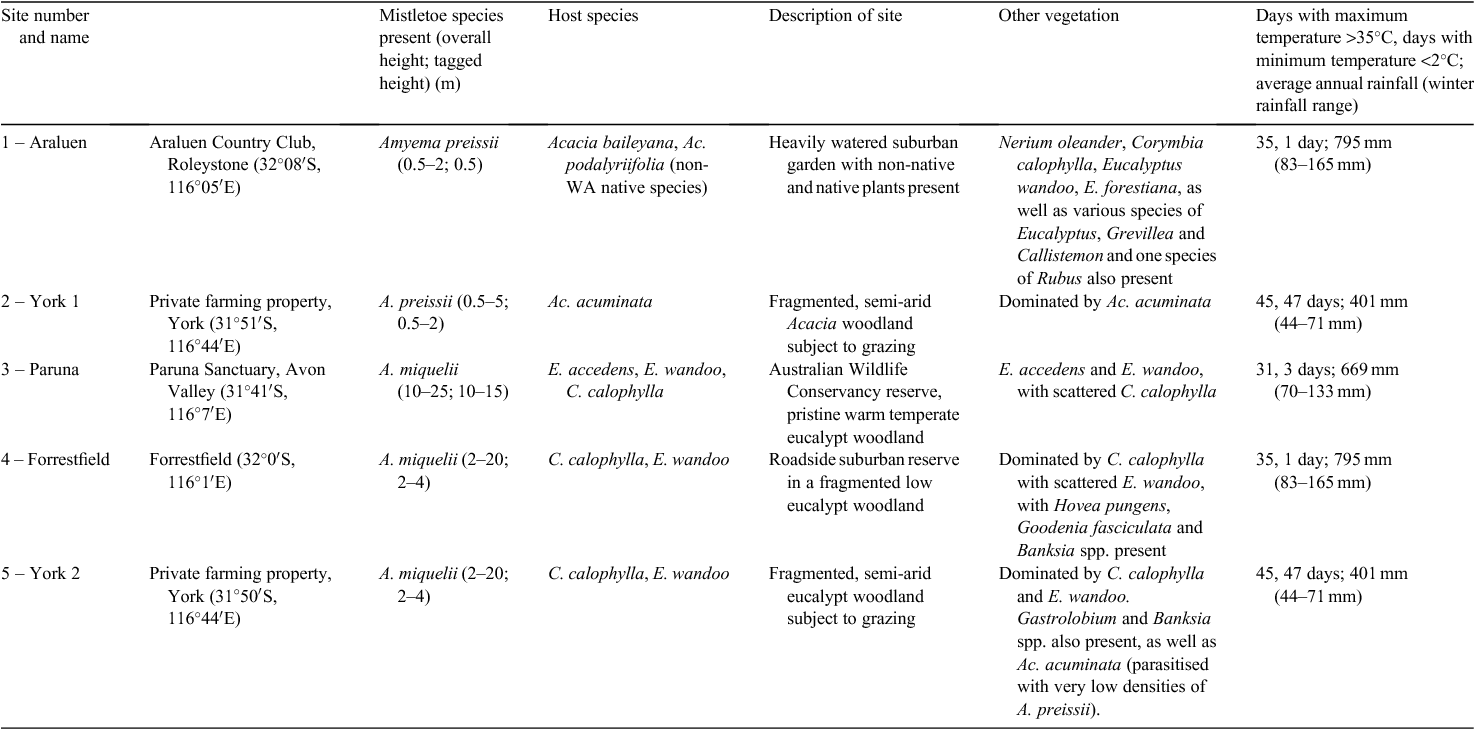
The study was conducted at five sites (each 100 × 200 m) in south-western WA where mistletoe was extremely abundant, between February 2010 and January 2011 (Table 1). Two sites contained Wireleaf Mistletoe parasitising Acacia hosts, and three contained Box Mistletoe parasitising Eucalyptus and Corymbia hosts. Mistletoe was the dominant food resource with few other sources of fruit or nectar present (Table 1), with the exception of the site at Araluen. Araluen is surrounded by a dense urban matrix, which includes flowering and fruiting plants in large gardens. Forrestfield is also surrounded by suburban matrix but the area is more rural, with larger property sizes and more native vegetation (Table 1). One of the sites (York 2) contained both species of mistletoe, but Wireleaf Mistletoe was present in extremely low densities on only two individual trees. In south-western WA, available records of flowering and fruiting suggested that both species of mistletoe have only brief periods of flowering and fruiting (A. N. Start, pers. comm.). In south-western WA, Wireleaf Mistletoe flowers January–March and fruits April–July, whereas Box Mistletoe has been recorded flowering March–April and fruiting June, November and December (A. N. Start, pers. comm.).
|
South-western WA experiences hot summers and cool wet winters. York (in the highly fragmented agricultural wheatbelt) is further inland than the other sites, and has a greater annual temperature range and lower rainfall than the other sites (Table 1).
Fruiting and flowering phenology
The flowering and fruiting phenologies of Wireleaf and Box Mistletoe were determined by counts of flowers (classed as bud, open or senescent) and fruit (classed as immature, unripe, ripe or bare, where only stalks remained after fruit had been removed) at intervals of ~5 weeks. At each site, 30-cm sections of tagged branches (measured proximally from the tip of the branch) of up to 21 randomly selected mistletoe plants were monitored for each survey period, as per Barea and Watson (2007). These branches ranged in height from 0.5 m above the ground to 15 m. Each tagged branch was monitored approximately every 5 weeks, with counts made of flowers (from buds through to senescent) and fruit (from immature through to bare). As the total number of flowers and fruits varied between branches, an index of relative abundance of flowers and fruit was calculated (expressed as the proportion of fruit and flowers per 30 cm of branch, summing to 1). The proportion was averaged across all mistletoe plants for each site or host. Mistletoes surveyed included Wireleaf Mistletoe parasitising Cootamundra Wattle (Acacia baileyana; n = 21 tagged branches), Queensland Silver Wattle (Ac. podalyriifolia; n = 21) and Jam (Ac. acuminata; n = 21), and Box Mistletoe parasitising Powderbark Wandoo (Eucalyptus accedens; n = 14) and Marri (Corymbia calophylla; n = 16 at York 2 and n = 18 at Forrestfield; Table 1).
