The impacts of fire on birds in Australia’s tropical savannas
J. C. Z. Woinarski A D E and S. Legge A B CA North Australian Biodiversity Hub, Charles Darwin University, Casuarina, NT 0909, Australia.
B Australian Wildlife Conservancy, Mornington Wildlife Sanctuary, PMB 925 Derby, WA 6728, Australia.
C Research School of Biology, College of Medicine, Biology and Environment, Australian National University, Canberra, ACT 0200, Australia.
D Present address: PO Box 148, Christmas Island, 6798, Australia.
E Corresponding author. Email: john.woinarski@cdu.edu.au
Emu 113(4) 319-352 https://doi.org/10.1071/MU12109
Submitted: 22 November 2012 Accepted: 11 June 2013 Published: 13 September 2013
Abstract
The ecology of Australia’s tropical savannas is shaped by the near-pervasive influence of fire. Constituting ~20% of Australia’s land area, tropical savannas contribute >75% of the area burnt in Australia each year. Across most of Australia’s tropical savannas, components of biodiversity are declining, including many species of birds. This review seeks to assess whether that decline is linked to current fire regimes. However, relevant studies are few, short-term and opportunistic, and indicate rather than demonstrate the effects of fire. There is no set of agreed paradigms for contextualising the relationship between birds and fire regimes in this region or for any management consequences. We conclude that the current fire regime is suboptimal for many species of birds, particularly for granivores, frugivores, hollow-dependent species and those that nest on or near the ground. For conservation reserves, we recommend that fire management protocols include the explicit targets that: (1) at least 25% of the savanna landscape is at least 3 years unburnt; (2) at least 5% is at least 10 years unburnt; (3) fire-sensitive non-savanna vegetation types are increasing or stable and (4) populations of selected hollow-nesting, ground-nesting, frugivorous and granivorous birds are increasing or stable. We also identify key knowledge gaps that currently inhibit conservation management.
Additional keywords: conservation, conservation management, frugivore, granivore, ground-nesting, hollow-nesting, management, pastoralism, rainforest, threatened species.
Introduction
A visitor new to the Australian tropical savannas could not help but be surprised, and perhaps disconcerted, by the preponderance of fire and burnt lands in the landscape and by the enthusiasm of land managers – even in conservation reserves – to light fires. Why is there so much fire, and given this characteristic, how is the biodiversity of this area faring? This review aims to set context for, integrate and interpret a disparate set of studies that relate to fire management and the conservation of birds in Australia’s tropical savannas.
Such consideration is timely and important because:
-
the Australian tropical savannas comprise a very significant component of the continent (1.9 × 106 km2, or ~20% of the Australian continental land-mass) (Fig. 1);
-
fire (managed or unmanaged) in this environment is near-pervasive;
-
much of the management of the area relates to fire, and most conservation managers continue to seek to use, control or manipulate fire, ostensibly to benefit biodiversity;
-
the management of fire may be more tractable than some other major threats to biodiversity in the region (e.g. predation by feral Cats, Felis catus);
-
in most tropical areas across the globe, large and rapidly increasing human populations are leading to extensive loss of natural vegetation, with this habitat loss being the major driver of declines of biodiversity; in contrast, with currently low human population density and little intensification of development in northern Australia, fire is probably the most important factor affecting the biodiversity of this region;
-
significant components of the biodiversity of the Australian tropical savannas are in decline, for reasons known or presumed to be associated with fire, or through causes that are not well resolved but may involve fire (e.g. Franklin et al. 2005; Woinarski et al. 2011);
-
the effects of fire are likely to become more severe with projected climate change (Williams et al. 2009);
-
this region contributes by far the largest extent of fires in Australia (~75% of the national total land area burnt annually; Russell-Smith et al. 2007);
-
fires in this region contribute much to Australia’s greenhouse gas emissions, and consequently offer scope for innovative solutions to Australia’s carbon budgeting;
-
this region, and particularly fire management within the region, provides some opportunity for integrating an enduring, Indigenous knowledge of land management with a western, scientific approach to land management and conservation, and to provide scope for Indigenous employment in biodiversity management and
-
there is no review of or operational manual for the management of fire in this region to benefit bird conservation.
Enhanced, and evidence-based, fire management in the region is a necessary, if perhaps not sufficient, requirement for the conservation of the bird species of the region. Our focus in this review is on the effects of fire on birds in the Australian tropical savannas, but we seek also to draw lessons for, or contrasts with, other faunal groups in this region and with the relationships of birds and fire in other Australian regions, and in tropical savannas elsewhere in the world. We seek specifically to address four questions:
-
Is the current fire regime in Australia’s tropical savannas disadvantageous to birds?
-
What is an optimal fire regime for this bird fauna?
-
Is that regime achievable?
-
What are the critical gaps in our knowledge that inhibit better fire management?
Australia’s tropical savannas
Tropical savannas are defined by climate and characterised by consequential vegetation structure. Worldwide they occur in areas exposed to strongly seasonal, wet–dry climates and frequent fires, and their ecological dynamics and distinctive vegetation structure are determined largely by fire and climate (Higgins et al. 2000; Van Langevelde et al. 2003; Bond and Keeley 2005; Bowman et al. 2009).
In Australia, the occurrence of tropical savannas is determined mostly by the influence of the summer monsoon, and these climatic conditions have been long-standing (Bowman et al. 2010). Across most of monsoonal Australia, the wet season extends from approximately December to April, and characteristically delivers ~90% of annual rainfall, with this dominance marginally less in the far east (Cape York Peninsula, Queensland) owing to topographic, orographic and maritime influences (Woinarski et al. 2007a). Although the wet season is mostly reliable, rainfall tallies and timing may vary appreciably between years, with substantial ecological impacts (Taylor and Tulloch 1985; Whitehead and Saalfeld 2000; Garnett and Williamson 2010). All environments in the Australian monsoonal tropics vary markedly between the wet and dry seasons, but this variation is most extreme for lowland flat areas (most notably the coastal floodplains), for which extensive flooding is an annual event. The pronounced seasonality of rainfall drives marked pulses in phenology, and hence resource availability, for many species of birds (and other animals). Rainfall tallies are highest in higher elevational areas of Cape York Peninsula and in the far north of the Northern Territory (NT), notably the Tiwi Islands, in parts of which annual rainfall exceeds 2000 mm. Average annual rainfall declines inland, with the southern edge of Australia’s tropical savannas approximately between the 500- and 1000-mm annual rainfall isohyets (Fig. 1). The geographical circumscription becomes somewhat indistinct in eastern Queensland, where the Wet Tropics (defined by high annual rainfall with less seasonality than the monsoonal tropics) forms a peripheral exclusion from the savannas, and the southern border of the tropical savannas merges untidily with areas less characterised by summer rainfall, approximately at the Brigalow Belt. Temperatures are high throughout the year (albeit lower in the dry season) and humidity is low through most of the dry season causing much of the grass layer to dry (cure), and become highly flammable. Cyclones are a frequent and, occasionally, ecosystem-altering event, especially in coastal regions (Bowman 1988; McQuade et al. 1996).
|
Most of the Australian tropical savannas are old and deeply weathered landscapes, with few fertile patches (with the notable exception being clay soils of the coastal floodplains and inland Mitchell grasslands). Extensive areas are flat and featureless, and there are no high mountain ranges, although there are deeply dissected areas in the Kimberley region of Western Australia, western Arnhem Land, NT, and parts of Cape York Peninsula (Williams 1991).
The Australian tropical savannas are sparsely populated: outside the few major towns, the average population density is ~0.1 individuals km–2 (Garnett et al. 2010). A high proportion (~30%) of the population is Indigenous, and Indigenous people dominate the population in the extensive areas outside the major towns. Regional economies are limited. The major industries are extensive pastoralism and mining, which is more localised and ephemeral, but fleetingly profitable. Pastoralism occupies over ~75% of the savannas, with large tracts managed by major agricultural conglomerates and significant areas owned and managed by Indigenous groups. Non-pastoral lands held collectively by Indigenous people constitute ~15% of the area, and conservation reserves ~6% (Garnett et al. 2010). Over the last 20–30 years there has been a substantial increase in the area of Indigenous lands managed for conservation, either through jointly managed national parks (such as Kakadu National Park) or as Indigenous Protected Areas (Moorcroft et al. 2012), and also an increase in the area of land managed for conservation by private organisations (such as the Australian Wildlife Conservancy).
Little of Australia’s tropical savannas has been cleared or otherwise substantially modified, although there are localised areas of intensive horticulture (notably in the Ord Irrigation Area of the eastern Kimberley region) and strip-mining (notably in eastern Arnhem Land, on Groote Eylandt and in parts of western Cape York Peninsula). Instead, habitat modification, largely driven by pastoralism and fire, has been more subtle, insidious and pervasive (Woinarski and Ash 2002; Woinarski and Fisher 2003; Woinarski et al. 2007a; Garnett et al. 2010).
In northern Australia, most non-savanna vegetation types (such as mangroves, rainforests, heathlands and riparian forests) are minor components of the landscape, mostly restricted to sites that are permanently moist (such as springs and river sides) or that provide some topographical protection from fire (such as gorges and other rugged rocky environments) (Fox et al. 2001). These highly fragmented environments now harbour much of the relictual biota of northern Australia and support biotic communities very different to the far more pervasive savanna woodlands (Specht 1958; Taylor and Dunlop 1985; Russell-Smith 1991; Russell-Smith et al. 1992; Woinarski 1993, 2004; Woinarski et al. 2000a).
The Australian tropical savannas blend distinctly Australian elements with those of tropical savannas of other continents. In coarse appearance, and in many ecological features, the vegetation structure resembles that of tropical savannas in Africa, India or South America, and some of the dominant grass species and many of the subdominant shrub genera are shared across continents to a greater degree than is the case for most Australian vegetation types (Taylor and Dunlop 1985). This resemblance is increasing with the introduction and rapid spread of African grasses to Australian tropical savannas (Cook and Dias 2006). However, in contrast to other tropical savannas, the dominant tree species of the Australian tropical savannas are mostly eucalypts (species of Eucalyptus and Corymbia), particularly the widely distributed Darwin Stringybark (Eucalyptus tetrodonta) and Darwin Woollybutt (E. miniata) (Bowman 1988; Woinarski 2004). The two dominant floristic elements of the Australian tropical savannas, grasses and eucalypts, have many ecological attributes that have allowed them to prosper over the long history of severely seasonal climates and frequent fire (Stocker 1966; Lacey and Whelan 1976; Bowman 1988).
Note that we use the terms ‘Australian tropical savannas’, ‘monsoonal Australia’ and ‘northern Australia’ almost interchangeably in this review. These terms apply broadly to the same area, but reflect vegetation, climatic and geographical bases. In general, our focus is on tropical savanna landscapes (dominated by savanna vegetation but including largely interstitial elements of other vegetation types) within areas exposed to monsoonal climates across northern Australia.
Birds and bird assemblages in monsoonal Australia
Birds are a prominent, distinctive and interesting feature of monsoonal Australia. Very broadly, the dominant vegetation type, savanna woodland, supports a bird community functionally similar to that of eucalypt woodlands of temperate Australia, but with regional taxonomic differentiation at the level of subspecies (e.g. Red-tailed Black-Cockatoo, Calyptorhynchus banksii macrorhynchus; Masked Owl, Tyto novaehollandiae kimberli and T. n. melvillensis; Crested Shrike-tit, Falcunculus frontatus whitei; Striated Pardalote, Pardalotus striatus uropygialis and P. s. melvillensis; Black-chinned Honeyeater, Melithreptus gularis laetior; Rufous Whistler, Pachycephala rufiventris falcata and P. r. pallida; Restless Flycatcher, Myiagra inquieta nana) or species (e.g. Northern Rosella, Platycercus venustus; Black-tailed Treecreeper, Climacteris melanura; Yellow-tinted Honeyeater, Lichenostomus flavescens; Northern Fantail, Rhipidura rufiventris; Great Bowerbird, Ptilonorhynchus nuchalis) (Woinarski et al. 1997). The bird assemblage in the northern woodlands is also less diverse in the guild of foliage-gleaning insectivorous bird species of the shrub layer (notably thornbills, Acanthiza spp., and scrub-wrens, Sericornis spp.) and those that forage in open ground (e.g. Australian Magpie, Gymnorhina tibicen; quail-thrush, Cinclosoma spp.; White-winged Chough, Corcorax melanoramphos). In contrast to such losses, the array of granivorous birds, particularly grassfinches, is particularly diverse in the Australian tropical savannas (Woinarski and Tidemann 1991; Franklin et al. 2000). The temperate eucalypt woodlands grade into taller and structurally diverse eucalypt forests (with distinctive bird species composition) in higher rainfall areas, but there is no analogous habitat in the Australian tropical savannas.
Vegetation types that contrast with the tropical savanna matrix add a further mix of bird species to the regional pool, but because of the small extent and patchiness of these habitats, their bird species richness is generally low. This is particularly so for rainforests and riparian forests, whose bird communities are attenuated from higher to lower rainfall areas of northern Australia, and from east (Cape York Peninsula) to west (Kimberley) (Johnstone and Burbidge 1991; Woinarski 1993; Woinarski et al. 2000a). A small number of bird species are closely associated with Melaleuca woodlands and forests (e.g. Bar-breasted Honeyeater, Ramsayornis fasciatus), hummock grasslands (grass-wrens, Amytornis spp.), tussock grasslands (Flock Bronzewing, Phaps histrionica) and heathlands (in part, White-streaked Honeyeater, Trichodere cockerelli). Wetlands are a prominent feature in parts of northern Australia but they, and their associated bird species, are not considered here.
Many species of birds are distributed extensively throughout monsoonal Australia. However, there is some gradational change in species composition across the (mostly) latitudinal rainfall gradient (with marginally more species in higher rainfall areas; Whitehead et al. 1992; Woinarski et al. 1999a) and small changes across the vast longitudinal span from the Kimberley region to Cape York Peninsula (Bowman et al. 2010). To some extent, this compositional variation with latitude is discontinuous, with minor biogeographical barriers, broadly corresponding with state and territory borders, in the Ord–Victoria and Carpentarian divides (Lee and Edwards 2008; Bowman et al. 2010; Eldridge et al. 2011), segregating some taxa at the level of subspecies (e.g. Partridge Pigeon, Geophaps smithii) or species (e.g. Long-tailed Finch, Poephila acuticauda; Black-throated Finch, P. cincta; Hooded Parrot, Psephotus dissimilis; Golden-shouldered Parrot, P. chrysopterygius; White-quilled Rock-pigeon, Petrophassa albipennis; Chestnut-quilled Rock-pigeon, P. rufipennis) across at least two of the three main northern Australian regions of the Kimberley, Top End and Cape York Peninsula (Ford 1978, 1987; Joseph and Omland 2009; Toon et al. 2010). There is little narrow-range endemicity among birds in monsoonal Australia, but in most of the few cases, such species coincide with areas of endemism identified for other components of the biota (Cracraft 1986, 1991; Woinarski et al. 2006, 2009a); examples include the Black Grasswren (Amytornis housei), White-throated Grasswren (A. woodwardi) and Carpentarian Grasswren (A. dorotheae).
The ecology of many bird species in monsoonal Australia is poorly known relative to that of birds in more populous parts of Australia (Noske and Franklin 1999). Notwithstanding the very marked climatic seasonality, patterns of breeding seasonality are mixed and many species have a long breeding season (Noske and Franklin 1999). Many species are sedentary, others disperse widely across and within the region, and some move to and from this region to other Australian regions or to the islands to Australia’s north, either regularly or irregularly (Morton and Brennan 1991; Woinarski et al. 2000b).
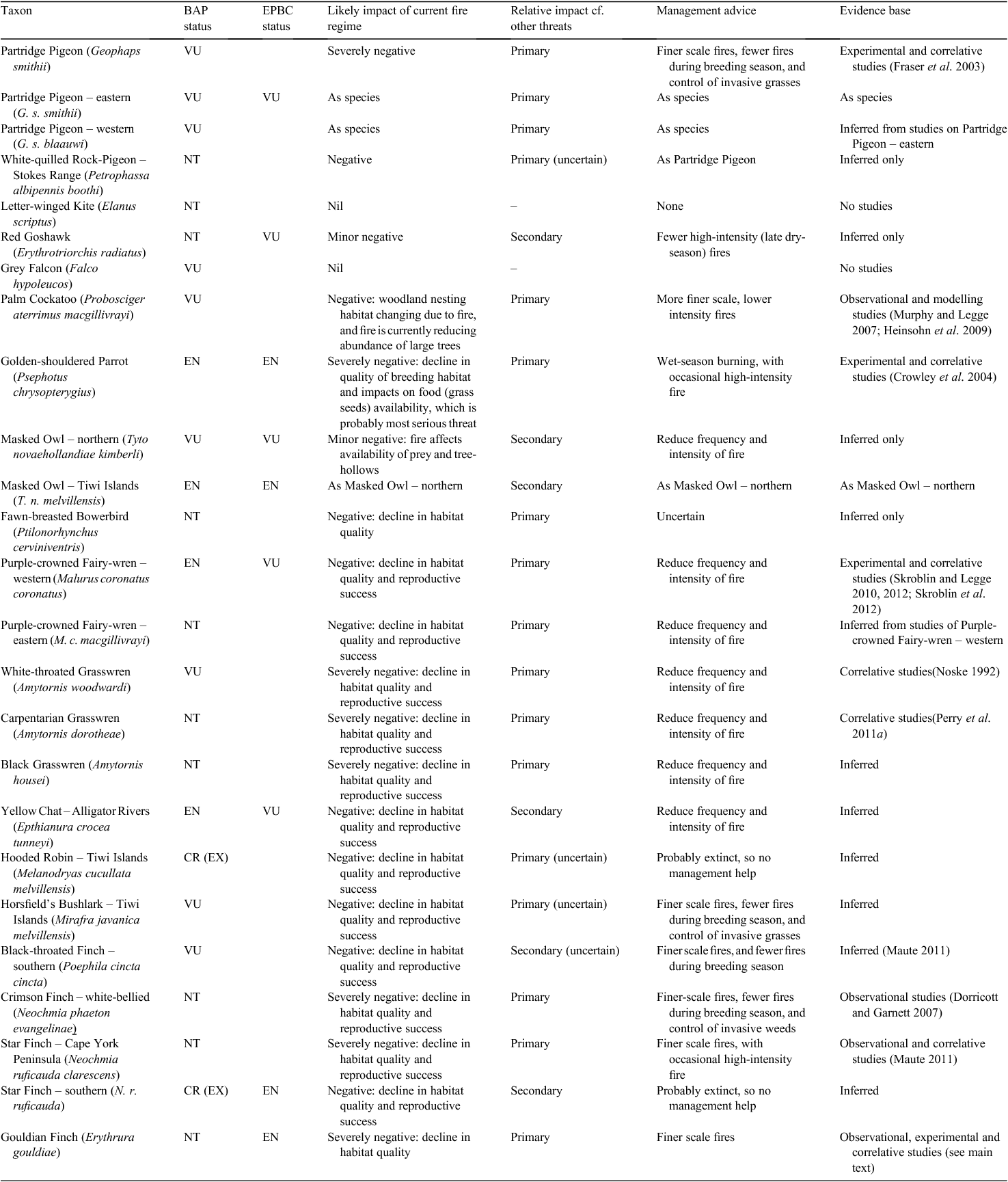
There have been no extinctions of bird species in this region since European settlement. However, the Tiwi Islands subspecies of Hooded Robin (Melanodryas cucullata melvillensis) is presumed to have become extinct over the last 20 years, possibly as a result of changed fire regimes (Garnett et al. 2011). Many others are in decline, notably including a large component of the diverse suite of granivores (Franklin 1999; Franklin et al. 2005). Table 1 lists all bird taxa occurring in this region that are currently considered threatened or near threatened.
|
Fire in monsoonal Australia
Fire
Fire is a complex factor. It perturbs environments in manners that are extraordinarily varied – perhaps more so than any other ecological factor – with its impacts varying from devastating to neutral or benign, to necessary, depending upon the perspective and ecological make-up of the organism or environment, the time-frame over which the impact is assessed, and the characteristics of any fire or fire regime. All fires are different, all species and environments respond differently to fire, and such responses may change depending upon spatial and temporal contexts. Furthermore, fire creates intricate and complex variation not only at any spot at any time but, by juxtaposition of spatial patterns of fire history, across broad contiguous landscape, and shapes continually shifting ecological patterning over time.
Fire is routinely initiated and used by landholders and managers for a wide range of objectives, and these objectives, and hence this use, may conflict between managers. Much fire is also initiated in an entirely unregulated manner and without strategic intent. In many cases, those using fire have little appreciation of the short-, medium- or long-term ecological consequences of that use. Fire is also a phenomenon existing at times beyond management control, with effects that may be detrimental to ecological or economic values.
The concept of fire needs deconstruction. One part of any definition relates to the characteristics of any individual fire; another part relates to the characteristics of fire patterning over time – the fire regime. The main parameters used to describe any single fire are its intensity, timing and extent (which may themselves be interlinked), and these parameters substantially influence the environmental impact of any fire. Any single fire may vary in its nature depending upon the time of day and the season. In turn, the intensity and extent of a fire may be linked to temperature and humidity, wind-strength, antecedent rainfall, fuel loads and their moisture content, topography, the previous history of fires at that site, and landscape connectivity. Low-intensity fires (e.g. <2 MW m–1) typically affect vegetation directly only at the ground and litter layer. Such fires may be patchy, leaving areas within the fire perimeter unburnt, and they typically move fairly slowly (facilitating control and also the ability of animals to escape it) (Williams et al. 2003a). In contrast, higher intensity fires (>10 MW m–1) may consume the forest canopy, kill many to most trees, destroy tree-hollows, have low patchiness (that is, they create a homogenous burnt landscape), and move rapidly and uncontrollably (Williams et al. 1999). In Australia’s tropical savannas, fire intensities typically range between 1 and 10 MW m–1 (Williams et al. 2003a), substantially less than the peak intensities of >100 MW m–1 experienced in severe bushfires in forests of temperate Australia (Williams et al. 2003a).
Different plant and animal species may respond idiosyncratically to any or all of these components of a single fire. The response will be influenced by intrinsic characteristics of the species (e.g. physiological tolerance, dietary or habitat flexibility, dispersal ability and site-fidelity), by the timing of the fire relative to the reproductive state of the species, and by extrinsic factors, such as the availability of sites that provide effective shelter during the fire or by the pattern of previous fires in the landscape. The effects of any fire may be direct (e.g. through immediate mortality) or delayed (e.g. individuals survive the fire but die subsequently because critical resources have been reduced by the fire), and may be modified by events after the fire (e.g. the extent and timing of subsequent rainfall). A single fire may kill many individuals of a species but this may not affect the viability of populations or the conservation security of a species. The latter is more influenced by the size of the fire relative to the area occupied by the species and, in some cases, the ability of the species and the landscape to allow for subsequent recolonisation of unfavourably burnt areas from unburnt refugia. Note that such examples should not colour the discussion unreasonably: it is important to recognise that there will always be species that benefit from, or require, fire, and that their conservation security relates inversely to the examples given.
As individual letters are to the alphabet or to the formation of words, individual fires are trivialities in themselves but collectively make up the infinite variety and ecological potency of fire regimes. It is the fire regime, not the fire, which moulds ecosystems, the traits of species and their conservation outlook. Fire regimes are defined across time and space, mostly by the incidence of fires (frequency or periodicity), by the scale, contrast and resolution of the mosaic of lands subjected to different fire histories, and by the variability in the types of fire that an area has been subjected to over time. Variation in these parameters is continuous, so the definition of a particular fire regime at a particular place is rarely fixed. Furthermore, our appreciation of the intricacy of fire regimes is constrained. For example, Gill et al. (2003) and Bradstock et al. (2005) note the ecological significance of the ‘invisible mosaic’: one can map readily all the areas burnt by a fire this year, but each patch in that array of recently burnt areas will be different because each may have been exposed to a different preceding constellation of fires – we see only the latest, most superficial, although perhaps the most ecologically important, manifestation of this labyrinthine historical variety. As for the discussion above about the impacts of individual fires, different plant and animal species respond idiosyncratically to the components of fire regimes. Many plant species, particularly those that reproduce only by seed, are much affected by the length of time between successive fires; if this interval is too short, individual plants may not be able to become mature and hence produce seeds that would allow for a post-fire generation. This is particularly the case for many heathland plants, including in monsoonal Australia (Russell-Smith et al. 1998, 2002; Russell-Smith 2006).
A history of fire in Australia’s tropical savannas
Fire has long been part of the monsoonal landscape. Before the arrival of humans, the high incidence of lightning strikes in the early, and often dry, storms of the wet season would have ensured that fires were frequent and sometimes extensive, and especially so as this timing corresponded to periods in which the fuel load was driest and most flammable, and temperatures were high. Accordingly, over an evolutionary time-scale, fire has been an important component of the ecology of the region, shaping the floristic composition and vegetation structure of monsoonal Australia and the ecological juxtaposition and relative extent of non-savanna plant elements within the broader savanna matrix (Bowman 2000). However, it is not straightforward to compare a pre-human fire regime with that operating under Indigenous management or the current fire management, because many other environmental factors (notably climate and herbivore density) changed substantially across these periods, and these changes themselves influence the patterning and impacts of fire.
Humans entered this landscape c. 60 000–40 000 years ago. Interpretation of the changes that they made to the landscape and its ecological processes is complex and contested (Bowman 1998; Preece 2002). But fire was and is the major tool of Aboriginal land managers. As a management tool, fire is far more controllable, benign and fine-scale when lit in the early to mid-dry season (in this region, approximately from April to July) than in the late dry season (August–October) (Russell-Smith and Edwards 2006). Consequently, it is likely that the fire regime changed from a pre-human one characterised by infrequent but extensive fires occurring mostly in the late dry season and sparked by lightning to a more variable regime that superimposed on this background fire regime a finer scale and more-frequent fire patterning dictated by human objectives. Inevitably such change would have benefitted some species and environments and disadvantaged others but the record of such change is sparse and open to differing interpretations: most notable in this argument is the role of Indigenous hunting and fire management in the extinction of the Australian megafauna (Johnson 2006). In turn, the loss of megafauna may itself have contributed to vegetational change and consequential changes in fire regime (Johnson 2006). This extinction event included at least some species of bird, including the giant mihirungs (Dromornithidae species) (Murray and Vickers-Rich 2004).
Fire regimes changed again with settlement by Europeans, mostly from the mid-19th century (Braithwaite and Estbergs 1985; Russell-Smith et al. 2003b). In many cases, these changes were undirected consequences of demographic and land-use changes rather than deliberate attempts to alter the fire regime. Across most of northern Australia, Indigenous land management was usurped. The most productive lands were taken over for cattle grazing, whereas in much of the more-rugged non-pastoral areas, Aboriginal people left their land (and the intricate day-to-day care necessary to maintain purposeful fire management) to congregate at missions and towns. (There are notable and important exceptions, such as in parts of western Arnhem Land, where the enduring link of traditional fire management has been mostly maintained, and that now provide irreplaceable insights into how and why fire was used traditionally and how the biota responded (Russell-Smith et al. 2009).) In the depopulated areas, fire management became less intensive and less purposeful, with the consequential outcome that the fire regime became characterised by unplanned and extensive fire (Bowman et al. 2001).
In pastoral areas, fire regimes became much influenced by the new factor of cattle. Many Aboriginal people worked as stockmen on pastoral properties and over two to three generations their knowledge of fire management probably became influenced by these pastoral perspectives on fire.
Regardless of the effects of humans on fire regimes, it is important to recognise that high temperatures and long dry seasons mean that fire is an unavoidable factor in the ecology of this region. This frames the region’s ecological attributes and management objectives differently to most other Australian landscapes for which decadal-scale ecological succession following fire may provide the ecological variability into which different species may fit. The fire-mediated variability in the Australian tropical savannas may be simpler but more nuanced than other regions of Australia, with landscape variability relating more to the scale of the mosaic of burnt and unburnt patches within any single year, to the serendipity of some landscape patches remaining unburnt for at least 3–5 years to allow for the growth of seedlings to escape the death-trap of small size, and to variation in plant responses (and hence resources for many birds) according to the month in which a fire occurs.
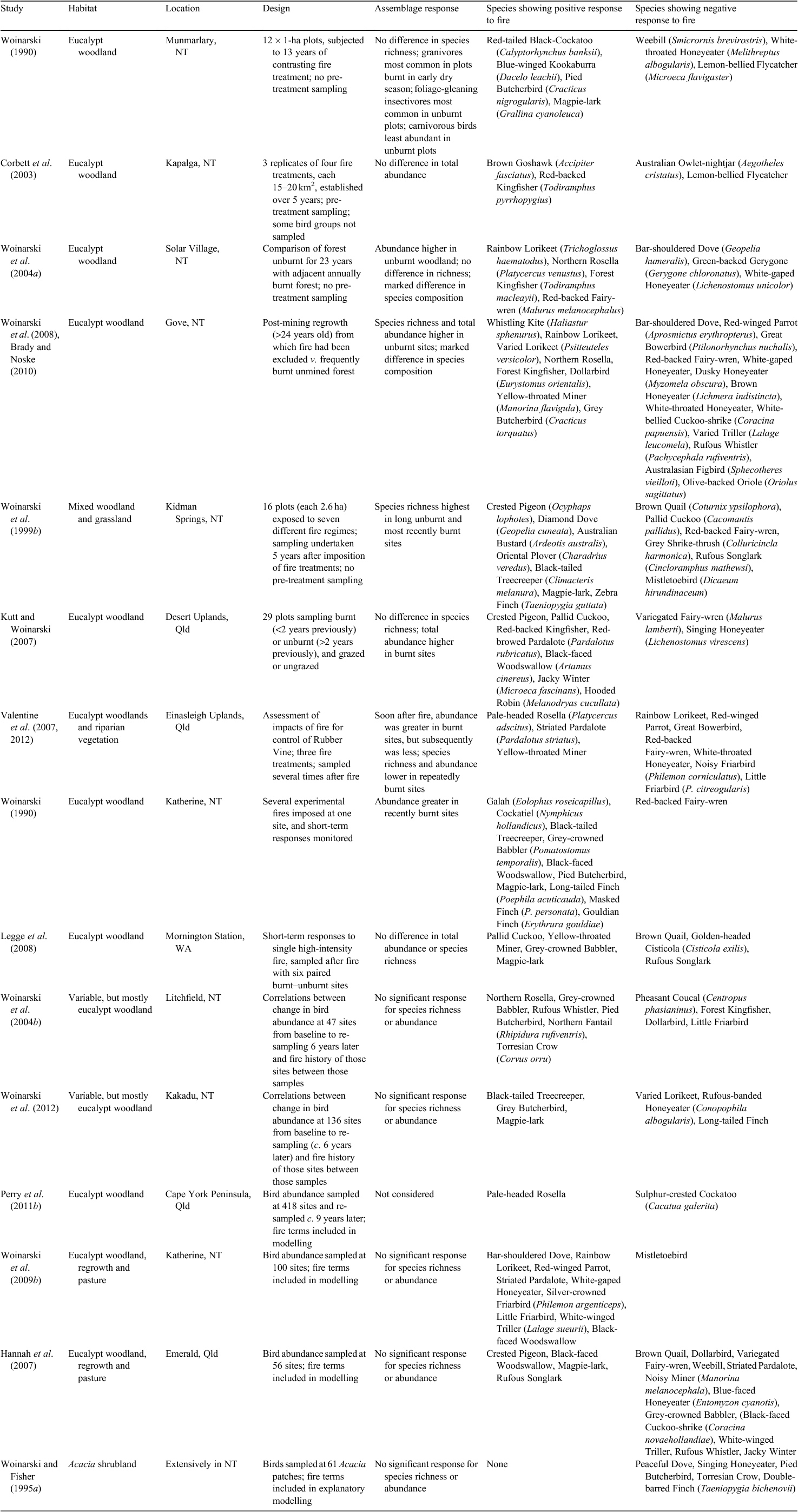
Much of the spatial and temporal patterning of fire is now readily resolved by continuous monitoring by satellite, with ever-increasing resolution. Characteristics of the current incidence of fires are summarised in Table 2. On average ~20% of the monsoonal tropics is burnt each year, with this proportion greater in years with more preceding rainfall and hence greater biomass of grass (Harris et al. 2008). Fire is most prevalent on Indigenous lands and conservation reserves (Franklin et al. 2008). Fire is more prevalent in the Kimberley and Top End than on Cape York Peninsula, and less in the tussock grasslands (which are of premium pastoral productivity) at the inland fringe of monsoonal Australia. By far the highest incidence of fires occurs in the dry season, with regional variation in this timing associated in part with land-use and with the extent of application of fire management. Notwithstanding attempts at pre-emptive burning, large fires (>1000 km2 in extent) at short intervals (<3 years) dominate the contemporary fire regime in many areas (Yates et al. 2008).
There are now many studies of the incidence of fire in northern Australia and of its effects upon vegetation (e.g. Bowman 1988; Russell-Smith et al. 2003a; Williams et al. 2003b), and these will not be considered in detail here. Such studies now comprise a large proportion of the ecological research undertaken in monsoonal Australia, and also of the fire research undertaken in Australia generally. Although with notable nuances, many studies have concluded that the savanna vegetation is rather resilient to fire, but that: (1) late dry-season fires or other high-intensity fires are likely to reduce the abundance of large trees and the tree-hollows that they support (Williams et al. 1999, 2003b; Woinarski and Westaway 2008; Yates et al. 2008; Russell-Smith et al. 2012a); (2) the shrub layer, which includes many pantropical plant species that bear fleshy fruits, is diminished or eliminated by frequent or high-intensity fires (Russell-Smith et al. 2003a; Williams et al. 2003b; Vigilante and Bowman 2004a; Woinarski et al. 2004a); (3) some vegetation types (notably rainforests and heathlands) and vegetation components (such as mistletoes and Northern Cypress-pine, Callitris columellaris var. intratropica) are more sensitive than tropical eucalypt woodlands to fire and diminish under regimes of frequent or high-intensity fires (Russell-Smith et al. 1998, 2002, 2010, 2012a, 2012b; Bowman et al. 2001; Russell-Smith 2006; Edwards and Russell-Smith 2009) and (4) against the prevailing trend, in some areas a reduced incidence of fire has resulted in ‘woody thickening’, an increased tree biomass within woodlands or an expansion of rainforests into woodlands, or woodlands to grasslands (Lewis 2002; Sharp and Bowman 2004; Russell-Smith et al. 2004a, 2004b). Many of these conclusions are based on research and monitoring over short periods but are also corroborated by multi-decadal scale observations (e.g. Lewis 2002; Russell-Smith et al. 2003b) and some limited archaeological evidence, notably records that indicate an appreciably greater pre-European incidence of fleshy fruited shrubs (Atchison et al. 2005; Atchison 2009).
Interactions of fire with other factors
Fire is itself a complex ecological factor but that complexity is compounded owing to its interactions with other factors that influence the ecology and conservation management of monsoonal Australia. These include pastoralism (or, more generally, grazing by introduced herbivores, farmed or feral), weeds, feral predators and climate change. These interactions may be synergistic (i.e. the two factors act in a compound, multiplicative manner), additive, negative, anarchic or unknown. These linkages may mean that the impacts of any fire or fire regime may differ in different settings, and may need to be contextualised according to the occurrence or prevalence of other factors of potential effect.
Worldwide, the dynamics of tropical savanna are influenced by the single and interactive influences of fire and herbivory (Bond and Keeley 2005). In Australian tropical savannas, the herbivory regime has changed very substantially since European settlement. Broadly, fire and pastoralism interact mostly through the reduction in fuel loads (grass) by livestock, and hence a propensity for fires on intensively grazed lands to be relatively infrequent and of low intensity. Such fire regimes may be beneficial for some components of biodiversity but any such advantage may be reduced or negated by the direct effects of pastoralism (most notably a reduction in ground cover, and trampling). Furthermore, most pastoralists dislike fire, as it consumes the food on which their stock depends and may destroy infrastructure. But some pastoralists use fire cautiously to create green pick – transient post-fire flushes of growth sought by cattle towards the increasingly barren end of the long dry seasons (Crowley et al. 2009) – with such localised high-intensity use potentially concentrating the harmful environmental impacts of livestock. Some pastoralists also burn off fuels in peripheral country deemed unsuitable for cattle, thereby reducing the risk of wild fires encroaching from these areas onto preferred pastures. These sacrifice areas often correspond with sites that would otherwise provide important refugia for biodiversity from the direct effects of pastoralism.