Bird species
Surveys of bird species presence at each site were carried out using a standardised search method (Watson 2003). We conducted a total of 53 survey days over the year: 11 at each of Araluen, York 1, Paruna and Forrestfield, and 9 at York 2. No surveys were conducted in July at any site.
We did not attempt to estimate bird species abundance. In each month, we conducted at least three 20-min surveys back-to-back, which were considered as one single survey. We used a stopping rule under which we continued to conduct 20-min surveys until the number of species seen in a single survey period of 20 min was less than or equal to the number of species seen in each of the two preceding sampling periods (Watson 2003, 2004). Surveys began within 90 min of sunrise (0503–0718 hours).
Statistical analysis
(1) Fruiting and flowering phenology
Differences in fruiting and flowering periods were compared among sites (and, for the Araluen site, between host species) and month by two-way analysis of variance (ANOVA), using Tukey–Kramer post hoc tests for unequal sample sizes as required. Proportions of ripe fruit and open flowers were arcsine square-root transformed before analysis to meet requirements of parametric statistics.
(2) Avian species richness
Avian species richness (total number of bird species recorded) was compared among sites and for months when flowers or fruit were present (open flower-class, ripe fruit-class) or absent (bud or senescent flower-classes, immature, unripe or bare fruit-classes) by two-way ANOVA, with Tukey–Kramer post hoc tests for unequal sample sizes as required.
(3) Avian community structure
Avian data, classified as presence (1) or absence (0) for each bird species for each monthly survey, were analysed by multidimensional scaling (MDS) using the program PAST 2.08b (Hammer et al. 2001). Avian community structure was then compared via two-way analysis of similarity (ANOSIM, Bray–Curtis similarity matrices; using PAST 2.08b), with site (numbered 1–5) and either flowering (comparing months with or without open flowers) or fruiting (comparing months with or without ripe fruit available) as independent factors. The ANOSIM test statistic (R) contrasts the differences among groups with variation within groups, with a large positive R (up to 1) signifying dissimilarity between groups; significance is calculated via permutation (Clarke 1993). Similarity percentage (SIMPER) was then used to assess which individual species were primarily responsible for observed differences (Clarke 1993) and then subsequently for feeding guilds (frugivore, nectarivore, insectivore, granivore and omnivore; see Supplementary material Table S1).
(4) Presence or absence of bird species
To examine the relationship of bird species presence and mistletoe flowering, we constructed, for each month, contingency tables of the presence–absence of individual bird species against the presence–absence of mistletoe flowers or fruit. These contingency tables were analysed for significance via Fisher’s exact probability using STATISTICA (StatSoft Inc. 2007), followed by a Bonferroni correction.
All other statistical tests were conducted using STATISTICA (StatSoft Inc. 2007).Statistical significance was set to α < 0.05. Results are presented as means ± standard deviation.
Results
In total, 41 species of bird were recorded over 53 survey days at the five sites (February 2010–January 2011), including two frugivores, the Mistletoebird and Silvereye (Zosterops lateralis), and six nectarivores, the Brown (Lichmera indistincta), New Holland (Phylidonyris novaehollandiae), Singing (Lichenostomus virescens) and White-cheeked (Phylidonyris niger) Honeyeaters, Red Wattlebird (Anthochaera carunculata) and Western Spinebill (Acanthorhynchus superciliosus) (Supplementary material Table S1).
Presence of mistletoe flowers
(1) Fruiting and flowering phenology
Flowers (and therefore nectar) of both species of mistletoe were available for 2–3 months at each site, spanning the austral summer and autumn (Wireleaf Mistletoe: December–February, Fig. 1a, c, e; Box Mistletoe: December–May, Fig. 1b, d, f), with maximum abundance at different times at each site (Fig. 1). A significant site × month interaction (F48,1014 = 59.66, P < 0.001) showed that flowering periods differed among sites and between mistletoe species. We note, however, that differences between hosts at the same site did not affect flowering or fruiting phenology (Wireleaf Mistletoe was monitored on Cootamundra Wattle and Queensland Silver Wattle at Araluen; see Fig. 1c, e).
(2) Avian species richness
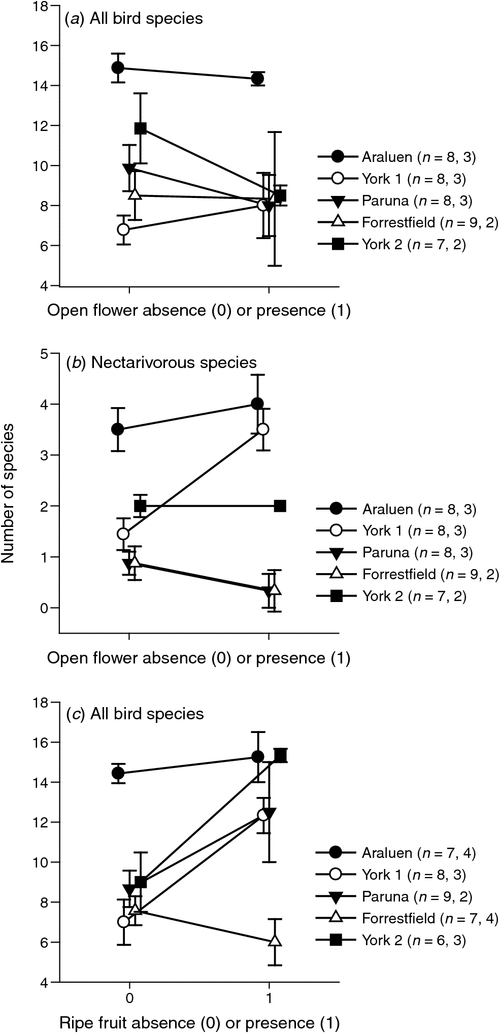
There were no significant differences in avian species richness (i.e. number of species recorded) between months when mistletoe was flowering or not (flowers present: 11.1 ± 4.1, n = 13 monthly surveys; flowers absent: 9.8 ± 4.0 bird species, n = 40 surveys; F1,43 < 0.01, P = 0.975), or among sites (F4,43 = 0.57, P = 0.686). However, a significant interaction term (site × presence of flowers: F4,43 = 6.49, P < 0.001; Fig. 2a), showed that Araluen had significantly higher avian species richness than three of the other four sites (not York 2) when flowers were not present (outside the summer months, Tukey–Kramer post hoc tests). There were also no significant differences in avian species richness when the nectarivorous species only (n = 6 species) were compared for months when mistletoe was flowering v. months when flowers were absent (flowers present: 1.9 ± 1.8, n = 13 monthly surveys; flowers absent: 1.7 ± 1.3 bird species, n = 40 surveys; F1,43 = 1.11, P = 0.297). There was a significant difference among sites (F4,43 = 20.71, P < 0.001); there was also a significant interaction term (site × presence of flowers: F4,43 = 2.80, P = 0.037, Fig. 2b) which shows that even when flowers were not present, Araluen had significantly more nectarivorous species than three of the other four sites (not York 2; Tukey–Kramer post hoc tests).
|
(3) Avian community structure
Bird community structure also varied among sites (two-way ANOSIM; site: R = 0.75, P < 0.001), but the presence of flowering mistletoe (comparing months with or without open flowers) did not have a significant effect on bird community structure (flowering: R = 0.08, P = 0.208).
(4) Presence or absence of bird species
The Grey Butcherbird (Cracticus torquatus) was the only bird species that was more likely to be recorded during months when mistletoe was flowering (Fisher’s exact test: P = 0.042), whereas the Western Gerygone (Gerygone fusca) was less likely to be recorded during these months (P = 0.042). However, as these differences were not significant after Bonferroni correction, these results will not be discussed further. During surveys, four species of nectarivorous honeyeater (Brown, New Holland, Singing, and White-cheeked honeyeaters) were directly observed feeding on flowers of both species of mistletoe.
Presence of ripe mistletoe fruit
(1) Fruiting and flowering phenology
Ripe fruit appeared some 2–7 months after flowering, and was available for 2–5 months, with maximum abundance occurring in winter (Wireleaf Mistletoe: June and August, Fig. 1a, c, e) and spring–summer (Box Mistletoe: November and January, Fig. 1b, d, f). A significant interaction term (site × month: F48,1014 = 26.66, P < 0.001) showed that fruiting periods differed among sites and between mistletoe species.
(2) Avian species richness
There were significantly more bird species present (i.e. greater species richness) during surveys when ripe mistletoe fruit was present than at other times (fruit present: 12.06 ± 4.27, n = 16 monthly surveys; fruit absent: 9.24 ± 3.65, n = 37 surveys; F1,43 = 7.94, P = 0.007). There was also a significant difference among sites (F4,43 = 13.60, P < 0.001) and a significant interaction term (site × month: F4,43 = 3.77, P = 0.010; Fig. 2c) with the two sites surrounded by urban matrix showing significantly higher (Araluen) or lower (Forrestfield) avian species richness (Fig. 2c).
(3) Avian community structure
Avian community structure was significantly different for months when ripe mistletoe fruit was present, compared with months when fruit was absent or unripe (two-way ANOSIM; fruiting: R = 0.28, P = 0.002), with significant differences also present among sites (site: R = 0.88, P < 0.001). The Mistletoebird made the greatest contribution of all species (a total of 41 species) to the difference between avian community structure for fruiting and non-fruiting months (6.3%, SIMPER). When analysed for the five feeding guilds (SIMPER, standardised by the number of species within each guild), the two frugivores contributed an average of 32.7% to the difference between fruiting and non-fruiting months, the six nectarivores 25.2%, the five omnivores 17.6%, the seven granivores 14.9% and the 21 insectivores 9.6%.
(4) Presence or absence of bird species
The Mistletoebird was the only species that was significantly more likely to be recorded during months when ripe mistletoe fruit was present (Fisher’s exact test: P < 0.001, significant after Bonferroni correction), although Mistletoebirds were also recorded at three sites (York 1, Paruna, York 2) during months when no ripe mistletoe fruits were recorded (periods of 2, 1 and 5 months for these sites respectively). The Mistletoebird and a parrot, the Australian Ringneck (Barnardius zonarius), were the only species of birds directly observed eating mistletoe fruit during our surveys (the Australian Ringneck feeding on Wireleaf Mistletoe; Mistletoebirds feeding on both species of mistletoe). Mistletoebirds were frequently observed probing the unripe green fruits of Box Mistletoe and pale-pink fruits of Wireleaf Mistletoe and then either rejecting (Box and Wireleaf Mistletoe) or occasionally ingesting the unripe fruit (Wireleaf Mistletoe only). Many other species were observed perching within mistletoe clumps, including the Weebill (Smicrornis brevirostris), Yellow-rumped Thornbill (Acanthiza chrysorrhoa), Striated Pardalote (Pardalotus striatus), Western Spinebill, Varied Sittella (Daphoenositta chrysoptera), Rufous Whistler (Pachycephala rufiventris), Grey Fantail (Rhipidura albiscapa), Red-capped Robin (Petroica goodenovii) and Silvereye.