In some areas, the robust native grasses have been deemed inadequate by pastoralists, who (together with agricultural research and support agencies) have introduced instead, over the last few decades, even more vigorous grasses from Africa in an attempt to increase productivity and profitability (Cook and Dias 2006). Many of these invasive grasses, notably Gamba Grass (Andropogon gayanus), Para Grass (Brachiaria mutica), Olive Hymenachne (Hymenachne amplexicaulis) and mission grasses (Pennisetum spp.), have proven to be an ecological disaster. Collectively, these grasses are now recognised as a Key Threatening Process under Australia’s Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth). Together, these grasses can occupy most habitats in monsoonal Australia and have invaded large areas, including many conservation reserves. They interact with fire synergistically, because their extremely high biomass and curing later in the season than native grasses results in fire intensities (and hence extent) many times greater than fires fuelled by native grasses (D’Antonio and Vitousek 1992; Rossiter et al. 2003). They also dramatically alter nutrient cycling (Rossiter-Rachor et al. 2009). Repeated episodes of such high-intensity fires result in depletion and eventual removal of tree cover and woody plants, transforming the savanna from open woodland to monospecific (or at least low diversity) non-native grasslands (Brooks et al. 2010; Setterfield et al. 2010). There are some contrasting cases: for some weed species, such as Rubber Vine (Cryptostegia grandiflora), fire (either single intense fires or a particular fire regime) may provide the most effective and economical mode of control (Radford et al. 2008).
The interaction of fire and feral predators is not yet well resolved. There is some evidence that introduced predators (across most of this system, feral Cats only) are more effective hunters in extensively and recently burnt areas because most shelter sites (e.g. hollow logs, dense grass tussocks) of their prey species have been removed (Oakwood 2000).
Global climate change is predicted to result in increasing temperatures and higher incidence of very hot days, and possibly increasing and more variable rainfall (which is likely to produce higher fuel loads) across much of the savannas. Hence, fires are likely to become more extensive and of higher intensity (Williams et al. 2009).
However, interactions are not only ecological. Fire forms part of the complex social and economic nature of northern Australia; it is not simply a factor that can be considered and managed solely within an ecological setting. Most acutely, this relates to Indigenous land management and the prerogative, or obligation, of Aboriginal landowners to set fires in a manner that is at least nominally traditional on their lands; it is a sign of ownership, affinity and cultural endurance. The management of fire also allows a basis for employment and income across remote lands and communities marked by few other economic opportunities. Such funding support for fire management is particularly the case through the recent proliferation of Indigenous Protected Areas across monsoonal (and inland) Australia (for which biodiversity conservation is at least an avowedly important justification and component), and through landmark agreements to offset industrial greenhouse gas emissions by broad-scale reduction of fire extent in the Australian savannas. At the very least, such initiatives serve to provide fire-management accountability, as fires must be rigorously documented against pre-defined performance expectations or financially binding contracts, sometimes with some secondary biodiversity benchmarking (e.g. Fitzsimons et al. 2012). To a limited but increasing extent, pastoralists are also entering this carbon market, with its chief instrument of fire management and consequential reduction of carbon-equivalent emissions (Douglass et al. 2011). Even without consideration of carbon accounting, there is increasing interest among pastoralists in the manner in, and extent to, which fire can influence economic production on pastoral lands (Dyer and Stafford Smith 2003).
A further pervading social and economic influence on fire management is that the tropical savanna landscapes of northern Australia have few people, few resources, low land prices and little infrastructure; hence there is little ability to control (particularly to suppress) fire, and often no substantial economic incentive to do so. However, to some extent, these characteristics allow for (indeed necessitate) broad-scale collaboration in fire management between clusters of neighbours and across a range of tenure types (e.g. the EcoFire program in the Kimberley; Legge et al. 2011, 2013).
Paradigms, assumptions and hypotheses
It would be helpful for fire management in monsoonal Australia if it was grounded in a firm and deep foundation of consistent evidence about its ecological fit and effect, and within a clear and unshakeable paradigmatic framework. But it is not. The evidence base is varied but far thinner than ideal, the paradigms of fire management remain sketchy and contested, and there remains uncertainty about the extent to which ecological understanding (and hence the direction of conservation management) can be generalised from savanna systems globally or from non-savanna systems elsewhere in Australia. In this section, we briefly outline some of the main hypotheses and paradigms that relate to the ecological role of fire, and of the management of fire and biodiversity in this system. These are not necessarily competing hypotheses, they vary in focus, and they are supported by variable levels of evidence, but together they provide the more-or-less intellectual framework for the issue of fire and birds in Australia’s tropical savannas.
Hypothesis 1
At any site, vegetation (and consequently animals) will show more or less predictable successional variation over time since fire, with some species associated with conditions soon after fire, some species associated with long periods since fire, and some species intermediate between these.
Successional models of ecosystem change following disturbance are well demonstrated in most temperate systems and some tropical systems, often in relation to fire, and may show considerable variability in the extent of the turnover in species composition, the distinctness and number of intermediate (seral) stages, and the rate and duration of change. However, succession is less evident in environments exposed to very frequent disturbance, from which late-successional species may have been long lost from the system, and disturbance-tolerant or disturbance-favoured species prevail. With fire and the tropical savannas of northern Australia, there has been some debate about the applicability of succession theory given the fire-proneness of the environment. Woinarski et al. (2004a) demonstrated some directional floristic, structural and faunal change at a site from which fire had been excluded for 23 years, with a weak tendency for increased manifestation of rainforest-associated elements, whereas weaker or no such directional changes were evident in other sites exposed to shorter fire-free intervals (Andersen et al. 2006).
Hypothesis 2
In this region, as a result of fire, savanna woodlands exert spatial dominance at the expense of other vegetation types less resilient to fire. If fire extent and frequency were reduced, these currently interstitial environments would increase to the net benefit of regional biodiversity.
All environments are dynamic and this dynamism may be most evident at the edges (ecotones) between contrasting vegetation types, as this frontier may ebb and flow with the dominant ecological drivers. In monsoonal Australia, fire is that driver, at least over the decadal scale at which we work. Savanna woodlands (or tropical eucalypt open forest) are by far the most extensive component in the landscape matrix, bordering or surrounding islands of rainforest, heathlands, Acacia shrublands and woodlands, and tussock and hummock grasslands. There is now abundant evidence that many of these borders are fluid, and that this fluidity is primarily a result of fire (Russell-Smith et al. 2004a, 2004b). Very broadly, rainforests may expand, at least towards limits imposed by other factors (soil, rainfall or other variables), into adjacent savanna woodlands when fire frequency or intensity is low, and retreat when it is high (Russell-Smith and Stanton 2002). With frequent high-intensity fires, the savanna woodlands themselves may show reduction in cover of trees and shrubs, resulting in expansion of grasslands, whereas regimes of infrequent fires may result in spread of woodlands into grasslands (Crowley and Garnett 1998). Note also that this dynamic is not driven solely by fire but also by global factors (notably increased atmospheric CO2), and increases in rainfall over the last century across much of north-western Australia. Together these factors tend to promote gradual increase in woody vegetation in this region (Banfai and Bowman 2006).
Hypothesis 3
Fire exclusion in this landscape will be futile or even counter-productive because it will inevitably lead to build-up of fuel and hence more intense fires.
This claim, that we must simply accept that the tropical savannas are fire-prone and not seek to invest in maintaining some areas in the landscape relatively long unburnt, is a defeatist argument. Loosely, the argument asserts that you can have either a regime of frequent fires of low intensity, or infrequent fires of high intensity. The scientific basis for the argument rests on the fact that some experimental long-term fire-exclusion plots happened to be burnt eventually by unplanned fire (Williams et al. 1999; Russell-Smith et al. 2003a) and, more persuasively, that detailed retrospective statistical analysis has shown that, across a large number of sample sites in the landscape, there was a positive correlation between the time since last fire and the intensity of the subsequent fire (Murphy and Russell-Smith 2010). The process underlying the argument is that fuel loads build up continuously the longer that an area is unburnt (Williams et al. 2003b). However, although there is evidence to support this contention for at least 5 years post-fire, it may be that over a longer time period fuel loads reach a maximum and then decline, as increasing shrub cover forces reduction in grass biomass. Furthermore, the argument hinges on the tension between the practicality and cost (through fire-breaks and active suppression) of seeking to maintain longer-unburnt patches and the valuation of such patches as population sources or required habitat for some species.
Hypothesis 4
The landscapes of northern Australia have been managed skilfully for 50 000 years by Indigenous practices (particularly fire management) and any current biodiversity decline may be best addressed by reversion to such practice (i.e. there is a consonance between biodiversity conservation and Indigenous land management).
This argument morphs uncomfortably between ecological and cultural spheres. Whereas Australians of European descent often view fire as destructive, and burnt areas as destroyed, an opposite perspective may be typical of Indigenous Australians, who may view fire instead as benign and necessary:
This earth, I never damage.
I look after. Fire is nothing, just clean up.
When you burn, new grass coming up.
That means good animal soon,
might be goanna, possum, wallaby
Burn him off, new grass coming up, new life all over. [Neidjie et al. 1986]
The ecological and conservation management component of the argument assumes in part that (1) Indigenous fire management aimed to optimise species richness (or that this result was a collateral benefit of other objectives); (2) biodiversity was maintained over the course of, and because of, the millennia of Indigenous management; (3) the current decline in biodiversity is primarily a result of the cessation of Indigenous land management; (4) detailed knowledge of Indigenous fire management has been maintained; (5) new components in the system (notably introduced plants and animals) will not pervert any continuation or resumption of the practice and outcomes of Indigenous land management and (6) such practice will not be incompatible with a landscape now spotted with infrastructure and alternative land-uses. All of these assumptions can be challenged. Furthermore, it may represent an almost symbolic process, given that the main objectives of traditional Indigenous fire management (to enhance food-producing plants and aid hunting) are no longer crucial matters dictating survival for most Indigenous landowners. Notwithstanding this uneasy foundation, there is some evidence that Indigenous fire management produces better biodiversity outcomes (or at least outcomes of benefit to some species) than a fire management vacuum (Price and Bowman 1994; Bowman et al. 2001; Yibarbuk et al. 2001; Whitehead et al. 2003; Vigilante and Bowman 2004b).
Hypothesis 5
Fire in this landscape is pervasive and natural, and, because of this pervasiveness, biodiversity elements in general are immune to its consequences.
This argument is similar to Hypothesis 3 above in its prevailing acceptance of frequent fire. It assumes that millennia of frequent fire have resulted in the adaptation to, or tolerance of, frequent fire by all species of plants and animals currently occurring in Australia’s tropical savannas. It asserts that, with the exception of a few atypical species (for which some peripheral tailored solution may be required), there is little conservation advantage in reducing fire frequency or intensity, and hence the (assumedly) large investment to do so would be unjustified, even if it was realisable. The evidence for it mostly relates to lack of clear differentiation in species complements across sites exposed to a small duration of contrasting fire regimes (e.g. Andersen et al. 2003a, 2003b, 2005). However, there is contrary evidence when these durations are longer (e.g. Woinarski et al. 2004a), and when the data from short-term experiments are extrapolated through modelling to longer terms (e.g. Pardon et al. 2003; Firth et al. 2010). A problem with the idea is that it is often the threatened species that are most disadvantaged by the current prevailing regime, so simply accepting that most species are tolerant of that regime doesn’t make for good conservation outcomes.
Hypothesis 6
Contemporary patterns of vegetation and fire regimes in this region are an aberration, an altered state and ecological trap, derived from a hiatus in Indigenous land management. A more benign and sustainable natural order will follow from broad-scale elimination (through wet season burning) of annual grasses.
This argument, applied particularly in the Darwin–Kakadu area of the NT, states that the understorey dominance of annual sorghum grasses (speargrass, Sarga spp.) is the nub of the fire ‘problem’, and that dominance probably represents a derived state arising fairly recently as a response to the collapse of Indigenous land management and the introduction of pastoralism. Furthermore, the argument recognises that the dominance of sorghum grasses has now become a self-reinforcing dynamic, with those annual grasses catalysing frequent hot fires to the relative detriment of their competitors (perennial grasses and herbs, with typically lower fuel loads). The argument is based in state-and-transition modelling and asserts that it is now difficult to return to the pre-settlement state of an understorey in which annual grasses are a minor component and in which fires are thus less prevalent or destructive. However, the argument runs, it may be possible to return to this preferred state through extensive use of fire in the early wet season, after the germination of the year’s cohort crop of annual grass seeds but before they themselves set seed. Such burning can remove annual plants over large areas, or at least over areas that may provide strategic fire-breaks (with reduced fuel loads). The argument becomes insubstantial in the many areas of the tropical savannas not dominated by annual native grasses.
Hypothesis 7
Biodiversity in any area will be maximised or optimised when a range of fire regimes (or times since fire) are present (the pyrodiversity begets biodiversity argument).
This is the most widely given and widely used advice that ecologists provide to conservation managers: essentially to seek to cover all options and cater for all species. It rests on the assumption that, given initially a fairly extensive uniform habitat, one can maximise the environmental variability that it can offer (and hence the biodiversity it can support) by manipulating fire in the system to maintain more-or-less equitable representation across all segments of a successional sequence. Thus, species associated with old-growth variants of the vegetation type, young regrowth variants and intermediate stages will all be maintained in the area.
The argument can be finessed further by tuning the spatial scale more finely, to propose that fire-mediated variability should be promoted not only across conservation reserves or broad regions but also at the scale of individual territories within those areas (Woinarski et al. 2005), thereby allowing individual animals the benefit of increased heterogeneity within their home-ranges, making it more likely that species richness will be optimised at any site as well as collectively across sites. Of course, such argument may be reduced to absurd if linked to species with very small territories or home-ranges (<1 ha).
Both variants of this argument have been linked to an idealised version of Indigenous fire management, whereby small areas of controlled fire are lit sequentially across the year on clan estates with the outcome of an extremely fine-scale mosaic (Yibarbuk et al. 2001).
There has been some recent argument, and a little evidence, against the pyrodiversity principle, including from Australia’s tropical savannas (Parr and Andersen 2006), mostly relating to definitional issues and the need for specificity in management objectives.
The pyrodiversity argument is sometimes misread as a justification for anarchy and laissez-faire – to let any fire regime happen. Instead, it requires managers to plan initiation, suppression and management of fire over time-scales from short to long, with that fire management explicitly linked to and defined by ongoing monitoring of the extent and spatial juxtaposition of the proportion of country across a sequence of post-fire ages.
Hypothesis 8
Conservation benefit may be delivered optimally by managers implementing the management needs (in this case, fire) of focal species, those of most particular significance for conservation or for ecosystem maintenance.
The focal species concept (Lambeck 1997) relies on the identification of one, or a small set of, individual species taken to be representative, or the superlative examples, of a broader ecological community; it requires detailed knowledge of the ecological requirements of that or those species; and it demands the resolute application of management to provide those ecological requirements. In Australia’s tropical savannas it has been used, for example, to promote fire management in the eucalypt lowlands to target the requirements of Partridge Pigeons (Fraser et al. 2003), in the sandstone plateau of Arnhem Land to target the requirements of heathland and rainforest plants (Petty et al. 2007), and in the savanna woodlands of Cape York Peninsula to benefit Golden-shouldered Parrot (Crowley et al. 2004). The advantage of the approach is that it provides for a clear objective, fairly unambiguous management advice, and a simple measure of performance success. The self-evident disadvantage is that all species are different and one, or a small set of, species will never fully represent the ecological requirements of a broader community of species. However, there is substantial commonality in the management requirements of many declining species in the monsoonal tropics. Typically, these species are those that respond negatively to regimes of frequent or high-intensity fire (Woinarski 2004; Andersen et al. 2005; Woinarski et al. 2009a) or to the effects of grazing by livestock. Broadly, conservation reserves have provided some refuge for the latter group of species (although feral stock may be widespread in such areas) but cannot provide refuge from unfavourable fire regimes.
In much of monsoonal Australia, long-unburnt habitat is a diminishing and small part of the landscape, even when long-unburnt is defined unambitiously as >10 years without fire (Woinarski 2004). In part, this argument links to the successional concept of Hypothesis 1 above but does not rely entirely on an assumption that long-unburnt vegetation will support a different suite of species to younger vegetation. Instead, long-unburnt vegetation may have the same species complement as younger vegetation but act as a source that provides opportunity for higher reproductive output, that can then replenish the species across the population sinks of the more pervasive, frequently burnt landscape.
Impacts of fire on birds
Framework for responses
Before considering the evidence base concerning the effects of fire on birds in monsoonal Australia, we describe how fires may affect birds (and their habitats and resources) in the short term (single fire-events), medium term and longer term.
In the short term, fire may kill individual birds. Given the fairly slow speeds and low intensities of most fires in monsoonal Australia, immediate mortality rates of adult birds are likely to be low. However effects on eggs or young may be substantial, especially for birds that nest in the grass and ground layer, with such effects related to the timing of fire vis-à-vis the breeding season for particular species. Species of birds with long incubation periods, such as the Emu (Dromaius novaehollandiae) may be at more risk than species with short nesting periods, and young of nidicolous species may be more affected than those of nidifugous species. Eggs, young and brooding adults of species that nest in shrubs, tree canopies or tree-hollows may also be lost when fires are more intense (typically in the late dry season), whereas the reproductive success of birds that nest in burrows (such as pardalotes, Pardalotus spp.) or termite mounds (such as Hooded Parrots and some kingfishers) may be little affected by fire. Fire may provide an immediate source of food for some birds that exploit invertebrates or vertebrates fleeing the fire, with at least some species of raptor and some aerial insectivores well known to track fires, congregating around the fire front (Braithwaite and Estbergs 1987). Raptors, bustards, corvids and other species may also forage in recently burnt areas, in the short term taking invertebrate or vertebrate prey killed or injured by the fire (in the days immediately after fire) and exploiting the more open ground layer to increase the efficiency of hunting for animals that survived the fire (in the weeks or months after fire). Dense grass and litter cover may also inhibit the foraging efficiency or movements of granivorous birds, and some fire may be beneficial in allowing such species to find fallen seeds of grasses and herbs more readily (Woinarski 1990; Dostine et al. 2001). However, fire may destroy some of these seeds (Watkinson et al. 1989) and fires of greater intensity destroy more seed. In the short term, fire may also influence the phenology and productivity of plant species, and hence of bird species that eat seeds, fruit or nectar (Crowley and Garnett 1999; Vigilante and Bowman 2004b). These responses vary appreciably between plant species: some species need fire to set seed or respond immediately to fire by flowering; for others flowering or seed set may be delayed by fire and productivity reduced (Williams et al. 2003b). Furthermore, these responses are moderated by the intensity and timing of fire. Given that there is likely to be substantial phenological synchrony between individual plants within a species at any site, a fire that burns part of that site may increase the time over which seeds, fruit or flowers of that plant species are available to birds beyond that brief peak available if the whole site was unburnt or the whole site was burnt.
In the medium term (~2–10 years), responses to fire regimes relate mainly to fairly subtle changes in plant species composition and vegetation structure and to the population-level consequences of the repeated effects of particular fire regimes on bird reproductive output. Where there is annual variability across space in the expression of fire, some subpopulations of any species of bird will be likely to be subjected to varyingly favourable fire regimes and some subpopulations of the same species will be subjected to unfavourable fire regimes. As long as the latter do not overwhelmingly dominate, it is likely that the species will persist in the landscape. Over the medium term, contrasting fire regimes are likely to result in increases in some plant species and decreases in others. This may be most evident in grasses, where single fires (notably for wet-season burning effects on annual grasses) or repeated consistent application of fire, or sustained exclusion of fires, may drastically alter grass species composition (Mott and Andrew 1985). For granivorous birds, such subtle changes can have significant effects on resource availability and habitat suitability, which in turn affects annual survival and reproductive success, and thence, over successive years, affects subpopulation persistence. The link is similar for the connection between fruit-producing plants (mostly shrubs) in the savannas and frugivorous birds, with frequent intense fire leading to reduced diversity, abundance, stature and fruit productivity of these plant species (Vigilante and Bowman 2004a), and consequently reduced diversity, abundance and persistence of frugivorous birds. In the medium term, with unfavourable fire regimes, some bird species may disappear from some sites.
Longer term responses of birds to fire regimes will be mediated mostly through effects on the extent and suitability of habitat as a history of fires (or of no fires) re-moulds the floristics and structural composition of the savannas and the islands of contrasting habitats interspersed within them. Over decades, if fires are frequent and intense, tree and shrub cover will decline and may be eliminated, and particularly susceptible (i.e. fire-sensitive) components, such as large trees, many non-eucalypt trees and obligate seeder plants, will disappear first. The savanna will be simplified and bird diversity will decline, with particular losses among frugivorous, ground-nesting, hollow-nesting and heathland species, and species associated with old clumps of spinifex (Triodia spp.) hummock grasses. Conversely, if the fire regime is of reduced intensity, extent and frequency, the abundance of broad-leaved shrubs will increase, ground litter will increase at the expense of grass cover, and the extent of Acacia shrublands and woodlands, Callitris woodlands, rainforests and heathlands will increase towards limits imposed by factors other than fire (e.g. Russell-Smith et al. 2004b; Brook and Bowman 2006). Over decades, centuries or millennia, the fire regime may also moderate the productivity of sites, with frequent intense fire incrementally consuming or transporting soil nutrients (Cook 2003). In the long term, with unfavourable fire regimes, some bird species will disappear from broad landscapes, and bird species composition will be changed.
Modes of research on fire and birds
In some areas of northern Australia, a profound and intimate knowledge of fire and its management has been retained by Indigenous landowners (e.g. Yibarbuk et al. 2001; Russell-Smith et al. 2009). This expertise provides a distinctive and substantial foundation for understanding the relationships between biodiversity and fire, and of Australian ecology in general. This knowledge has been documented for some mammal and plant species (e.g. Telfer and Garde 2006). However, for birds, there has been little record and transfer of such knowledge to the scientific literature; one notable exception is for the Partridge Pigeon (Fraser et al. 2003).
More conventional scientific approaches include experimental (manipulative) and correlative studies, detailed autecological studies and modelling. In time-series (or longitudinal manipulative) experiments, a small set of contrasting fire regimes is imposed upon sites that are initially comparable, and the consequential divergence (from a pre-impact baseline) over time is measured. This approach has been used worldwide, and has documented the effects of fire in many environments (e.g. du Toit et al. 2003). Its application and utility is constrained by the cost of establishment and ongoing imposition of the experimental regimes, the difficulty of avoiding spoiling of the design by unwanted wildfire, the requirement for studies to extend over many years, and the simplification of chaotic real-world fire regimes to a small set of rigidly imposed, artificial abstractions. Most of the few such studies in Australian tropical savannas have involved fairly small experimental plots (which constrains interpretability of results for the many species of birds that are fairly mobile or have large home-ranges) or have been of fairly brief experimental duration (which also reduces the interpretability of results, as medium- to long-term effects are not realised) (Williams et al. 2003c). In many cases, sampling of birds in such studies has been added by happenstance to studies designed to examine other environmental components that are more amenable to small-scale experimentation (e.g. Woinarski et al. 1999b).
Correlative studies seek instead to sample sites at the same time across a set of burnt and unburnt areas or a spectrum of natural variation in fire regimes (space-for-time replication or ‘chronosequence’). The disadvantage of this approach is that sampled sites are not necessarily matched for all variables other than fire regimes, and that the fire regimes at sampled sites may not have been as crisply defined as for experimental manipulation. The advantages of the approach are that it can readily increase sample size and reduce the experimental duration (and hence cost) of the studies. Although this approach has been widely used elsewhere in Australia (e.g. Haslem et al. 2011), there are few examples of its use in the Australian tropical savannas, perhaps because frequent fire mercurially shifts fire histories across this landscape, because there are few sites in the landscape that are long-unburnt, rendering most potential contrasts in fire history rather muted, and because, at most sites, with contrasting fire history there are conflating influences relating to topography or land-use. A variation on this research design is a monitoring approach that samples every site twice or more, and compares the divergence between the results of the baseline and subsequent sampling of each site with the fire history that occurred at that site in the between-sampling interval.
Some autecological studies of particular bird species have included consideration of responses to fire. In one example, Ziembicki (2009) used satellite telemetry to examine landscape-scale movements of the Australian Bustard (Ardeotis australis) at sites in the Victoria River district of the NT. He found that Bustards would move over at least tens of kilometres to forage in burnt areas, with this response following almost immediately after the fire and sustained for several weeks.
Another approach applies life-history and ecological attributes to characterise species according to their likely sensitivity to fire. This approach has been widely used for plants and some faunal groups (e.g. Whelan et al. 2002), and an analogous study has used foraging and life-history attributes to predict the response of birds in Australia’s tropical savannas to pastoral impacts (Martin et al. 2005). In part, this approach may simply be systematised common sense – for example, all other things being equal, ground-nesting birds may be expected to be more affected by ground-level fires than those that nest in the canopy. But modelling can be a powerful tool to extrapolate, over decadal or longer time periods, results (such as fire-related impacts on survival or reproductive output) derived from single brief studies to assessments of longer-term population viability. Some exquisite, and useful, models have been developed that predict the long-term responses of vegetation to different fire regimes in northern Australia, with further elaborations that include the interactive effects of invasive grasses, grazing by livestock and climate change (Liedloff and Cook 2007). Other models have extrapolated long-term population viability for individual species from short-term studies of fire responses, including for the Brush-tailed Rabbit-rat (Conilurus penicillatus; Firth et al. 2010), Northern Brown Bandicoot (Isoodon macrourus; Pardon et al. 2003) and Northern Cypress-pine (Price and Bowman 1994) in monsoonal Australia. Heinsohn et al. (2009) modelled population viability of the Palm Cockatoo (Probosciger aterrimus) on Cape York Peninsula based on long-term (fire-related) trends in the availability of hollows. Reside et al. (2012) modelled the responses of several species of savanna birds to fire regimes under projected climate change.
A comparison of current and historical status has also been used to infer the effects of changing fire regimes on birds, but this is a blunt tool given the limited and imprecise historical baseline concerning the status of individual species of bird and of historic fire regimes, and the large range of potentially conflated or alternative explanatory factors. Nonetheless, the approach may provide some insights. For example, Garnett and Crowley (1995) compared early accounts of the abundance of Black Treecreeper (Climacteris picumnus melanota) on Cape York Peninsula with its present highly localised and sparse distribution there, and interpreted this change as being driven largely by changed fire regimes, with this argument supported in part by its now apparently relictual distribution associated with sites better protected from fire.
Evidence base: fire and bird assemblages
Perhaps surprisingly, given the pervasiveness of fire in northern Australian landscapes, there are few studies that have focussed on the responses of bird assemblages to fire, and these few studies have constraints that limit their ability to be used for generalisation. Most studies have been brief, and report on samples that compare abundance of species at a single time, rather than relative habitat suitability over the course of a year or lifetime of a bird.
We attempt to collate the findings of these studies in Table 3, which summarises the responses observed in bird community studies. This tabulation provides a simplistic collation, because the studies are so varied in duration, methodology, environments considered, range of fire regimes included, and format of results, and we simplify responses to an unsubtle essence: to whether the species was (or became) more or less abundant at sites that were exposed to more (or more recent) fire. Elsewhere in this paper we note that the definition of fire regimes is complex and the responses of birds may be subtle, so we recognise that we lose appreciable detail in such simplification. The studies below are ordered broadly by methodological approaches.
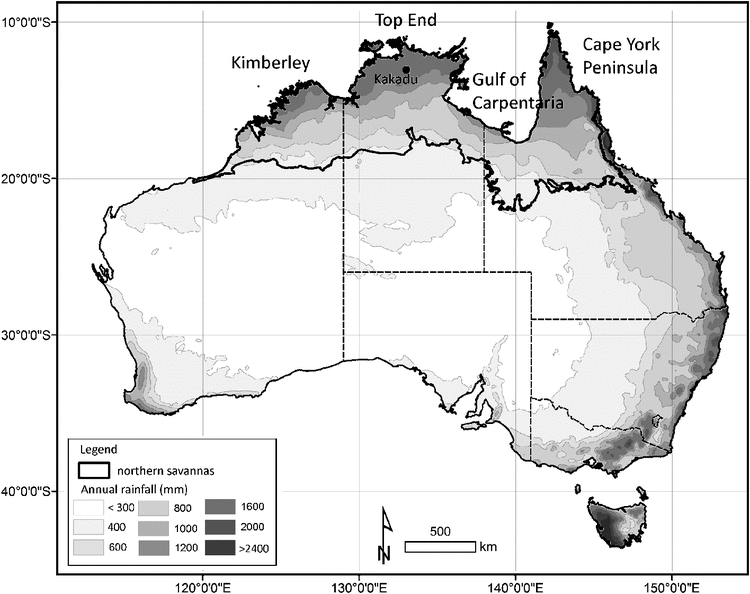
Woinarski (1990) sampled bird assemblages in 12 experimental plots already exposed to 13 years of contrasting fire regimes (unburnt, biennially burnt, annually burnt in the early dry season, and annually burnt in the late dry season) in eucalypt forests and woodlands at Munmarlary, near Kakadu National Park. The experiment was initially designed to examine the responses of plants, and the small plot-size (1 ha) undoubtedly compromised the value of the design for examining the responses of birds. Six species of bird (from 11 species recorded in sufficient numbers) showed significant variation between fire treatments (see Table 3).
In eucalypt woodlands at Kapalga, within Kakadu National Park, Corbett et al. (2003) examined the responses of birds in larger plots (15–20 km2) over which four experimental treatments (unburnt, burnt each year in the early dry season, burnt each year in the late dry season, and burnt each year progressively through the season; each with three replicates) were imposed for 5 years. This study included pre-treatment sampling and focussed on five groups: forest passerines, diurnal raptors, other diurnal predatory birds, nocturnal predatory birds and ground birds, but excluded some suites of birds, notably honeyeaters, parrots, finches and some other common birds. Of 25 species considered, five showed significant fire-treatment effects.
At Solar Village, near Darwin, Woinarski et al. (2004a) contrasted the abundance of bird species in a tropical eucalypt forest site (of >30 ha) that had been protected from fire for 23 years, with adjacent eucalypt forest that had been burnt annually over that period. At this site, the contrast of vegetation was far more marked than for the Kapalga study, and responses of birds were accordingly more pronounced. Of 27 species of bird considered, 16 showed a significant treatment effect. The bird species composition in the long-unburnt forest was trending towards that characteristic of monsoon rainforests, although this progression was fairly sedate.
There are few such long-unburnt sites in the tropical savannas of northern Australia, but some rehabilitated forests established after extensive strip-mining are protected from fire. At one such site at Gove, in north-eastern Arnhem Land, Brady and Noske (2010) and Woinarski et al. (2008) sampled birds across a range of regrowth ages and in adjacent unmined open forests. There were stark differences in bird assemblages between fire-excluded forest sites at least 24 years after mining and nearby frequently burnt sites. Although this result may be influenced in part by the effects of mining, the very strong similarity in bird responses to those observed at the fire-excluded Solar Village sites suggests that response to fire is an influential component in the results.
In a study similar to the Munmarlary design but in a lower rainfall area in the Victoria River district of the NT, Woinarski et al. (1999b) sampled birds at 16 plots (each 2.6 ha) in two contrasting woodland and grassland environments exposed to seven different experimental fire regimes (unburnt, burnt in the early dry season at intervals of 2, 4 and 6 years, and burnt in the late dry season at intervals of 2, 4 and 6 years). Sampling was undertaken 5 years after experimental establishment (and hence before all treatments had been fully imposed). Of 30 bird species considered, 14 were significantly associated with fire regime. This study had design constraints similar to that of the Munmarlary study: the plot-size was small and there was no pre-treatment sampling.
In the Desert Uplands of northern Queensland, Kutt and Woinarski (2007) sampled birds at a series of sites of contrasting histories of fire (burnt <2 years or >2 years before sampling) and livestock grazing. Of 35 bird species considered, six showed significant fire effects. In this study, the effect of fire regime on birds was greater than that of grazing treatment.
In the Einasleigh Uplands of Queensland, Valentine et al. (2007, 2012) examined bird responses to a range of management fires designed to control Rubber Vine. The study included replicates in different creek systems, two habitat types, and, initially, three fire treatments (no fire, fire in the dry season and fire in the wet season). Sampling occurred at several periods after fire treatments, but there was no pre-treatment baseline. The responses of species changed appreciably over the course of the study, with more markedly detrimental responses at sites that were burnt more than once.
Woinarski (1990) monitored short-term responses (with sampling over a period of 7 months) to several fires (two in the early dry season and one in the late dry season) on parts of a study site in a eucalypt woodland near Katherine. Of 30 species considered, 22 showed significant short-term responses to fire, with most increasing in abundance in the recently burnt areas.
Short-term responses to a single, high-intensity, late dry-season fire were also considered by Legge et al. (2008) in the central Kimberley. This study included no before-fire sampling but six closely paired replicate sites in burnt and unburnt areas were sampled 1 month after the fire. Three bird species (all grass-dwellers) were ‘overwhelmingly recorded’ in unburnt sites, and four species (none of them grass-dwellers) were more abundant in burnt sites.
Woinarski et al. (2004b, 2012) sampled birds at many sites in Litchfield and Kakadu National Parks, re-sampled the same sites in the same manner 5–8 years after initial sampling, and then related change in abundance at each site to the fire history of the sites in the intervening period. At Litchfield, of 44 bird species considered, the change in abundance of 10 species was related significantly to fire history. For Kakadu, of 91 bird species considered, only six species showed any significant relationship with fire history. These studies afforded little insight: there was little clear association between changes in bird abundance and fire history, in part because many other uncontrolled factors may have influenced abundance at any site at the time of sampling.
On Cape York Peninsula, Perry et al. (2011b) used a study design broadly similar to that of Woinarski et al. (2004b, 2012) at Litchfield and Kakadu National Parks (above), with a large set of plots sampled initially in 2000 and re-sampled in 2008. Fire was an important component in the explanatory models for four species that declined significantly over this period but for only two species was this relationship clearly directional. Although far from definitive, results from this study were consistent with earlier conclusions that ongoing regional declines of some species of bird, such as the Bush Stone-curlew (Burhinus grallarius) and Black Treecreeper, were a result of changed fire regimes and associated changes in vegetation structure (Garnett and Crowley 1995; Kutt et al. 2005).
Reside et al. (2012) modelled a large database of distributional records for 44 bird species across the Australian tropical savannas. The resulting models were then used to simulate likely responses to a markedly increased frequency of late dry-season fires associated with projected climate change. Fire parameters made little contribution (3–4%) to distributional models. With a simulated regime of more-frequent and higher-intensity fires, most species showed a predicted decline in range. The approach has some constraints. For example, the data used were pooled over the period 1997–2008, as were the fire data, so many distributional records of birds preceded the fire histories that were used to explain them. Furthermore, as typical of climate-based modelling, the models and predicted distributions are limited because they have no primary consideration of habitat or historical factors. As an example, the model and predicted distribution for one of the four showcased species, White-streaked Honeyeater, extends far beyond its known geographical range. A further problem with such coarse-grained modelling is that species associated with particular landscape features are poorly represented. In this case, these included the Gouldian Finch (Erythrura gouldiae) and riparian bird species such as Purple-crowned Fairy-wren (Malurus coronatus) and Crimson Finch (Neochmia phaeton) that the modelling implausibly predicted to be favoured by increased fire frequency, with the hopeful explanation that ‘these species may be more resilient to frequent fires owing to their proximity to water courses’. More detailed autecological research suggests instead that increases in fire frequency will seriously disadvantage such species (Skroblin and Legge 2010, 2012; Legge et al. 2011).
In two studies within pastoral settings, near Katherine (Woinarski et al. 2009b) and near Emerald in central Queensland (Hannah et al. 2007), bird assemblages were compared across a series of cleared areas, regrowth and intact eucalypt woodlands, with a range of disturbance factors (including fire and grazing) superimposed on that variation. In the Katherine study, of 15 species considered, the abundance of 10 was significantly related to fire. This study concluded that ‘fire, weeds and grazing [were] … in many cases … a more important determinant of faunal attributes than was whether or not the quadrat had been cleared’. In the Emerald study, of 40 species considered, the abundance of 16 was significantly related to fire. Trends across these studies were notably divergent, with far more species negatively associated with fire in the Emerald area, perhaps simply because this area exhibited a more marked contrast in fire regimes than the Katherine study area, where almost all sites were exposed to frequent fire.
Woinarski and Fisher (1995a) examined bird assemblages in the distinctive Lancewood (Acacia shirleyi) woodlands of lower rainfall areas of monsoonal NT. Birds were sampled in many Lancewood patches, with each sampled site scored for the incidence or impact of fire. Of 45 bird species considered, the abundance of five was significantly related to fire history, with all of these more common in patches less affected by fire. These ‘islands’ of Acacia woodlands, with their characteristic, fairly open ground layer, are being diminished and degraded under current fire regimes, with gradual invasion and replacement by the dominant eucalypt woodlands and dense grassy understoreys (Russell-Smith et al. 2010). A suite of bird species, including the Apostlebird (Struthidea cinerea), Hooded Robin, Rufous Whistler, Grey-crowned Babbler (Pomatostomus temporalis) and Tawny Frogmouth (Podargus strigoides), that are associated more with these Acacia woodlands than eucalypt savannas, is likely to be disadvantaged by this fire-driven change to vegetation (Woinarski and Fisher 1995b).