Discussion
In this study, we recorded greater avian species richness and altered avian community structure in the presence of fruiting mistletoe, and a greater chance of sighting Mistletoebirds during months when ripe fruit was present. Can mistletoes in south-western WA therefore be described as a keystone resource? We discuss the findings of our study and records from the literature in relation to the five criteria of keystone plant identified by Peres (2000) and Kotliar (2000): reliability and abundance of resources, degree of consumer specificity, temporal redundancy and resources (functions) not otherwise present.
Reliability of mistletoe resources
The Wireleaf Mistletoe and Box Mistletoe are important resources for avian communities in south-western WA because they are widely distributed as well as having extended flowering and fruiting periods. Wireleaf Mistletoe and Box Mistletoe each had flowers or fruit available for about half the year (Wireleaf Mistletoe 6–7 months and Box Mistletoe 5 months) and across all sites timing of flowering and fruiting was staggered so that resources were available over all months of the year. We predicted that there would be a greater number of bird species present at our study sites when mistletoe flowers or fruit were available, and that avian community structure would reflect the availability of resources. The presence of ripe mistletoe fruit was correlated with significantly higher avian species richness and altered community structure. Importantly, the patterns of presence of fruit did not coincide across our survey sites (maximum 100 km apart), and yet the presence of bird species was associated with site-specific timing of the presence of fruit. Therefore, although we are not able to discount entirely ecosystem-wide effects such as the detectability of birds (Field et al. 2002), spring and winter migration (see review on partial migration by Chan 2001) and surges driven by rainfall that attract locally mobile species (as well as promoting recruitment and fruiting of mistletoes; Reid 1987; Yan and Reid 1995), the findings of the present study are consistent with those of previous studies that have observed a positive link between increased species richness and density of mistletoes (Turner 1991; Bennetts et al. 1996), and manipulative studies comparing the avifauna of two adjacent woodland remnants, one of which had been manually cleared of mistletoe (Watson 2002; Watson and Herring 2012). Reid (1986) reported that mistletoe is one of the few reliable sources of fruit in eucalypt forests of south-eastern Australia, and although we only recorded fruiting phenology over 1 year, anecdotal observations (K. Napier, pers. obs.) suggest that mistletoe fruit is predictably available at each site from year to year, and is therefore extremely reliable. Mistletoes may therefore fulfil the criterion of resource reliability to sustain consumers such as the Mistletoebird.
Abundance of mistletoe
Crude measures of mistletoe abundance at each site in the present study indicated that mistletoe is super-abundant in each of the study sites, typical of fragmented landscapes in south-western WA. With the caveat that resource patch density (sensu Peres 2000) was not measured in the present study, mistletoes in south-western WA do appear to fulfil the criterion of high abundance (sensu Peres 2000) because of their provision of abundant nectar and fruit resources. The attribute of resource abundance as a criterion for keystone species status is seen as secondary to the redundancy, reliability and specificity of a given resource (Peres 2000).
Degree of consumer specificity
Peres (2000) classified resources as ranging from extremely generalised, defined as being consumed by ≥50% of the species in an avian community, to extremely specialised, defined as being consumed by ≤5% of the species in the community. Mistletoes promote biodiversity by providing plentiful resources such as nutrient-rich fruit, nectar and leaf-litter, as well as sheltered nesting sites and site for abundant foliar arthropods (Watson 2001; Cooney et al. 2006; Watson et al. 2011). Although the influence of these resources on the structure of an avian community can be difficult to elucidate (Watson et al. 2011), several studies have described the link between the presence of aerial mistletoes and greater avian species richness (e.g. Turner 1991; Bennetts et al. 1996; Watson 2002; Watson and Herring 2012). The present study clearly indicates a correlation between the presence of bird species and the temporal availability of ripe mistletoe fruit. We had predicted that frugivorous birds would be more likely to be present when ripe mistletoe fruit was available. Not surprisingly, presence of the only mistletoe fruit specialist found in WA, the Mistletoebird, was associated with the presence of fruiting mistletoe, and Mistletoebirds were observed feeding on the fruit of both species of mistletoe. Australian Ringneck parrots, which are opportunistic feeders (Higgins 1999), were observed ingesting fruits of Wireleaf Mistletoe in our study and have been previously recorded feeding on the fruits of at least two other mistletoes, Amyema quandang and Harlequin Mistletoe (Lysiana exocarpi) (Forde 1986; Watson 2011; Western Australian Herbarium 2011). Several other species of bird (nectarivorous, granivorous and insectivorous) that are known to eat mistletoe fruits, either regularly or opportunistically, were recorded in the present study, including Singing Honeyeaters, Red Wattlebirds, Silvereyes and Yellow-rumped Thornbill (see Reid 1986 and references therein), although none was observed to feed on mistletoe fruit during this study. These species did not show patterns of occurrence in relation to the presence of mistletoe fruit individually, but there was a significant increase in avian species richness and changes in the structure of the avian community in months when mistletoe fruit was present compared with months when it was not.