Some studies of fire-sensitive habitats have examined the effects of fire on birds less directly. Birds that rely on riparian vegetation and scattered rainforest pockets that thread and punctuate the savanna matrix may be particularly vulnerable to changes in fire patterns that compromise the continuity of their (naturally) patchily distributed habitat. Many frugivorous birds forage across a large number of scattered rainforest patches. Surveys of birds in rainforest patches in the NT showed that a range of frugivores were sensitive to isolation of patches (Price et al. 1999). For example, the Rose-crowned Fruit-dove (Ptilonopus regina), Pied Imperial-Pigeon (Ducula bicolor) and Eastern Koel (Eudynamys orientalis) were absent from patches where the cumulative area of rainforest within a 50-km radius was <0.4%. Price et al. (1999) extrapolated these results to show that even minor reductions in the total area of rainforest would have substantial effects on the abundance and persistence of many frugivorous birds. Repeated intense fires through the savanna matrix will penetrate and damage the perimeters of rainforest pockets; over time, such reduction in rainforest area (McKenzie and Belbin 1991) will have disproportionate consequences for a suite of bird species that overfly the matrix.
Together, this set of studies provides some insight into the responses of bird assemblages to fire in Australia’s tropical savannas, but all of the individual studies have constraints and there are few commonalities in ecological interpretation of results across the studies (Table 3). In this regard, there are too few studies, which are so notably heterogeneous in the fire regimes considered, in study design or in the duration of the study response, to allow for a robust meta-analysis. For some species there is sufficient consistency to allow for reasonably confident ascription of population-level responses to a range of fire regimes; for example, the abundance of Variegated Fairy-wrens (Malurus lamberti), Weebills (Smicrornis brevirostris), Dusky Honeyeaters (Myzomela obscura), Rufous Whistlers, White-throated Honeyeaters (Melithreptus albogularis) and Lemon-bellied Flycatchers (Microeca flavigaster) (mostly species associated with shrubby understoreys) decreases with regimes of frequent or high-intensity fire, whereas that of Crested Pigeons (Ocyphaps lophotes), Forest Kingfishers (Todiramphus macleayii), Northern Rosellas, Yellow-throated Miners (Manorina flavigula), Black-faced Woodswallows (Artamus cinereus), Grey Butcherbirds (Cracticus torquatus) and Magpie-larks (Grallina cyanoleuca) (mostly species associated with open ground or woodlands with a very open understorey) increases. The tendency for Yellow-throated Miners and butcherbirds to increase in savanna woodlands that are frequently burnt may compound the direct impacts of such fire regimes on bird assemblages, as these despotic birds may themselves reduce the diversity and abundance of other bird species, particularly of smaller insectivorous birds and those associated with shrubby understoreys (e.g. Kutt et al. 2012).
Evidence base: fire and individual species of bird
A few studies have focussed in more detail on the effects of the frequency, scale and intensity of fire on individual species of birds. Most of the species considered are dependent on the grass layer or on fire-sensitive vegetation. Arguably these species are a biased set, in that they were chosen for study based on a presupposition that they were sensitive to fire. Nevertheless, they illustrate the range of potential responses to changes in fire patterns.
Grass-dwellers
Six species of fairy-wrens (Malurus spp.) and grasswrens (Amytornis spp.) are found in the tropical savannas. In a landscape dominated by grass, these grassland birds are an unusual fit. Only one of the six species (the Red-backed Fairy-wren, M. melanocephalus) occurs extensively across the lowland savanna country. Three others (the grasswrens) are highly restricted, associated not so much with the tall grassy savanna but rather with complex, rocky (and hence somewhat fire-sheltered) environments and the hummock grasslands (‘spinifex’, Triodia spp.) that these support. The Variegated Fairy-wren has a patchy distribution largely exclusive of the dominant, tall-grass savanna, occurring instead mainly in some rocky ranges and Acacia woodlands supporting open or short-grass understoreys (Woinarski and Fisher 1995b). The Purple-crowned Fairy-wren is restricted to narrow bands of riparian vegetation. Since European settlement, the main fabric of the habitats of this grass-dwelling guild has been diminished by cattle grazing and extensive fire.
Focal studies have been carried out on four of these species, anecdotal information exists for a fifth species, and the sixth has been included within community-level studies. In all cases, increases in the frequency and scale of fire, especially high-intensity fire, are associated with declines or changes in behaviour that are expected to lead to decline. All three northern grasswrens are sensitive to the availability of mature spinifex, in which they live, shelter, forage, and nest (Noske 1992; Woinarski et al. 2007b, 2012; Cawardine et al. 2011). Extensive surveys have shown that Carpentarian Grasswrens are more likely to occur in old-growth spinifex rather than frequently or recently burnt areas, and to occur in landscapes with physical features that reduce the spread of fire, such as large areas of rock, high densities of gullies and areas distant from roads, from which most fires are lit (Harrington et al. 2011; Perry et al. 2011a).
In the Kimberley, Murphy et al. (2010) examined the response of individual Red-backed Fairy-wrens to fire. Fires did not cause immediate mortality, but Fairy-wrens living in territories affected by fires changed their ranging behaviour to include unburnt habitat. With low-intensity fires, unburnt habitat was often available within the original territory, whereas birds in territories affected by higher intensity fires had to relocate to the edge of the firescar. Moreover, Fairy-wrens in areas affected by high-intensity fires had fewer breeding attempts and lower nesting success, and hence lower reproductive output, than Fairy-wrens in unburnt areas. This study reveals a mechanism for population decline credible for any grass-dwelling bird: they can modify their ranging behaviour in response to a shifting distribution of unburnt vegetation in space and time but there will be species-specific thresholds of scale beyond which their dispersal abilities are limiting and aspects of their biology are compromised. Specifically, an increase in the frequency of extensive, high-intensity fires is the key regime shift that will most strongly affect habitat connectivity through space and time.
The Purple-crowned Fairy-wren has an extremely intermittently distributed habitat, and fire may markedly reduce the area of habitat and increase fragmentation (Skroblin et al. 2012). In this study in the Kimberley region, suitable riparian vegetation lined only 11% of the 2700 km of surveyed waterways, and was distributed as 342 discrete patches. The Purple-crowned Fairy-wren was absent from stretches of riparian vegetation that was degraded by cattle trampling, fire and invasion of weeds (Skroblin and Legge 2010, 2012). Conversely, exclusion of fire and cattle from riparian areas on one Kimberley property led to a marked increase in population of Fairy-wrens over 6 years (Legge et al. 2011). The regional distributional survey also showed that Purple-crowned Fairy-wrens were absent from small, isolated patches of habitat, suggesting an upper limit to the distance for inter-patch dispersal. This distributional information, supported by genetic analysis (Skroblin et al. 2012), indicated that compromising habitat connectivity (for example, by damaging riparian vegetation with repeated intense fires) will rapidly accelerate the processes of population isolation and local extinctions (Skroblin and Legge 2013).
Granivorous birds
Approximately one-third of seed-eating bird species of the tropical savannas have declined since European settlement (Franklin 1999; Franklin et al. 2005). Grazing by introduced herbivores and changed fire patterns are implicated, because both of these factors affect the productivity, species composition and seed yields of the grass layer (Mott and Andrew 1985; Crowley and Garnett 1999). Population declines have been most marked in areas of low and variable rainfall, and in landscapes that are topographically simple (Franklin et al. 2005); both features are associated with higher grazing pressure and more marked changes in fire pattern.
Studies, particularly of the Gouldian Finch, have shown that the persistence of granivorous birds in a landscape depends upon the intricate spatial and temporal patterning of fire (Dostine et al. 2001; Dostine and Franklin 2002; Woinarski et al. 2005; Lewis 2007). During the wet season, grass-seed specialists rely on a sequence of perennial grasses as their seed successively becomes available, with variation in this availability related to previous fire and also moderated by highly localised early wet-season rainfall events. During the dry season they switch to the massive seed store produced by annuals at the end of the wet season, which lies dormant on the ground until the next rains. Interruptions to any part of that sequence as a result of extensive fire (or grazing) can spell starvation (Dostine and Franklin 2002). In the central Kimberley, frequent extensive fires have been shown to lead to declines in the physical condition of Gouldian Finches during the early to mid-wet season (presumed to be a result of resource depletion) compared with other sympatric species of finch and with Gouldian Finches in adjacent areas with less-frequent fires. Moreover, intensive fine-scale fire management over 6 years led to a significant improvement in the condition of Gouldian Finches at this time of year (Maute 2011; S. Legge, unpubl. data). Frequent extensive fire was reducing the seed available from key species of grass that Gouldian Finches relied on during this period, including some species of spinifex that require several years after fire to reach maturity (Craig 1992; Armstrong and Legge 2011).
Studies on the Golden-shouldered Parrot on Cape York Peninsula have illustrated how pastoralism has precipitated a cascading series of effects on savanna ecosystems, with consequential effects for granivorous species. Cattle preferentially graze a suite of grass species, including Cockatoo Grass (Alloteropsis semialata), which is a key food source for the Parrot; grazing reduces seed yields in this grass (Crowley and Garnett 2001). Most significantly, pastoralism is associated with a key shift in fire patterns. In this case, the incidence of small-scale, high-intensity fires in the late dry season to early wet season (‘storm burns’) has decreased in some parts of Cape York Peninsula, owing to a combination of fuel reduction caused by livestock and fire suppression by pastoralists. This has led to woodland invasion of small pockets of grassland (Crowley et al. 2009), which alters habitat quality for an array of bird species. The thickening woodlands probably facilitate predation by butcherbirds and other predatory species on nesting and feeding Golden-shouldered Parrots (Garnett et al. 2010).
A study of the Partridge Pigeon in Kakadu National Park, using radio-telemetry, dietary analysis, assessment of breeding success and manipulation of fire and grazing regimes (Fraser et al. 2003), showed a complex response to fire. This species nests (in the early dry season) and forages (for fallen seeds) on the ground, and is thus much affected by individual fires and fire regimes. Fire has three main effects: (1) it can destroy eggs, young and possibly brooding adults, because nests are in highly flammable sites (often under grass tussocks); (2) it can expose birds to more predation and (3) it can increase foraging efficiency, with birds able to walk more easily and being able to detect fallen seed more readily on burnt ground. So, fire is both advantageous and disadvantageous, and an optimal regime is one that produces a fine-scale mosaic of burnt and unburnt patches at the scale of individual home-ranges. Broad-scale decline of Partridge Pigeons indicates that the current fire regimes (probably coupled with impacts of pastoralism) are suboptimal for this species.
Hollow-nesting birds
Taylor et al. (2003) reported that 18% of forest-dependent bird species in monsoonal Australia were obligate or frequent hollow nesters. In this environment, the creation and destruction of hollows is strongly linked to fire. Fire damages the trunk and roots of trees, and such damage allows ingress to fungi, microorganisms and termites, which then cause extensive hollowing of the tree. Over half of savanna trees are ‘piped’ (hollowed out) by termites (Werner and Prior 2007), providing numerous hollows useful to nesting birds. Birds that nest in large hollows require old trees. In areas exposed to frequent, intense fires, there are few such trees because their hollowness makes them more vulnerable to mortality in any single fire event (Braithwaite and Estbergs 1985; Lonsdale and Braithwaite 1991; Bowman and Panton 1995). Research on Cape York Peninsula found strong competition between large parrots for large hollows (Heinsohn et al. 2003) and reported that fire was the primary cause of loss of nesting hollows (Murphy and Legge 2007). In this study, the key fire pattern that was damaging was frequent fires, especially of fires of high intensity.
The Gouldian Finch is affected by fire not only through effects on food resources but also through effects on availability of nesting hollows, at least in some areas. In the eastern Kimberley, Brazill-Boast et al. (2010, 2011a, 2011b) showed that suitable hollows, even the fairly small hollows used by finches, were likely to be a limiting resource. They considered that the abundance of hollows (and hence the suitability of an area as breeding habitat) was influenced by fire regimes, with more frequent fire likely to lead to fewer hollows.
Frugivorous birds and birds associated with shrubby understoreys
In savanna woodlands, most birds are associated with one of the two dominant structural components of the vegetation, the tree overstorey or the grass understorey. Although some assemblage studies have indicated an association of some bird species (e.g. Northern Fantail and White-throated Honeyeater) with the increased cover of woody shrubs promoted by fire exclusion, there have been no targeted autecological studies of such species. Price (1998) considered the use of rainforest patches and the surrounding tropical savanna matrix by some frugivorous birds (notably Great Bowerbird; Yellow Oriole, Oriolus flavocinctus; Australasian Figbird, Sphecotheres vieilloti), and concluded that the savanna provided important fruit resources for frugivores otherwise mostly associated with rainforests, at times when few rainforest fruits were available (mostly in the dry season). Hence, a regime of frequent or high-intensity fires in the savanna, which will lead to diminution of the production of fleshy fruits (Vigilante and Bowman 2004a), is likely to have spill-over consequences for the abutting rainforest bird assemblages, even if the fires do not directly damage the rainforests.
Contrasts and commonalities
Tropical savannas on other continents
Tropical savannas are most extensive in Australia, India, Central and South America and Africa. Declines in tropical savanna birds have been recorded from all continents (Butchart et al. 2004) but elsewhere changes wrought by habitat loss are usually more immediate and pressing issues than shifts in fire patterns (Woinarski et al. 2007a).
The most notable long-term research on fire and biodiversity in savanna areas has been conducted in South Africa’s Kruger National Park, where a series of operational approaches have been integrated with research programs to investigate the impacts of fire on vegetation and fauna (van Wilgen et al. 2007; van Wilgen and Biggs 2011). These studies have focussed more on large mammals than birds, but Mills (2004) and van Wilgen et al. (2007) reported that bird assemblages were generally resilient to a single fire event, with only fairly small changes in the abundance of some species (especially ground-dwelling and granivorous species) and no changes in species richness or community composition. Other studies in Africa and South America have focussed mainly on short-term or small-scale responses of bird assemblages to fire (e.g. Barnard 1987; Cavalcanti and Alves 1997; Cintra and Sanaiotti 2005; Gregory et al. 2010; and see Parr and Chown 2003). These studies have illustrated that some bird species favour fire-disturbed areas, at least in the short-term. This evidence has been used to support the claim that current trends towards fire suppression (and suppression of other disturbance factors like small-scale grazing of stock) in these regions may be a key threat for some species, including Neotropical and Nearctic migrants (Gregory et al. 2010). Note that this conservation management concern about current trends for fire suppression in parts of the African and American savannas differs from the shifts in fire patterns of most widespread concern in the Australian tropical savannas (i.e. more-frequent, extensive fire).
Some autecological studies in Africa and South America have more congruence with studies on Australian savanna birds, identifying the importance of long-unburnt vegetation for grass-dependent bird species (e.g. Malan 1998; Jansen et al. 1999) and potential mechanisms for population decline. On the southern edge of the tropics in Argentina, extensive and frequent savanna fires affected the settlement rates, and therefore the breeding success, of female Strange-tailed Tyrants (Alectrurus risora), which prefer unburnt savanna vegetation as nesting sites (Di Giacomo et al. 2011), whereas fire suppression may lead to vegetation thickening and loss of preferred nesting habitat. The study thus mirrors the case of the Red-backed Fairy-wren in Australian savannas (Murphy et al. 2010), and reiterates that nuances in the spatio-temporal patterning of savanna fires may interact with the ecologies of species to affect population trajectories. In another comparable example, Parker and Willis (1997) documented decline of three small grassland flycatchers (Rufous-sided Pygmy-Tyrant, Euscarthmus rufomarginatus; Bearded Tachuri, Polystictus pectoralis; Sharp-tailed Tyrant, Culicivora caudacuta) in cerrado vegetation in tropical South America. Their changing status was associated with changed fire regimes, with all species requiring large savanna areas with patches of varying ages since fire. These species were disadvantaged on ranches that were burnt annually and in reserves where a low fire frequency resulted in transformation of grasslands to shrublands.
One major intercontinental contrast is in the response of tropical savannas to exclusion of fire. In northern Australia, vegetation (and fauna) responses are far more muted and gradual (Woinarski et al. 2004a; Andersen et al. 2006, 2007) than is the case in other tropical savannas, such as high-rainfall and high-productivity areas of western Africa, where substantial transformation of savanna to rainforest may occur after 10 or more years of fire exclusion (Hopkins 1983; Furley et al. 1992). This contrast is in part a result of the low soil fertility and more intense dry season in the Australian savannas and, in some cases, to the more recent anthropogenic derivation of savanna environments on other continents. Regardless of the explanation, the outcome is that fire effects in the Australian tropical savannas may not be as crisply defined and conspicuous as those elsewhere.
Birds and fire elsewhere in Australia
Most Australian fires occur in the tropical savannas. However, the responses of birds to fire are more sharply defined in some other Australian environments. There are several reasons for this: in part it is a result of a longer history of research in other regions; because in most other Australian environments it is fairly straightforward to study a more marked contrast in fire history; because fires in most other environments are of substantially higher intensity and, hence, effects; and because fires in most other environments occur at a substantially lower frequency, allowing for a more clearly defined successional process. Furthermore, in those parts of Australia with more people and economic assets in the landscape than is the case for the tropical savannas, fire may be a higher profile and more contentious issue, and hence there is a demand for information on fire. There are other regional contrasts: the environmental consequences of fire regimes in most other areas of Australia are more affected by habitat fragmentation, a longer period following the cessation of Indigenous fire management, and the loss of knowledge of that management. These latter issues have stimulated considerable speculation and historical interpretation about the appearance and ecological patterning of, particularly temperate, Australia before European settlement, about whether or not it is desirable or practical to attempt to return to such an ecological state, and the role of changed fire management in this transition (e.g. Gammage 2011).
The known impacts of fire on Australian birds have been reviewed by Woinarski (1999) and Woinarski and Recher (1997). Those reviews concluded that bird species associated with older seral stages or requiring old hollow-bearing trees are generally declining under current fire regimes. This pattern is most marked in coastal heathlands, mallee woodlands and shrublands, montane eucalypt forests and hummock grasslands.
Fire was recognised as a current threat for 47 of the 236 Australian threatened bird taxa listed by Garnett and Crowley (2000), surpassed only by land clearance (60 taxa) and grazing by livestock (65 taxa). Bird species associated with long unburnt vegetation include Malleefowl (Leipoa ocellata), the abundance of which generally increases in mallee woodlands to at least 40–60 years after fire (Benshemesh 1990; Watson et al. 2012a), Noisy Scrub-bird (Atrichornis clamosus) (Burbidge 2003), Eastern Bristlebird (Dasyornis brachypterus) (Bain et al. 2008) and Black-eared Miner (Manorina melanotis) (Clarke et al. 2005). Ecological traits associated with such preference include limited dispersal ability (rendering subpopulations particularly susceptible to extirpation in a single extensive fire, with such areas then unlikely to be recolonised), a propensity for foraging in dense leaf litter, and a propensity for foraging on the ground or in low shrubs. Many of the bird species associated with older successional stages are the enduring remnants of a more ancient avifauna, often with low reproductive potential but fairly long-lived; such species are living to a slower rhythm than that this continent currently offers.
Across Australia, an unusually high proportion of birds nest only in tree-hollows (Gibbons and Lindenmayer 2002). This requirement hinges closely on fire and its management. Eucalypts generally have to be several hundred years old before they can form large hollows (Mawson and Long 1994). Across much of temperate, arid and semi-arid Australia, hollows are a limiting resource for many bird species (and mammals and other fauna), with their abundance declining as a result of changed fire regimes, clearing and forestry activities.
In general, the strong pattern between many bird species and time since fire is mediated through the impacts of fire on plant species composition and vegetation structure: birds are passengers on the vegetation succession train. However, to conserve bird species that are fire-sensitive, it is not sufficient to manage simply for vegetation age (Clarke 2008) because spatial characteristics are also important (Watson et al. 2012b). Furthermore, even if vegetation was of suitable age for any particular species of bird, their presence or abundance may be dictated more, or influenced to some degree by, the abundance of introduced predators. For example, in an area in which introduced predators were present, Eastern Bristlebirds showed a strong association with older vegetation, which provided more protection from predation, whereas in areas where predators were effectively controlled birds also used younger vegetation (Lindenmayer et al. 2009).
Fire regimes may influence the location of boundaries between vegetation types. One such example is the dynamic edge of rainforest and wet sclerophyll (eucalypt) forests in the Wet Tropics of north-eastern Queensland. Over recent decades, fire has largely been excluded from this system, and in response the rainforest has expanded and the narrow band of wet sclerophyll forest has retreated. This result has disadvantaged species associated with the small area of wet sclerophyll forest in this region (including Eastern Yellow Robin, Eopsaltria australis, and habitat-specialist threatened mammals, such as the Northern Bettong, Bettongia tropica, and Yellow-bellied Glider, Petaurus australis, Winter et al. 2004). This outcome is considered to be a direct consequence of loss of the previous Indigenous management of regular fire that checked rainforest expansion (Harrington and Sanderson 1994; Russell-Smith and Stanton 2002).
To what extent are current fire regimes in the tropical savannas worse for birds than the current fire regimes elsewhere in Australia? This is a tricky question to answer. We suspect that there are probably more examples in temperate Australia where managers specifically attempt to apply fire regimes designed to enhance habitat suitability for birds. Such examples include fire suppression or exclusion, or small-scale burning to enhance heterogeneity and resource production, for the Orange-bellied Parrot (Neophema chrysogaster), Ground Parrot (Pezoporus wallicus), Noisy Scrub-bird, Western Whipbird (Psophodes nigrogularis) and Eastern Bristlebird (Burbidge 2003; Garnett et al. 2011). There are few comparable examples of such purposeful fire management in the tropical savannas. However, we suspect that generally there has been far more divergence between pre-European and post-European fire management in temperate, arid and semi-arid Australia than in the tropical savannas, so the extent to which the pre-European environmental equilibrium has been upset, and the consequences for fire-sensitive bird species, is probably substantially greater in the rest of Australia than in the tropical savannas. Also, the long history of frequent fire in the tropical savannas may have sharply winnowed its bird fauna, long ago removing those species with most finicky fire requirements or dependence upon long-unburnt vegetation; in contrast, some such species have persisted (albeit mostly in now relictual habitats) elsewhere in Australia. Furthermore, across landscapes that are more populous and rich in economic assets than the tropical savannas, fire is managed mainly for reasons other than biodiversity conservation. Across large scales, such management will increasingly render habitats unsuitable for an increasing proportion of bird species (Clarke 2008).
Fire and other fauna in Australian tropical savannas
Much as for birds, no pervasive, clearly etched response of other faunal groups to fire in the Australian tropical savannas has been described, nor clear direction for the management of fire for the conservation of this fauna. As with birds, the orthodox view is that, with a few odd exceptions, other faunal groups are resilient to fire, with most support for this opinion deriving from the relatively subdued variation in responses of many animal species to the landscape-scale Kapalga fire experiment (Andersen et al. 2003a, 2005).
Some of the studies of bird assemblages considered above also considered responses of other faunal groups within the same design, at the same site and at the same time, substantially facilitating response comparisons among faunal groups. In some cases, small experimental plot-sizes made it far more likely that responses will be meaningful and evident for groups other than birds.
Ant assemblages are perhaps the most studied, in part a reflection of their extraordinary diversity, abundance and ecological dominance in the Australian monsoon tropics. Higher ant species richness in more-frequently burnt savanna sites has been reported consistently across studies in northern Australia, and matches typically higher ant species richness in savanna than in monsoon rainforest sites in this landscape, in marked contrast to tropical environments on other continents (Andersen 1991; Andersen et al. 2006, 2007). Broadly similar results and interpretations were described for beetle and grasshopper assemblages (Orgeas and Andersen 2001; Andersen et al. 2003a). Nonetheless, although there is a need to consider assemblage traits in such speciose groups, there is also a case for particular attention being paid to individual species. One marked example is that of Leichhardt’s Grasshopper (Petasida ephippigera), a spectacular and fairly immobile species restricted to sandstone escarpments of the Top End, and dependent on a narrow range of plant species (Pityrodia spp.) as food plants. Pityrodia is highly fire-sensitive, as is the Grasshopper, which cannot flee fire and cannot readily disperse across wide landscapes to recolonise previously burnt patches. Hence repeated fires across its sandstone heathland environments result in a spatial series of extirpations, with these local losses aggregating to become landscape-wide losses as fire frequency and extent increase (Barrow 2009).
Similar concerns have been expressed for land-snails, another group with low dispersal ability and narrow endemicity. The fire-prone and seasonally parched savannas are not ideal habitat for this group but they occur discontinuously across the region in disjunct refugia, such as rainforest patches. At one site near Katherine, Braby et al. (2011) chronicled fire-driven savanna expansion to the detriment of narrowly endemic land-snail species and their habitat, with extinction of these species a likely consequence of continuation of the current fire regime.
The responses of reptiles to fire regimes are broadly comparable to those of birds but with somewhat more marked assemblage-scale impacts. This is probably because of the small home-ranges of most species and a sensitivity of many reptiles to the extent and type of ground cover that may be substantially influenced by fire (Trainor and Woinarski 1994; Woinarski et al. 1999b, 2004a; Corbett et al. 2003). Reptile assemblages have been shown to be not greatly affected by a single low-intensity fire (Nicholson et al. 2006) but show more marked responses to a single high-intensity fire (Legge et al. 2008). As with other faunal groups, some reptile species are advantaged by regimes of frequent or high-intensity fires and some species advantaged by reduced fire frequencies and intensities. Again, as with other faunal groups, there may be more insight in considering the responses of individual species rather than the chaotic and ostensibly compensating responses within assemblages. The iconic Frilled Lizard (Chlamydosaurus kingii) is advantaged by some fire because dense grass inhibits its foraging but it suffers elevated rates of mortality in high-intensity fires, such that population viability requires a regime of patchy and fairly low-intensity fires (Griffiths and Christian 1996; Brook and Griffiths 2004), a response pattern that is broadly analogous to that of the Partridge Pigeon. Few reptiles in northern Australia are threatened, but the Yellow-snouted Gecko (Lucasium occultum) is considered vulnerable, with its status largely linked to fires that are too frequent or intense and too regularly destroy the leaf-litter layer in which it shelters and forages (Woinarski et al. 2007b).
Responses of mammals to fire in the Australian tropical savannas are more well-defined than for birds, and there is more evidence of detriment in the current fire regimes (McKenzie 1981; Woinarski et al. 2001, 2010; 2011). This disparity probably reflects the more-limited dispersal ability of most terrestrial mammals compared with birds. Mammals may also be more disadvantaged because of their greater dependence upon shelter resources that may be incinerated or diminished by fire (notably fallen logs, tree-hollows and dense ground-layer vegetation), the likelihood that they may be more susceptible than birds to more efficient post-fire hunting by feral predators, the importance of fleshy fruits in the diet of a higher proportion of mammals than birds, and possibly by the greater handicap to locomotion of mammals than birds of an unbroken dense grass layer.
The sharper response of mammals to fire compared with birds has been shown by coincident sampling of birds and mammals after single fires (Legge et al. 2008) and across multiple fire events (Woinarski et al. 2012). Detailed autecological studies of some species of mammal has allowed for population viability modelling that predicts the most marked declines (to at least local extinction) for regimes of frequent or high-intensity fires and least decline in regimes characterised by less fire and smaller, patchier fires (Pardon et al. 2003; Firth et al. 2010). Mammals with a preference for shrubby understoreys, notably the partly frugivorous Common Brushtail Possum (Trichosurus vulpecula) and Black-footed Tree-rat (Mesembriomys gouldii), are far more abundant in infrequently burnt areas (Kerle 1985; Friend and Taylor 1985; Friend 1987; Woinarski et al. 2004a), in accord with the increased abundance, cover and fruit productivity of understorey shrubs in areas subjected to less-frequent and intense fires.
In other cases with some parallels to the responses of bird species, some mammal species, notably the Delicate Mouse (Pseudomys delicatulus) are more abundant in more frequently and intensively burnt tropical savanna landscapes (Corbett et al. 2003), and some mammal species, particularly macropods, may be attracted to recently burnt landscapes, to feed on green pick (Telfer and Garde 2006; Murphy and Bowman 2007).
Conclusions
We sought to distil the available research so as to derive conclusions about the responses of birds to fire in this region. This task has proven challenging, for several reasons. The body of most relevant research is limited, largely opportunistic and not strategically developed. The effect of fire in this environment is fairly subtle and incremental, most likely taking many years or decades to reveal ecologically profound consequences. The mobility and plasticity of birds renders it harder to discern ecologically significant responses to fire than is the case for plants. There is no coherent intellectual context within which to frame the elements of a compelling ecological conclusion, and any such framework may be coloured by parables about Indigenous land management, and a widely held dogma that most of the biodiversity components in the region are resilient to fire.
In our introduction, we posed four questions:
-
Is the current fire regime in Australia’s tropical savannas disadvantageous to birds?
-
What is an optimal fire regime for this bird fauna?
-
Is that regime achievable?
-
What are the critical knowledge gaps that inhibit such better fire management?
We answer the first question here and the remaining three questions in the following sections.
Broadly, savanna bird assemblages appear to be somewhat resilient to changes in fire patterns, in that shifts in fire pattern produce some changes in the abundance of some bird species but not major changes in species richness or wholesale restructuring of the community. However, that conclusion is severely tempered by caveats about time-frames and does not well accommodate the end-point of ongoing inexorable incremental decline. There is enough evidence from the existing research to conclude that many species of birds (and other vertebrates and plants) are declining across substantial parts of this region and that the current fire regimes are contributing to that decline and in some cases are the major driver of it (Table 1).
There is some ecological commonality in the groups of bird species that are detrimentally fire-affected and of the largely overlapping set of birds now listed as threatened. The set mostly comprises grass-dwellers, frugivores, riparian specialists, obligate hollow-nesters and granivores (especially those mostly consuming grass seeds). Most of these species have specific habitat requirements that are subverted by prevailing fire regimes. These include combinations of:
-
unburnt (in some cases, long-unburnt) vegetation, at the scale of a home-range or within dispersal distance;
-
fire-sensitive vegetation appropriately distributed and retained at the scale of subpopulations or dispersal capacities, or both (for riparian specialists, rainforest frugivores) or
-
a variety of grasses, in a spectrum of ages, distributed across the savanna matrix (wide-ranging granivorous species).
For species with these requirements, a syndrome of frequent, extensive or high-intensity fires (the prevalent contemporary fire pattern) leads to population declines. In at least some cases, this fire regime is the major factor responsible for current declines; in many other cases, its impact is now linked almost inextricably with changes associated with the prevailing land-use of pastoralism (or the impacts of feral animals and weeds; Petty and Werner 2010) to such an extent that it is difficult to tease apart the relative effects of these factors. As indicated in the following section, we also conclude that there is sufficient evidence now available to establish fire-management programs that redress the threat and can deliver better conservation outcomes.
Recommendations for management
There are many tenures, managers and management objectives in the Australian tropical savannas, which renders complex the regional coordination of management of broad-scale factors such as fire. Our recommendations here are primarily aimed at those managers responsible for delivering biodiversity conservation outcomes.
Much current fire management is failing. We suspect that in part this is because it is untargeted, because it emphasises practice rather than outcomes, because it is geared negatively (i.e. to apply fire to limit risks of worse fire), because there is so little accountability associated with getting it wrong, and because the case for fire-driven erosion of conservation values has not been sufficiently well made. In framing the task of fire management, we recommend clear objectives and measurable outcomes (Parr et al. 2009). For managers of lands that espouse to conserve biodiversity, the most relevant objective for fire management is to maintain viable populations of all species but particularly those species and environments that are disadvantaged by the current pervasive fire regime. In most cases, such species and environments can be most readily retained by decreasing fire frequency, extent and intensity.
Operational targets for prescribed burning should have an ecological justification, and the spatial outcomes of fire management should be measured with reference to metrics that reflect that underlying ecology. Recognising that such recommendations rest on an incomplete knowledge base, these fire-pattern targets should be partnered with well-designed monitoring programs (Parr and Andersen 2006; Legge et al. 2011).
The age-profile and distribution of unburnt vegetation and the protection of fire-sensitive vegetation (rainforest, riparian) will be critical in determining whether fire-sensitive bird species persist in the landscape. In most cases, we can make only educated guesses at the appropriate scale and age-profile (the heterogeneity in time and space) to aim for, and we lack information on the thresholds (of fire scale, intensity, frequency, heterogeneity, in a single year and over many years) beyond which population viability is compromised. Over time, our understanding of thresholds will improve and we may be able to identify more precise spatial fire-pattern goals (see Biggs and Rogers 2003 for an example from Kruger National Park). We recognise that our proposed targets may be challenging. However, in the absence of targets set to deliver biodiversity benefits, fire management is likely to be based instead on targets unrelated to, or antithetical to, biodiversity conservation, or it may continue to be based on least-cost operational or current practice, or it may simply be purposeless and anarchic. The main challenge we anticipate is to insinuate these targets within a current management setting that is firmly anchored in the premise that a high proportion of the land should be burnt every year in a controlled manner in order to stave off uncontrolled and more destructive fires. A targeted biodiversity fire management objective is not necessarily incompatible with this established mode but their integration will require more skilful, strategic and purposeful spatial planning (e.g. Andersen et al. 2003b).
The targets that we propose are:
-
at least 25% of the savanna landscape (at property or regional scale) is at least 3 years unburnt and, in some contexts, such as rocky landscapes or lower rainfall areas, the target proportion should be much higher;
-
at least 5% of the savanna landscape is at least 10 years unburnt (and again, in some contexts this proportion should be much higher);
-
the average size of burnt patches is <1 km2 (as recommended also by Radford (2012), based on the ecology and conservation management of mammals);
-
within tropical eucalypt forest, the basal area of understorey shrubs is >1 m2 ha–1;
-
the boundaries of rainforest patches and other fire-sensitive non-savanna vegetation types are increasing or stable and
-
populations of selected hollow-nesting, ground-nesting and granivorous birds are increasing or stable.
These are measurable and achievable and their realisation is likely to result in conservation benefits to birds in this region. These management prescriptions rest on the premise that conservation managers and other landholders have access to, and are adept at interpreting, archives of satellite-based fire mapping, and that this library is recognised to be a key guide to pre-emptive burning and suppression. This is increasingly the case across most of the tropical savannas and fire management is becoming increasingly evidence-based and sophisticated (Russell-Smith et al. 2009; Legge et al. 2013) and there is increasing capability and precision in the quantification and interpretation of fire metrics (e.g. Price et al. 2005; Legge et al. 2013).
As shown by success with the broad-scale Kimberley EcoFire program (Legge et al. 2011, 2013) and the West Arnhem Land Fire Abatement program (Whitehead et al. 2009), a strategic prescribed burning program can be achieved, with intensive management effort, with its success dependent also on education and engagement programs to reduce haphazard ignition behaviour from all sectors of the community. Furthermore, management is not simply about the on-ground practice of fire ignition or suppression; it needs also a sympathetic and appropriate policy setting, adequate resourcing, a system of accountability and regulation, broad community support and involvement, a linked monitoring program, and mechanisms to facilitate cross-tenure collaboration.
Any consideration aimed at improving fire management in the Australian tropical savannas must also encompass the need to control invasive pasture grasses. In partnership with fire, these have the potential to rapidly transform and degrade all Australian tropical savanna ecosystems. Their management is complex and challenging but will require regulation to constrain any deliberate spread, biosecurity protocols in conservation reserves, research aimed at more effective control techniques, and sustained and intensive management of existing stands in areas of high conservation value.
Key priorities for further research
In this review, we have noted particularly and frequently the limitation of existing knowledge. The major gaps in our collective knowledge that inhibit better management are described below.
Are there ideal or threshold landscape mosaics, relating to metrics about patch-size, inter-patch limits and vegetation age distributions?
Fire-imposed landscape variability is desirable but it is not yet possible to define an ideal level and scale of patchiness, or to indicate whether there are any thresholds in such heterogeneity beyond which local populations are no longer viable. This may be best addressed with focussed studies on how the behaviour, breeding success, movements and survival of individuals of exemplar species are affected by different fire-pattern attributes (e.g. Murphy et al. 2010; Di Giacomo et al. 2011) and extrapolative modelling of results from such studies.
What are the impacts of prescribed (preventative) burning?
In order to use prescribed (preventative) fire sensibly, it needs to be viewed as part of a continuum of potential fire patterns, and to examine more systematically its positive and negative impacts. Notwithstanding the perception of its advocates, prescribed burning is unlikely to be benign: many birds nest on the ground and in the grass layer at the end of the wet season and early dry season, the time of most prescribed burning. The limited post-fire regrowth on freshly burnt areas is highly attractive to herbivores, and the interaction of fire and grazing can lead to a degraded landscape. Because most prescribed burning occurs in the early dry season, the physical habitat changes produced persist for many months before the next growing season – this includes substantially reduced cover, reduced resources, and exposing the soil surface to scorching temperatures for a prolonged period.