Many nectarivorous species, as well as insectivorous and generalist species, feed on the nectar of Australian mistletoes (see Watson 2001). In the present study, although four nectarivorous species of honeyeater were observed feeding on the nectar of both species of mistletoe, we did not find that the presence of these species at our study sites was linked with the presence of mistletoe flowers. Many insectivorous species were also observed perching in clumps of mistletoe, which may reflect enhanced foraging opportunities presented by the abundance of insects associated with mistletoes (Turner 1991; Burns et al. 2011; Watson 2011; Watson et al. 2011).
In summary, the literature suggests support for the criterion of consumer specificity proposed by Peres (2000), although in the present study only the presence of a single species (the Mistletoebird) was positively correlated with fruiting mistletoe; mistletoe, in this study, would therefore be classified as an extremely specialised resource, and fails to meet this criterion as it is not consumed by a large proportion of the bird assemblage with which they coexist (i.e. extremely generalised resource). This criterion requires further investigation.
Temporal redundancy of mistletoe
Under the keystone criterion of temporal redundancy density (sensu Peres 2000), a resource may be considered entirely indispensable if it is available during periods of overall resource scarcity. Mistletoes in the fragmented landscapes assessed in this study may then be considered a low-redundancy resource as they were often the only source of fleshy fruit and nectar available (with the exception of the Araluen site, where blackberry (Rubus sp.) was present; see Table 1), and would therefore be considered entirely indispensable. Temporal and spatial fluctuations within and among habitats owing to individual movements and population processes occur in most avian communities (Malizia 2001). The distribution and abundance of food resources, in particular, influences the movements of many birds (Levey 1988). The foraging efficiency of nectarivores and frugivores is also affected by the temporal pattern of availability of flowers and fruit: if the occurrence of nectar and fruit are temporally and spatially predictable, animals may retain this information and visit plants with available resources without random, undirected searching (Wright 2005). In South Australia, A. quandang (Reid 1990) exhibited continuous production of ripe fruit in local plant communities owing to overlap in successive annual fruit crops, whereas the fruiting and flowering by Wireleaf Mistletoe and Box Mistletoe at each of our study sites was fairly distinct. However, across all five sites examined in the present study, nectar or ripe fruit, or both, was available for every month of the year owing to staggered flowering and fruiting of the two species, as well as geographical variation in timing of flowering and fruiting. In SA, the continuous availability of fruit produced by A. quandang was able to sustain permanent populations of Mistletoebirds (Reid 1990) but it appears that the distinct, but staggered fruiting phenology of Wireleaf Mistletoe and Box Mistletoe in south-western WA supports locally mobile populations of Mistletoebirds, and a similar result was also recorded in another study in South Australia (Yan 1993). We note that Mistletoebirds were present at three sites during months when no ripe mistletoe fruits were recorded, and that this may be attributed to slight differences in fruiting and flowering phenology that were not captured by our survey methods (e.g. ripe fruit present on mistletoe plants that were not monitored during surveys), or Mistletoebirds feeding on the unripe fruits of Wireleaf Mistletoe. With the caveat that fruiting and flowering phenology was recorded for only 1 year, mistletoe appears to be a temporally reliable source of fruit and nectar (see also Yan 1993). Through the provision of fruit and nectar resources, these mistletoes act to sustain mobile populations of Mistletoebirds and assist to sustain permanent populations of nectarivorous birds in the local area throughout the year.
Resources (functions) not otherwise present
Approximately 18 × 106 ha, or 87%, of the agricultural region of WA has been cleared and in the wheatbelt the percentage is estimated to be even higher (93%; DEP 1997). For example, 22 districts in the wheatbelt have <10% native vegetation cover remaining and the few patches of remaining native vegetation exist in fragmented and isolated patches (Shepherd et al. 2001). Similar environmental disturbance has occurred in agricultural landscapes across Australia. The quality of food resources in these fragmented landscapes is an important consideration in terms of maintaining fauna species. For example, Norton et al. (1995) predicted that in extremely fragmented habitats (such as the heavily cleared wheatbelt), mistletoes would eventually become extinct owing to regional declines in key avian pollinators and dispersers (Saunders 1993), as has been recorded in the wheatbelt area north of the town of Kellerberrin in WA (Norton et al. 1995). Loranthaceous mistletoes naturally have a patchy distribution across the landscape, owing to the patterns of bird dispersal (Reid and Lange 1988; Reid et al. 1995) and to narrow microsite tolerances coupled with host specificity (Knutson 1983; Yan and Reid 1995). The distribution of Australian mistletoes in fragmented habitats is likely to be dependent on the distribution of mistletoes before landscape fragmentation and the effect of that on avian pollinators and dispersers (Norton et al. 1995). Mistletoes have become more abundant in fragmented habitats in south-eastern Australia (Reid et al. 1994; Watson 2001) and tend to be either super-abundant or absent in fragmented areas of south-western WA (Norton et al. 1995). The presence of these plants (and the resources they provide) in fragmented, otherwise resource-poor habitats, may therefore counteract the detrimental effects caused by habitat fragmentation (Kelly et al. 2000; Watson 2002) and may further support their recognition as important resources for birds. Mistletoes therefore play a unique role in the fragmented landscapes examined in the present study in their provision of vital food resources to Mistletoebirds.
Our findings of increased species richness and changes in the structure of the avian community in response to flowering and fruiting of mistletoes show that Wireleaf Mistletoe and Box Mistletoe may provide important food and shelter resources for birds in fragmented south-western WA woodlands. However, whereas mistletoes produce highly reliable, low-redundancy fruit resources that play a unique role in these fragmented landscapes, we failed to find evidence that mistletoe nectar and fruit are consumed by a wide range of birds. Instead, the only species of bird reliant on these food resources is the specialist Mistletoebird. The potential for the keystone status of mistletoes in south-western WA requires further investigation, with comprehensive experimental mistletoe removal tests such as those performed by Watson (2002) and Watson and Herring (2012) recommended.
Acknowledgments
We are indebted to Tony Start (Western Australian Herbarium, WA Department of Environment and Conservation), for kindly sharing his data and knowledge of all things related to mistletoe; and to Simon Cherriman, whose tree climbing skills were invaluable while tagging mistletoe plants at Paruna. We also sincerely thank the staff at the Araluen Country Club and Resort, Dr Manda Page and Jo Kuiper from the Australian Wildlife Conservancy, and Peter Monger and family for allowing access to their sites. We thank the Associate Editor, David Watson, and three anonymous reviewers whose comments greatly improved previous versions of this manuscript. Fieldwork was approved by the Murdoch University Animal Ethics Committee (approval R2175/08), and supported by the Holsworth Wildlife Research Endowment to KRN.
References
Baker, H. G., Baker, I., and Hodges, S. A. (1998). Sugar compositions of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica 30, 559–586.| Sugar compositions of nectars and fruits consumed by birds and bats in the tropics and subtropics.Crossref | GoogleScholarGoogle Scholar |
Barea, L. P. (2008). Interactions between frugivores and their resources: case studies with the Painted Honeyeater Grantiella picta. Ph.D. Thesis, Charles Sturt University, Albury, NSW.
Barea, L. P., and Watson, D. (2007). Temporal variation in food resources determines onset of breeding in an Australian mistletoe specialist. Emu 107, 203–209.
| Temporal variation in food resources determines onset of breeding in an Australian mistletoe specialist.Crossref | GoogleScholarGoogle Scholar |
Barlow, B. A. (1984). Loranthaceae. In ‘Flora of Australia. Vol. 22’. (Ed. A. S. George.) pp. 68–131. (Australian Government Publishing Service: Canberra.)
Barlow, B. A. (1992). Conspectus of the genus Amyema Tieghem (Loranthaceae). Blumea 36, 293–381.
Barrett, G. W., Silcocks, A., Barry, S., Cunningham, R., and Poulter, R. (2003). ‘The New Atlas of Australian Birds.’ (Royal Australasian Ornithologists Union: Melbourne.)
Bennetts, R. E., White, G. C., Hawksworth, F. G., and Severs, S. E. (1996). The influence of dwarf mistletoe on bird communities in Colorado ponderosa pine forests. Ecological Applications 6, 899–909.
| The influence of dwarf mistletoe on bird communities in Colorado ponderosa pine forests.Crossref | GoogleScholarGoogle Scholar |
BirdLife International (2012). Grantiella picta. In ‘IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2’. Available at http://www.iucnredlist.org/details/106005373/0 [Verified 14 May 2013].
Brown, E. M., Burbidge, A. H., Dell, J., Edinger, D., Hopper, S. D., and Wills, R. T. (1997). ‘Pollination in Western Australia: A Database of Animals Visiting Flowers.’ (Western Australian Naturalists Club: Perth.)
Bureau of Meteorology (2011). Climate statistics for Australian locations. (Bureau of Meteorology: Melbourne.) Available at http://www.bom.gov.au/climate/data/index.shtml [Verified 5 May 2013].
Burns, A. E., Cunningham, S. A., and Watson, D. M. (2011). Arthropod assemblages in tree canopies: a comparison of orders on box mistletoe (Amyema miquelii) and its host eucalypts. Australian Journal of Entomology 50, 221–230.
Calder, D. M. (1983). Mistletoes in focus: an introduction. In ‘The Biology of Mistletoes’. (Eds. D. M. Calder and P. Bernhardt.) pp. 1–18. (Academic Press: Sydney.)
Chan, K. (2001). Partial migration in Australian landbirds: a review. Emu 101, 281–292.
| Partial migration in Australian landbirds: a review.Crossref | GoogleScholarGoogle Scholar |
Clarke, K. R. (1993). Non-parametric multivariate analysis of changes in community structure. Australian Journal of Ecology 18, 117–143.
| Non-parametric multivariate analysis of changes in community structure.Crossref | GoogleScholarGoogle Scholar |
Cooney, S. J. N., Watson, D. M., and Young, J. (2006). Mistletoe nesting in Australian birds: a review. Emu 106, 1–12.
| Mistletoe nesting in Australian birds: a review.Crossref | GoogleScholarGoogle Scholar |
Davidar, P. (1985). Ecological interactions between mistletoes and their avian pollinators in south India. Journal of the Bombay Natural History Society 82, 45–60.
Davidson, N. J., True, K. C., and Pate, J. S. (1989). Water relations of the parasite : host relationship between the mistletoe Amyema linophyllum (Fenzl) Tieghem and Casuarina obesa Miq. Oecologia 80, 321–330.
| Water relations of the parasite : host relationship between the mistletoe Amyema linophyllum (Fenzl) Tieghem and Casuarina obesa Miq.Crossref | GoogleScholarGoogle Scholar |
DEP (1997). ‘State of the Environment Reference Group draft working papers. Section 4, Land.’ (State of the Environment Reporting Unit, Department of Environmental Protection: Perth.)
Field, S. A., Tyre, A. J., and Possingham, H. P. (2002). Estimating bird species richness: how should repeat surveys be organized in time? Austral Ecology 27, 624–629.
| Estimating bird species richness: how should repeat surveys be organized in time?Crossref | GoogleScholarGoogle Scholar |
Forde, N. (1986). Relationships between birds and fruits in temperate Australia. In ‘The Dynamic Partnership: Birds and Plants in Southern Australia’. (Eds. H. A. Ford and D. C. Paton.) pp. 42–58. (Government Printer: Adelaide.)