How do interactions between fire and other potential threats operate, and which interactions are of most concern? How large is the effect of fire on birds relative to other threats?
The effects of fire are context-dependent. We need to determine whether, when and why the coincidence of other threatening processes cause additive, interactive or multiplicative consequences. Management of fire will be enhanced when these interactions are understood and factored into management practice and objectives.
We have concluded that fire is a major factor affecting threatened birds in the tropical savannas but there is currently little quantitative evidence on which we can assess its effect relative to other factors. Current increases in the extent of private conservation reserves (mostly as properties formerly used for pastoralism) will allow more scope for manipulative experimental designs that can tease apart the relative impacts of different threatening factors and, hence, more precisely determine priorities of management responses.
Can we craft and impose tailored solutions for fire-sensitive bird species?
Currently, there are explicitly tailored fire-management guidelines for few species in the Australian tropical savannas. As a consequence, with some notable exceptions (e.g. Golden-shouldered Parrot; Crowley et al. 2004), consideration of the responses of bird species to fire regimes is typically not a component of fire-management guidelines or, if it is included, it is addressed only through very generic guidelines. This review has shown that the few detailed autecological studies of individual species in this region have provided significant insight from which management prescriptions could and should be readily derived. For other threatened species, more strategic research is required, from which tailored fire-management guidelines can be developed and mandated for use in relevant broader fire-management planning and practice.
Are patches that are long-unburnt explosive?
Over at least several years, fuel loads increase with time since fire. As such, many managers view patches of longer unburnt vegetation with discomfort, concerned that they will wick fires of heightened intensity. However, such patches are important for the conservation of savanna birds. This disparity of perceptions needs to be addressed or it will stymie enhanced fire management. We advocate research that seeks to assess whether there is a post-fire age-threshold beyond which fuel accumulation or combustibility no longer increases and to identify management options for protecting older patches from combustion.
What are the most practical mechanisms to reduce fire extent and frequency?
Current dogma is that fire is needed to reduce fire. This assertion may be valid but it comes with the consequence that much land is burnt each year in prescribed burning campaigns. It may be instructive to consider the extent to which prescribed burning can be reduced (e.g. through delivery of more strategic fire-breaks) without compromising the risks of high-intensity fire. Such cost-benefit analysis should include options for actions that are not currently conventional practice in the region (notably including more strategic delivery of fire suppression).
How can we best link objectives with practice?
Fire management consumes much of the tightly constrained budget of conservation managers (and managers of other land types) in the Australian tropical savannas. However, there has been little assessment of the cost efficiency of different operational options for achieving specific management objectives. In other words, assuming we know what we want to achieve in terms of fire pattern, what is the most practical and cost-effective way to achieve it? For example, a fire-pattern analysis of different operational approaches in Kruger National Park was valuable because it showed not only that managers can influence some aspects of a fire pattern (including the intensity, extent and seasonality of fire) more easily than others (the overall area burnt by the end of each year), but also which operational paradigm achieved these changes most effectively (van Wilgen et al. 2004).
How can we best monitor progress and management impact?
Any fire-management program should be complemented with a monitoring program that measures progress towards targets. There are current monitoring programs for some regional fire-management programs (e.g. Legge et al. 2013) but these typically assess fire indices rather than biodiversity outcomes (which are often assumed). In part, this reflects the ease and immediacy of quantifying fire indices, whereas biodiversity measures may be more difficult and costly to assess and may take longer to show meaningful responses. It is critical to design systematic monitoring programs that include clear assessments of the links between management, fire indices and biodiversity responses, in a manner that allows for regular refinement of management practice.
How can we optimise trade-offs between fire management for biodiversity, pastoralism, cultural needs and other factors?
Much of Australia’s tropical savannas is managed primarily for purposes other than biodiversity conservation but all lands in this largely natural landscape contribute to regional-scale conservation outcomes (Woinarski et al. 2013). In some cases, the optimal fire-management prescriptions for biodiversity conservation may deliver suboptimal results for pastoral production or other objectives. Some cost-benefit and optimisation analyses are needed to establish acceptable trade-offs, to better tailor fire-management guidelines that achieve reasonable biodiversity conservation outcomes on lands devoted primarily to pastoralism or other land-use objectives, and to price stewardship payments for landholders to bridge the divide between fire management for potentially competing outcomes.
We note that the delivery of this set of research priorities should lead to considerable refinement in the objectives and operation of fire management in this region. However, our conclusion is that there is already sufficient evidence available to show that the current prevalent fire regime is detrimental to biodiversity in Australia’s tropical savannas. This evidence base is sufficient to plan now for improved fire management, with such improvement mainly requiring a reduction in fire frequency and extent across most savanna landscapes.
Acknowledgements
We thank Kate Buchanan and Leo Joseph for inviting this contribution. For this review in particular we acknowledge Ian Rowley, whose insightful research allows us to see the world from a bird’s perspective, and for his interest in northern Australia. We thank many colleagues with whom we have worked including Alan Andersen, Shaun Ansell, David Bowman, Andrew Cockburn, Garry Cook, Gay Crowley, Andrew Edwards, Ron Firth, Alaric Fisher, Atticus Fleming, Don Franklin, Stephen Garnett, Tony Griffiths, Joanne Heathcote, Chris Johnson, John Kanowski, Alex Kutt, Kim Maute, Steve Murphy, Jeremy Russell-Smith, Anja Skroblin, Peter Whitehead and Dick Williams. Our research has been supported by the Tropical Savannas Cooperative Research Centre, National Environmental Research Program North Australian Hub, Australian Wildlife Conservancy, Australian Research Council, the Western Australian government’s Kimberley Science and Conservation Strategy, and Parks Australia. We thank Andrew Edwards for the provision of data in Table 2. We are most grateful for the particularly helpful reviews of Allan Burbidge, Hugh Ford and Ian Radford, and for the editorial advice and patience of Mike Lawes, Chris Zalewski and Kate Buchanan.
References
Andersen, A. N. (1991). Responses of ground-foraging ant communities to three experimental fire regimes in a savanna forest of tropical Australia. Biotropica 23, 575–585.| Responses of ground-foraging ant communities to three experimental fire regimes in a savanna forest of tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Orgeas, J., Blanche, R. D., and Lowe, L. M. (2003a). Terrestrial insects. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 107–125. (Springer-Verlag: New York.)
Andersen, A. N., Cook, G. D., and Williams, R. J. (2003b). Synthesis: fire ecology and adaptive conservation management. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 153–164. (Springer-Verlag: New York.)
Andersen, A. N., Cook, G. D., Corbett, L. K., Douglas, M. M., Eager, R. W., Russell-Smith, J., Setterfield, S. A., Williams, R. J., and Woinarski, J. C. Z. (2005). Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology 30, 155–167.
| Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., Hertog, T., and Woinarski, J. C. Z. (2006). Long-term fire exclusion and ant community structure in an Australian tropical savanna: congruence with vegetation succession. Journal of Biogeography 33, 823–832.
| Long-term fire exclusion and ant community structure in an Australian tropical savanna: congruence with vegetation succession.Crossref | GoogleScholarGoogle Scholar |
Andersen, A. N., van Ingen, L. T., and Campos, R. I. (2007). Contrasting rainforest and savanna ant faunas in monsoonal northern Australia: a rainforest patch in a tropical savanna landscape. Australian Journal of Zoology 55, 363–369.
| Contrasting rainforest and savanna ant faunas in monsoonal northern Australia: a rainforest patch in a tropical savanna landscape.Crossref | GoogleScholarGoogle Scholar |
Armstrong, G., and Legge, S. (2011). The post-fire response of an obligate seeding Triodia species (Poaceae) in the fire-prone Kimberley, north-west Australia. International Journal of Wildland Fire 20, 974–981.
| The post-fire response of an obligate seeding Triodia species (Poaceae) in the fire-prone Kimberley, north-west Australia.Crossref | GoogleScholarGoogle Scholar |
Atchison, J. (2009). Human impacts on Persoonia falcata. Perspectives on post-contact vegetation change in the Keep River region, Australia, from contemporary vegetation surveys. Vegetation History and Archaeobotany 18, 147–157.
| Human impacts on Persoonia falcata. Perspectives on post-contact vegetation change in the Keep River region, Australia, from contemporary vegetation surveys.Crossref | GoogleScholarGoogle Scholar |
Atchison, J., Head, L., and Fullagar, R. (2005). Archaeobotany of fruit seed processing in a monsoon savanna environment: evidence from the Keep River region, Northern Territory, Australia. Journal of Archaeological Science 32, 167–181.
| Archaeobotany of fruit seed processing in a monsoon savanna environment: evidence from the Keep River region, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Bain, D. W., Baker, J. R., French, K. O., and Whelan, R. J. (2008). Post-fire recovery of Eastern Bristlebirds (Dasyornis brachypterus) is context-dependent. Wildlife Research 35, 44–49.
| Post-fire recovery of Eastern Bristlebirds (Dasyornis brachypterus) is context-dependent.Crossref | GoogleScholarGoogle Scholar |
Banfai, D. S., and Bowman, D. M. J. S. (2006). Forty years of lowland monsoon rainforest expansion in Kakadu National Park, northern Australia. Biological Conservation 131, 553–565.
| Forty years of lowland monsoon rainforest expansion in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Barnard, P. (1987). Foraging site selection by three raptors in relation to grassland burning in a montane habitat. African Journal of Ecology 25, 35–45.
| Foraging site selection by three raptors in relation to grassland burning in a montane habitat.Crossref | GoogleScholarGoogle Scholar |
Barrow, P. (2009). Impact of fire regimes on Leichhardt’s grasshoppers. In ‘Kakadu National Park Landscape Symposia Series 2007–2009. Symposium 3: Fire Management’, 23–24 April 2008, Kakadu National Park, NT. Supervising Scientist Internal Report 566. (Eds S. Atkins and S. Winderlich.) pp. 147–149. (Department of the Environment, Water, Heritage and the Arts, Supervising Scientist Division: Darwin.)
Benshemesh, J. (1990). Management of Malleefowl – with regard to fire. In ‘The Mallee Lands: A Conservation Perspective’. (Eds J. C. Noble, P. J. Joss and G. K. Jones.) pp. 206–211. (CSIRO Australia: Melbourne.)
Biggs, H. C., and Rogers, K. H. (2003). An adaptive system to link science, monitoring and management in practice. In ‘The Kruger Experience: Ecology and Management of Savanna Heterogeneity’. (Eds J. T. du Toit, K. H. Rogers and H. C. Biggs.) pp. 59–80. (Island Press: Washington, DC.)
Bond, W. J., and Keeley, J. E. (2005). Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20, 387–394.
| Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S. (1988). Stability amid turmoil: towards an ecology of north Australian eucalypt forests. Proceedings of the Ecological Society of Australia 15, 149–158.
Bowman, D. M. J. S. (1998). The impact of Aboriginal landscape burning on the Australian biota. New Phytologist 140, 385–410.
| The impact of Aboriginal landscape burning on the Australian biota.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S. (2000). ‘Australian Rainforests: Islands of Green in a Land of Fire’. (Cambridge University Press: Cambridge, UK.)
Bowman, D. M. J. S., and Panton, W. J. (1995). Munmarlary revisited: response of a north Australian Eucalyptus tetrodonta savanna protected from fire for 20 years. Australian Journal of Ecology 20, 526–531.
| Munmarlary revisited: response of a north Australian Eucalyptus tetrodonta savanna protected from fire for 20 years.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S., Price, O., Whitehead, P. J., and Walsh, A. (2001). The ‘wilderness effect’ and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia. Australian Journal of Botany 49, 665–672.
| The ‘wilderness effect’ and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S., Balch, J. K., Artaxo, P., Bond, W. J., Carlson, J. M., Cochrane, M. A., D’Antonio, C. M., DeFries, R. S., Doyle, J. C., Harrison, S. P., Johnston, F. H., Keeley, J. E., Krawchuk, M. A., Kull, C. A., Marston, J. B., Moritz, M. A., Prentice, I. C., Roos, C. I., Scott, A. C., Swetnam, T. W., van der Werf, G. R., and Pyne, S. J. (2009). Fire in the Earth system. Science 324, 481–484.
| Fire in the Earth system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvVGmtb8%3D&md5=239ae8b35535a2046ba7ec84a59a1070CAS |
Bowman, D. M. J. S., Brown, G. K., Braby, M. F., Brown, J. R., Cook, L. G., Crisp, M. D., Ford, F., Haberle, S., Hughes, J., Isagi, Y., Joseph, L., McBride, J., Nelson, G., and Ladiges, P. Y. (2010). Biogeography of the Australian monsoon tropics. Journal of Biogeography 37, 201–216.
| Biogeography of the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Braby, M. F., Willan, R. C., Woinarski, J. C. Z., and Kessner, V. (2011). Land snails associated with limestone outcrops in northern Australia – a potential bioindicator group. Northern Territory Naturalist 23, 2–17.
Bradstock, R. A., Bedward, M., Gill, A. M., and Cohn, J. S. (2005). Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitats and animals. Wildlife Research 32, 409–423.
| Which mosaic? A landscape ecological approach for evaluating interactions between fire regimes, habitats and animals.Crossref | GoogleScholarGoogle Scholar |
Brady, C. J., and Noske, R. A. (2010). Succession in bird and plant communities over a 24-year chronosequence of mine rehabilitation in the Australian monsoon tropics. Restoration Ecology 18, 855–864.
| Succession in bird and plant communities over a 24-year chronosequence of mine rehabilitation in the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Braithwaite, R., and Estbergs, J. (1985). Fire patterns and woody vegetation trends in the Alligator Rivers region of northern Australia. In ‘Ecology and Management of the World’s Savannas’. (Eds J. Tothill and J. Mott.) pp. 359–364. (Australian Academy of Science: Canberra.)
Braithwaite, R. W., and Estbergs, J. A. (1987). Fire-birds of the Top End. Australian Natural History 22, 299–302.
Brazill-Boast, J., Pryke, S. R., and Griffith, S. C. (2010). Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches. Emu 110, 170–177.
| Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches.Crossref | GoogleScholarGoogle Scholar |
Brazill-Boast, J., Pryke, S. R., and Griffith, S. C. (2011a). Selection of breeding habitat by the endangered Gouldian Finch (Erythrura gouldiae) at two spatial scales. Emu 111, 304–311.
| Selection of breeding habitat by the endangered Gouldian Finch (Erythrura gouldiae) at two spatial scales.Crossref | GoogleScholarGoogle Scholar |
Brazill-Boast, J., van Rooij, E., Pryke, S. R., and Griffith, S. C. (2011b). Interference from Long-tailed Finches constrains reproduction in the endangered Gouldian Finch. Journal of Animal Ecology 80, 39–48.
| Interference from Long-tailed Finches constrains reproduction in the endangered Gouldian Finch.Crossref | GoogleScholarGoogle Scholar | 20880021PubMed |
Brook, B. W., and Bowman, D. M. J. S. (2006). Postcards from the past: charting the landscape-scale conversion of tropical Australian savanna to closed forest during the 20th century. Landscape Ecology 21, 1253–1266.
| Postcards from the past: charting the landscape-scale conversion of tropical Australian savanna to closed forest during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Brook, B. W., and Griffiths, A. D. (2004). Frillneck Lizard Chlamydosaurus kingii in northern Australia: determining optimal fire management regimes. In ‘Species Conservation and Management: Case Studies’. (Eds H. R. Akcakaya, M. A. Burgman, O. Kindvall, P. Sjogren-Gulve, J. Hatfield and M. A. McCarthy.) pp. 312–325. (Oxford University Press: Oxford, UK.)
Brooks, K. J., Setterfield, S. A., and Douglas, M. M. (2010). Exotic grass invasions: applying a conceptual framework to the dynamics of degradation and restoration in Australia’s tropical savannas. Restoration Ecology 18, 188–197.
| Exotic grass invasions: applying a conceptual framework to the dynamics of degradation and restoration in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. H. (2003). Birds and fire in the Mediterranean climate of south-west Western Australia. In ‘Fire in Ecosystems of South-west Western Australia: Impacts and Management’. (Eds I. Abbott and N. Burrows.) pp. 321–347. (Backhuys: Leiden, the Netherlands.)
Butchart, S. H. M., Stattersfield, A. J., Bennun, L. A., Shutes, S. M., Akcakaya, H. R., Baillie, J. E. M., Stuart, S. N., Hilton-Taylor, C., and Mace, G. (2004). Measuring global trends in the status of biodiversity: Red List indices for birds. PLoS Biology 2, e383.
| Measuring global trends in the status of biodiversity: Red List indices for birds.Crossref | GoogleScholarGoogle Scholar |
Cavalcanti, R. B., and Alves, M. A. (1997). Effects of fire on savanna birds in central Brazil. Ornitologia Neotropical 8, 85–87.
Cawardine, J., O’Conner, T., Legge, S., Mackey, B., Possingham, H., and Martin, T. (2011). ‘Priority Threat Management to Protect Kimberley Wildlife’. (CSIRO Ecosystems Sciences: Canberra.)
Cintra, R., and Sanaiotti, T. M. (2005). Fire effects on the composition of a bird community in an Amazonian savanna (Brazil). Brazilian Journal of Biology 65, 683–695.
| Fire effects on the composition of a bird community in an Amazonian savanna (Brazil).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD287ltVSrug%3D%3D&md5=6a36f52734de9b27035635d99f811130CAS |
Clarke, M. F. (2008). Catering for the needs of fauna in fire management: science of just wishful thinking? Wildlife Research 35, 385–394.
| Catering for the needs of fauna in fire management: science of just wishful thinking?Crossref | GoogleScholarGoogle Scholar |
Clarke, R. H., Boulton, R. L., and Clarke, M. F. (2005). Estimating population size of the Black-eared Miner, with an assessment of landscape-scale habitat requirements. Pacific Conservation Biology 11, 174–188.
Cook, G. D. (2003). Fuel dynamics, nutrients, and atmospheric chemistry. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 47–58. (Springer-Verlag: New York.)
Cook, G. D., and Dias, L. (2006). It was no accident: deliberate plant introductions by Australian government agencies during the 20th century. Australian Journal of Botany 54, 601–625.
| It was no accident: deliberate plant introductions by Australian government agencies during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Corbett, L. K., Andersen, A. N., and Muller, W. J. (2003). Terrestrial vertebrates. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 126–152. (Springer-Verlag: New York.)