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, 1–9.
Hawkeswood, T. J. (1981). Notes on the pollination of Nuytsia floribunda (Labill.) R. Br. (Loranthaceae) and some literature reviewed. Western Australian Naturalist 15, 17–21.
Hawksworth, F. G., and Wiens, D. (1996). ‘Dwarf Mistletoes: Biology, Pathology, and Systematics’, 2nd edn. (USDA Forest Service: Washington, DC.)
Higgins, P. J. (Ed.) (1999). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 4: Parrots to Dollarbird.’ (Oxford University Press: Melbourne.)
Keast, A. (1958). The influence of ecology on variation in the Mistletoebird (Dicaeum hirundinaceum). Emu 58, 195–206.
| The influence of ecology on variation in the Mistletoebird (Dicaeum hirundinaceum).Crossref | GoogleScholarGoogle Scholar |
Kelly, D., Ladley, J. J., Robertson, A. W., and Norton, D. A. (2000). Limited forest fragmentation improves reproduction in the declining New Zealand mistletoe Peraxilla tetrapetala (Loranthaceae). In ‘Genetics, Demography and Viability of Fragmented Populations’. (Eds. A. Young and G. Clarke.) pp. 241–252. (Cambridge University Press: Cambridge, UK.)
Knutson, D. M. (1983). Physiology of mistletoe parasitism and disease responses in the host. In ‘The Biology of Mistletoes’. (Eds. D. M. Calder and P. Bernhardt.) pp. 295–316. (Academic Press: Sydney.)
Kotliar, N. B. (2000). Application of the new keystone-species concept to Prairie Dogs: how well does it work? Conservation Biology 14, 1715–1721.
| Application of the new keystone-species concept to Prairie Dogs: how well does it work?Crossref | GoogleScholarGoogle Scholar |
Kuijt, J. (1969). ‘The Biology of Parasitic Flowering Plants.’ (University of California Press: Berkeley, CA.)
Ladley, J. J., Kelly, D., and Robertson, A. W. (1997). Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae). New Zealand Journal of Botany 35, 345–360.
| Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae).Crossref | GoogleScholarGoogle Scholar |
Levey, D. J. (1988). Spatial and temporal variation in Costa Rican fruit and fruit-eating bird abundance. Ecological Monographs 58, 251–269.
| Spatial and temporal variation in Costa Rican fruit and fruit-eating bird abundance.Crossref | GoogleScholarGoogle Scholar |
López de Buen, L., and Ornelas, J. F. (2001). Seed dispersal of the mistletoe Psittacanthus schiedeanus by birds in central Veracruz, Mexico. Biotropica 33, 487–494.
Malizia, L. R. (2001). Seasonal fluctuations of birds, fruits, and flowers in a subtropical forest of Argentina. Condor 103, 45–61.
| Seasonal fluctuations of birds, fruits, and flowers in a subtropical forest of Argentina.Crossref | GoogleScholarGoogle Scholar |
Mathiasen, R. L., Nickrent, D. L., Shaw, D. C., and Watson, D. M. (2008). Mistletoes: pathology, systematics, ecology, and management. Plant Disease 92, 988–1006.
| Mistletoes: pathology, systematics, ecology, and management.Crossref | GoogleScholarGoogle Scholar |
Norton, D. A., Hobbs, R. J., and Atkins, L. (1995). Fragmentation, disturbance, and plant distribution: mistletoes in woodland remnants in the Western Australian wheatbelt. Conservation Biology 9, 426–438.
| Fragmentation, disturbance, and plant distribution: mistletoes in woodland remnants in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Paton, D. C., and Ford, H. A. (1977). Pollination by birds of native plants in South Australia. Emu 77, 73–85.
| Pollination by birds of native plants in South Australia.Crossref | GoogleScholarGoogle Scholar |
Peres, C. A. (2000). Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal of Tropical Ecology 16, 287–317.
| Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods.Crossref | GoogleScholarGoogle Scholar |
Power, M. E., Tilman, D., Estes, J. A., Menge, B. A., Bond, W. J., Mills, L. C., Daily, G., Castilla, J. C., Lubchenco, J., and Paine, R. T. (1996). Challenges in the quest for keystones. Bioscience 46, 609–620.
| Challenges in the quest for keystones.Crossref | GoogleScholarGoogle Scholar |
Rawsthorne, J., Watson, D. M., and Roshier, D. A. (2012). The restricted seed rain of a mistletoe specialist. Journal of Avian Biology 43, 9–14.
| The restricted seed rain of a mistletoe specialist.Crossref | GoogleScholarGoogle Scholar |
Reid, N. (1986). Pollination and seed dispersal of mistletoes (Loranthaceae) by birds in southern Australia. In ‘The Dynamic Partnership: Birds and Plants in Southern Australia’. (Eds. H. A. Ford and D. C. Paton.) pp. 64–84. (Government Printer: Adelaide.)
Reid, N. (1987). The mistletoebird and Australian mistletoes: co-evolution or coincidence? Emu 87, 130–131.
| The mistletoebird and Australian mistletoes: co-evolution or coincidence?Crossref | GoogleScholarGoogle Scholar |
Reid, N. (1990). Mutualistic interdependence between mistletoes (Amyema quandang), and honeyeaters and mistletoebirds in an arid woodland. Australian Journal of Ecology 15, 175–190.
| Mutualistic interdependence between mistletoes (Amyema quandang), and honeyeaters and mistletoebirds in an arid woodland.Crossref | GoogleScholarGoogle Scholar |
Reid, N. (1991). Coevolution of mistletoes and frugivorous birds? Australian Journal of Ecology 16, 457–469.
| Coevolution of mistletoes and frugivorous birds?Crossref | GoogleScholarGoogle Scholar |
Reid, N., and Lange, R. T. (1988). Host specificity, dispersion and persistence through drought of two arid zone mistletoes. Australian Journal of Botany 36, 299–313.
| Host specificity, dispersion and persistence through drought of two arid zone mistletoes.Crossref | GoogleScholarGoogle Scholar |
Reid, N., Yan, Z., and Fittler, J. (1994). Impact of mistletoes (Amyema miquelii) on host (Eucalyptus blakelyi and Eucalyptus melliodora) survival and growth in temperate Australia. Forest Ecology and Management 70, 55–65.
| Impact of mistletoes (Amyema miquelii) on host (Eucalyptus blakelyi and Eucalyptus melliodora) survival and growth in temperate Australia.Crossref | GoogleScholarGoogle Scholar |
Reid, N., Stafford Smith, M., and Yan, Z. (1995). Ecology and population biology of mistletoes. In ‘Forest Canopies’. (Eds. M. D. Lowman and N. M. Nadkarn.) pp. 285–310. (Academic Press: San Diego, CA.)
Robertson, A. W., Kelly, D., Ladley, J. J., and Sparrow, A. D. (1999). Effects of pollinator loss on endemic New Zealand mistletoes (Loranthaceae). Conservation Biology 13, 499–508.
| Effects of pollinator loss on endemic New Zealand mistletoes (Loranthaceae).Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A. (1993). Comunity-based observer scheme to assess avian responses to habitat reduction and fragmentation in south-western Australia. Biological Conservation 64, 203–218.
| Comunity-based observer scheme to assess avian responses to habitat reduction and fragmentation in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Shepherd, D. P., Beeston, G. R., and Hopkins, A. J. M. (2001). Native vegetation in Western Australia. Technical report 249, Department of Agriculture, Western Australia, Perth.
Start, A. N. (2011). Fire responses and survival strategies of mistletoes (Loranthaceae) in an arid environment in Western Australia. Australian Journal of Botany 59, 533–542.
| Fire responses and survival strategies of mistletoes (Loranthaceae) in an arid environment in Western Australia.Crossref | GoogleScholarGoogle Scholar |
StatSoft Inc. (2007). STATISTICA (data analysis software system), version 8.0. Tulsa, OK.
Stiles, F. G., and Freeman, C. E. (1993). Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25, 191–205.
| Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Turner, R. J. (1991). Mistletoe in eucalypt forest – a resource for birds. Australian Forestry 54, 226–235.
Watson, D. M. (2001). Mistletoe – a keystone resource in forests and woodlands worldwide. Annual Review of Ecology and Systematics 32, 219–249.
| Mistletoe – a keystone resource in forests and woodlands worldwide.Crossref | GoogleScholarGoogle Scholar |
Watson, D. M. (2002). Effects of mistletoe on diversity: a case-study from southern New South Wales. Emu 102, 275–281.
| Effects of mistletoe on diversity: a case-study from southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Watson, D. M. (2003). The ‘standardized search’: an improved way to conduct bird surveys. Austral Ecology 28, 515–525.
| The ‘standardized search’: an improved way to conduct bird surveys.Crossref | GoogleScholarGoogle Scholar |
Watson, D. M. (2004). Comparative evaluation of new approaches to bird surveys. Wildlife Research 31, 1–11.
| Comparative evaluation of new approaches to bird surveys.Crossref | GoogleScholarGoogle Scholar |
Watson, D. M. (2011). ‘Mistletoes of Southern Australia.’ (CSIRO Publishing: Melbourne.)
Watson, D. M., and Herring, M. (2012). Mistletoe as a keystone resource: an experimental test. Proceedings of the Royal Society of London B. Biological Sciences 279, 3853–3860.
| Mistletoe as a keystone resource: an experimental test.Crossref | GoogleScholarGoogle Scholar |
Watson, D. M., McGregor, H. W., and Spooner, P. G. (2011). Hemiparasitic shrubs increase resource availability and multi-trophic diversity of eucalypt forest birds. Functional Ecology 25, 889–899.
| Hemiparasitic shrubs increase resource availability and multi-trophic diversity of eucalypt forest birds.Crossref | GoogleScholarGoogle Scholar |
Western Australian Herbarium (2011). ‘FloraBase – the Western Australian Flora’, ver. 2.6, 25 August 2011. (Department of Environment and Conservation.) Available at http://florabase.dec.wa.gov.au/ [Verified 5 May 2013].
Wright, D. D. (2005). Diet, keystone resources and altitudinal movement of Dwarf Cassowaries in relation to fruiting phenology in a Papua New Guinean rainforest. In ‘Tropical Fruits and Frugivores: The Search for Strong Interactions’. (Eds. J. L. Dew and J. P. Boubli.) pp. 205–236. (Springer: Dordrecht, the Netherlands.)
Yan, Z. (1993). Seed dispersal of Amyema preissii and Lysiana exocarpi by Mistletoebirds and Spiny-cheeked Honeyeaters. Emu 93, 214–219.
| Seed dispersal of Amyema preissii and Lysiana exocarpi by Mistletoebirds and Spiny-cheeked Honeyeaters.Crossref | GoogleScholarGoogle Scholar |
Yan, Z., and Reid, N. (1995). Mistletoe (Amyema miquelii and A. pendulum) seedling establishment on eucalypt hosts in eastern Australia. Journal of Applied Ecology 32, 778–784.
| Mistletoe (Amyema miquelii and A. pendulum) seedling establishment on eucalypt hosts in eastern Australia.Crossref | GoogleScholarGoogle Scholar |