Cracraft, J. (1986). Origin and evolution of continental biotas: speciation and historical congruence within the Australian avifauna. Evolution 40, 977–996.
| Origin and evolution of continental biotas: speciation and historical congruence within the Australian avifauna.Crossref | GoogleScholarGoogle Scholar |
Cracraft, J. (1991). Patterns of diversification within continental biotas: hierarchical congruence among the areas of endemism of Australian vertebrates. Australian Systematic Botany 4, 211–227.
| Patterns of diversification within continental biotas: hierarchical congruence among the areas of endemism of Australian vertebrates.Crossref | GoogleScholarGoogle Scholar |
Craig, A. B. (1992). Aspects of post-fire regeneration in Soft Spinifex (Triodia pungens) communities near Newman, Western Australia. Western Australian Department of Agriculture, Kununurra, WA.
Crowley, G. M., and Garnett, S. T. (1998). Vegetation change in the grasslands and grassy woodlands of east-central Cape York Peninsula, Australia. Pacific Conservation Biology 4, 132–148.
Crowley, G., and Garnett, S. (1999). Seeds of the annual grasses Schizachyrium spp. as a food resource for tropical granivorous birds. Australian Journal of Ecology 24, 208–220.
| Seeds of the annual grasses Schizachyrium spp. as a food resource for tropical granivorous birds.Crossref | GoogleScholarGoogle Scholar |
Crowley, G. M., and Garnett, S. T. (2001). Growth, seed production and effect of defoliation in an early flowering perennial grass, Alloteropsis semialata (Poaceae), on Cape York Peninsula, Australia. Australian Journal of Botany 49, 735–743.
| Growth, seed production and effect of defoliation in an early flowering perennial grass, Alloteropsis semialata (Poaceae), on Cape York Peninsula, Australia.Crossref | GoogleScholarGoogle Scholar |
Crowley, G., Garnett, S. T., and Shepherd, S. (2004). Management guidelines for Golden-shouldered Parrot conservation. Queensland Parks and Wildlife Service, Brisbane.
Crowley, G., Garnett, S., and Shephard, S. (2009). Impact of storm-burns on Melaleuca viridiflora invasion of grasslands and grassy woodlands on Cape York Peninsula, Australia. Austral Ecology 34, 196–209.
| Impact of storm-burns on Melaleuca viridiflora invasion of grasslands and grassy woodlands on Cape York Peninsula, Australia.Crossref | GoogleScholarGoogle Scholar |
D’Antonio, C., and Vitousek, P. (1992). Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23, 63–87.
Di Giacomo, A., Di Giacomo, A., and Reboreda, J. (2011). Effects of grassland burning on reproductive success of globally threatened Strange-tailed Tyrants Alectrurus risora. Bird Conservation International 21, 411–422.
| Effects of grassland burning on reproductive success of globally threatened Strange-tailed Tyrants Alectrurus risora.Crossref | GoogleScholarGoogle Scholar |
Dorricott, K. E., and Garnett, S. T. (2007). National recovery plan for the white-bellied subspecies of the Crimson Finch Neochmia phaeton evangelinae and the northern subspecies of the Star Finch Neochmia ruficauda clarescens. Queensland Parks and Wildlife Service, Brisbane. Available at http://www.environment.gov.au/biodiversity/threatened/publications/n-evangelinae-n-clarescens.html [Verified 21 July 2013].
Dostine, P. L., and Franklin, D. C. (2002). A comparison of the diet of three finch species in the Yinberrie Hills area, Northern Territory. Emu 102, 159–164.
| A comparison of the diet of three finch species in the Yinberrie Hills area, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Dostine, P. L., Johnson, G. C., Franklin, D. C., Zhang, Y., and Hempel, C. (2001). Seasonal use of savanna landscapes by the Gouldian Finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern Territory. Wildlife Research 28, 445–458.
| Seasonal use of savanna landscapes by the Gouldian Finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Douglass, L. L., Possingham, H. P., Cawardine, J., Klein, C. J., Roxburgh, S. H., Russell-Smith, J., and Wilson, K. A. (2011). The effects of carbon credits on savanna land management and priorities for biodiversity conservation. PLoS ONE 6, e23843.
| The effects of carbon credits on savanna land management and priorities for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1OmsrzN&md5=b0872d9ee63c285df49d69494d478864CAS | 21935363PubMed |
du Toit, J. T., Rogers, K. H., and Biggs, H. C. (2003). ‘The Kruger Experience: Ecology and Management of Savanna Heterogeneity.’ (Island Press: Washington.)
Dyer, R., and Stafford Smith, M. (2003). Ecological and economic assessment of prescribed burning impacts in semi-arid pastoral lands of northern Australia. International Journal of Wildland Fire 12, 403–413.
| Ecological and economic assessment of prescribed burning impacts in semi-arid pastoral lands of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Edwards, A. C., and Russell-Smith, J. (2009). Ecological thresholds and the status of fire-sensitive vegetation in western Arnhem Land, northern Australia: implications for management. International Journal of Wildland Fire 18, 127–146.
| Ecological thresholds and the status of fire-sensitive vegetation in western Arnhem Land, northern Australia: implications for management.Crossref | GoogleScholarGoogle Scholar |
Eldridge, M. D. B., Potter, S., and Cooper, S. J. B. (2011). Biogeographic barriers in north-western Australia: an overview and standardisation of nomenclature. Australian Journal of Zoology 59, 270–272.
| Biogeographic barriers in north-western Australia: an overview and standardisation of nomenclature.Crossref | GoogleScholarGoogle Scholar |
Firth, R. S. C., Brook, B. W., Woinarski, J. C. Z., and Fordham, D. A. (2010). Decline and likely extinction of a northern Australian native rodent, the Brush-tailed Rabbit-rat Conilurus penicillatus. Biological Conservation 143, 1193–1201.
| Decline and likely extinction of a northern Australian native rodent, the Brush-tailed Rabbit-rat Conilurus penicillatus.Crossref | GoogleScholarGoogle Scholar |
Fitzsimons, J., Russell-Smith, J., James, G., Vigilante, T., Lipsett-Moore, G., Morrison, J., and Looker, M. (2012). Insights into the biodiversity and social benchmarking components of the Northern Australian fire management and carbon abatement programmes. Ecological Management & Restoration 13, 51–57.
| Insights into the biodiversity and social benchmarking components of the Northern Australian fire management and carbon abatement programmes.Crossref | GoogleScholarGoogle Scholar |
Ford, J. (1978). Geographical isolation and morphological and habitat differentiation between birds of the Kimberley and the Northern Territory. Emu 78, 25–35.
| Geographical isolation and morphological and habitat differentiation between birds of the Kimberley and the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Ford, J. (1987). Minor isolates and minor geographic barriers in avian speciation in continental Australia. Emu 87, 90–102.
| Minor isolates and minor geographic barriers in avian speciation in continental Australia.Crossref | GoogleScholarGoogle Scholar |
Fox, I. D., Neldner, V. J., Wilson, G. W., Bannink, P. J., Wilson, B. A., Brocklehurst, P. S., Clark, M. J., Dickinson, K. J. M., Beard, J. S., Hopkins, A. J. M., Beeston, G. R., Harvey, J. M., Thompson, E. J., Ryan, T. S., Thompson, S. L., Butler, D. W., Cartan, H., Addicott, E. P., Bailey, L. P., Cumming, R. J., Johnson, D. C., Schmeider, M., Stephens, K. M., and Bean, A. R. (2001). ‘The Vegetation of the Australian Tropical Savannas.’ (Environment Protection Agency: Brisbane.)
Franklin, D. C. (1999). Evidence of disarray amongst granivorous bird assemblages in the savannas of northern Australia, a region of sparse human settlement. Biological Conservation 90, 53–68.
| Evidence of disarray amongst granivorous bird assemblages in the savannas of northern Australia, a region of sparse human settlement.Crossref | GoogleScholarGoogle Scholar |
Franklin, D. C., Woinarski, J. C. Z., and Noske, R. A. (2000). Geographic patterning of species richness among granivorous birds in Australia. Journal of Biogeography 27, 829–842.
| Geographic patterning of species richness among granivorous birds in Australia.Crossref | GoogleScholarGoogle Scholar |
Franklin, D. C., Whitehead, P. J., Pardon, G., Matthews, J., McMahon, P., and McIntyre, D. (2005). Geographic patterns and correlates of the decline of granivorous birds in northern Australia. Wildlife Research 32, 399–408.
| Geographic patterns and correlates of the decline of granivorous birds in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Franklin, D. C., Petty, A. M., Williamson, G. J., Brook, B. W., and Bowman, D. M. J. S. (2008). Monitoring contrasting land management in the savanna landscapes of northern Australia. Environmental Management 41, 501–515.
| Monitoring contrasting land management in the savanna landscapes of northern Australia.Crossref | GoogleScholarGoogle Scholar | 17828595PubMed |
Fraser, F., Lawson, V., Morrison, S., Christopherson, P., McGreggor, S., and Rawlinson, M. (2003). Fire management experiment for the declining Partridge Pigeon, Kakadu National Park. Ecological Management & Restoration 4, 94–102.
| Fire management experiment for the declining Partridge Pigeon, Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
Friend, G. R. (1987). Population ecology of Mesembriomys gouldii (Rodentia : Muridae) in the wet-dry tropics of the Northern Territory. Australian Wildlife Research 14, 293–303.
| Population ecology of Mesembriomys gouldii (Rodentia : Muridae) in the wet-dry tropics of the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Friend, G. R., and Taylor, J. A. (1985). Habitat preferences of small mammals in tropical open-forest of the Northern Territory. Australian Journal of Ecology 10, 173–185.
| Habitat preferences of small mammals in tropical open-forest of the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Furley, P. A., Proctor, J., and Ratter, J. A. (1992). ‘Nature and Dynamics of Forest–Savanna Boundaries.’ (Chapman & Hall: London.)
Gammage, B. (2011). ‘The Biggest Estate on Earth: How Aborigines Made Australia.’ (Allen & Unwin: Sydney.)
Garnett, S. T., and Crowley, G. M. (1995). The decline of the Black Treecreeper Climacteris picumnus melanota on Cape York Peninsula. Emu 95, 66–68.
| The decline of the Black Treecreeper Climacteris picumnus melanota on Cape York Peninsula.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., and Crowley, G. M. (2000). ‘The Action Plan for Australian Birds 2000.’ (Environment Australia: Canberra.)
Garnett, S. T., and Williamson, G. J. (2010). Spatial and temporal variation in precipitation at the start of the rainy season in tropical Australia. Rangeland Journal 32, 215–226.
| Spatial and temporal variation in precipitation at the start of the rainy season in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., Woinarski, J. C. Z., Crowley, G. M., and Kutt, A. S. (2010). Biodiversity conservation in Australian tropical rangelands. In ‘Wild Rangelands: Conserving Wildlife While Maintaining Livestock in Semi-arid Ecosystems’. (Eds J. du Toit, R. Kock and J. Deutsch.) pp. 191–234. (Wiley-Blackwell: Chichester, UK.)
Garnett, S. T., Szabo, J. K., and Dutson, G. (2011). ‘The Action Plan for Australian Birds 2010.’ (CSIRO Publishing: Melbourne.)
Gibbons, P., and Lindenmayer, D. (2002). ‘Tree Hollows and Wildlife Conservation in Australia.’ (CSIRO Publishing: Melbourne.)
Gill, A. M., Allan, G., and Yates, C. (2003). Fire-created patchiness in Australian savannas. International Journal of Wildland Fire 12, 323–331.
| Fire-created patchiness in Australian savannas.Crossref | GoogleScholarGoogle Scholar |
Gregory, N. C., Sensenig, R. L., and Wilcove, D. S. (2010). Effects of controlled fire and livestock grazing on bird communities in East African savannas. Conservation Biology 24, 1606–1616.
| Effects of controlled fire and livestock grazing on bird communities in East African savannas.Crossref | GoogleScholarGoogle Scholar | 20561002PubMed |
Griffiths, A. D., and Christian, K. A. (1996). The effects of fire on the Frillneck Lizard (Chlamydosaurus kingii) in northern Australia. Australian Journal of Ecology 21, 386–398.
| The effects of fire on the Frillneck Lizard (Chlamydosaurus kingii) in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Hannah, D., Woinarski, J. C. Z., Catterall, C. P., McCosker, J. C., Thurgate, N. Y., and Fensham, R. J. (2007). Impacts of clearing, fragmentation and disturbance on the bird fauna of eucalypt savanna woodlands in central Queensland, Australia. Austral Ecology 32, 261–276.
| Impacts of clearing, fragmentation and disturbance on the bird fauna of eucalypt savanna woodlands in central Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Harrington, G. N., and Sanderson, K. D. (1994). Recent contraction of wet sclerophyll forest in the wet tropics of Queensland due to invasion by rainforest. Pacific Conservation Biology 1, 319–327.
Harrington, G., Freeman, A., Murphy, S., Venables, B., and Edwards, C. (2011). Carpentarian Grasswren survey: Boodjamulla National Park 15–30 June 2011. Birds Australia North Queensland.
Harris, S., Tapper, N., Packham, D., Orlove, B., and Nicholls, N. (2008). The relationship between the monsoonal summer rain and dry-season fire activity of northern Australia. International Journal of Wildland Fire 17, 674–684.
| The relationship between the monsoonal summer rain and dry-season fire activity of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Haslem, A., Kelly, L. T., Nimmo, D. G., Watson, S. J., Kenny, S. A., Taylor, R. S., Avitabile, S. C., Callister, K. E., Spence-Bailey, L. M., Clarke, M. F., and Bennett, A. F. (2011). Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire. Journal of Applied Ecology 48, 247–256.
| Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire.Crossref | GoogleScholarGoogle Scholar |
Heinsohn, R., Murphy, S., and Legge, S. (2003). Overlap and competition for nest hollows among Eclectus Parrots, Palm Cockatoos and Sulphur-crested Cockatoos. Australian Journal of Zoology 51, 81–94.
| Overlap and competition for nest hollows among Eclectus Parrots, Palm Cockatoos and Sulphur-crested Cockatoos.Crossref | GoogleScholarGoogle Scholar |
Heinsohn, R., Zeriga, T., Murphy, S. A., Igag, P., Legge, S., and Mack, A. L. (2009). Do Palm Cockatoos (Probosciger aterrimus) have long enough lifespans to support their low reproductive success? Emu 109, 183–191.
| Do Palm Cockatoos (Probosciger aterrimus) have long enough lifespans to support their low reproductive success?Crossref | GoogleScholarGoogle Scholar |
Higgins, S. I., Bond, W. J., and Trollope, W. S. W. (2000). Fire, resprouting and variability: a recipe for grass-tree coexistence in savanna. Journal of Ecology 88, 213–229.
| Fire, resprouting and variability: a recipe for grass-tree coexistence in savanna.Crossref | GoogleScholarGoogle Scholar |
Hopkins, B. (1983). Successional processes. In ‘Ecosystems of the World. Vol. 13: Tropical Savannas’. (Ed. F. Bourliere.) pp. 605–616. (Elsevier: Amsterdam.)
Jansen, R., Little, R. M., and Crowe, T. M. (1999). Implications of grazing and burning of grasslands on the sustainable use of francolins (Francolinus spp.) and on overall bird conservation in the highlands of Mpumulanga Province, South Africa. Biodiversity and Conservation 8, 587–602.
| Implications of grazing and burning of grasslands on the sustainable use of francolins (Francolinus spp.) and on overall bird conservation in the highlands of Mpumulanga Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. (2006). ‘Australia’s Mammal Extinctions: A 50 000 Year History.’ (Cambridge University Press: New York.)
Johnstone, R. E., and Burbidge, A. H. (1991). The avifauna of Kimberley rainforests. In ‘Kimberley Rainforests of Australia’. (Eds N. L. McKenzie, R. B. Johnston and P. G. Kendrick.) pp. 361–391. (Surrey Beatty & Sons: Sydney.)
Joseph, L., and Omland, K. (2009). Phylogeography: its development and impact in Australo-Papuan ornithology with special reference to paraphyly in Australian birds. Emu 109, 1–23.
| Phylogeography: its development and impact in Australo-Papuan ornithology with special reference to paraphyly in Australian birds.Crossref | GoogleScholarGoogle Scholar |
Kerle, J. A. (1985). Habitat preference and diet of the Northern Brushtail Possum Trichosurus vulpecula in the Alligator Rivers region, N.T. Proceedings of the Ecological Society of Australia 13, 161–176.
Kutt, A. S., and Woinarski, J. C. Z. (2007). Vegetation and the vertebrate fauna assemblage pattern in response to grazing and fire in a tropical savanna woodland in north-eastern Australia. Journal of Tropical Ecology 23, 95–106.
| Vegetation and the vertebrate fauna assemblage pattern in response to grazing and fire in a tropical savanna woodland in north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Kutt, A. S., Bolitho, E. E., Retallick, R. W. R., and Kemp, J. E. (2005). Pattern and change in the terrestrial vertebrate fauna of the Pennefather River, Gulf of Carpentaria, Cape York Peninsula. In ‘Gulf of Carpentaria Scientific Study Report’. Geography Monograph Series number 10. pp. 261–300. (Royal Geographical Society of Queensland Inc.: Brisbane.)
Kutt, A. S., Vanderduys, E. P., Perry, J. J., and Perkins, G. C. (2012). Do miners (Manorina spp.) affect bird assemblages in continuous savanna woodlands in north-eastern Australia? Austral Ecology 37, 779–788.
| Do miners (Manorina spp.) affect bird assemblages in continuous savanna woodlands in north-eastern Australia?Crossref | GoogleScholarGoogle Scholar |
Lacey, C. J., and Whelan, P. (1976). Observations on the ecological significance of vegetative reproduction in the Katherine–Darwin region of the Northern Territory. Australian Forestry 39, 131–139.
| Observations on the ecological significance of vegetative reproduction in the Katherine–Darwin region of the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Lambeck, R. J. (1997). Focal species: a multi-species umbrella for nature conservation. Conservation Biology 11, 849–856.
| Focal species: a multi-species umbrella for nature conservation.Crossref | GoogleScholarGoogle Scholar |
Lee, J. Y., and Edwards, S. V. (2008). Divergence across Australia’s Carpentarian Barrier: statistical phylogeography of the Red-backed Fairy-wren (Malurus melanocephalus). Evolution 62, 3117–3134.
| Divergence across Australia’s Carpentarian Barrier: statistical phylogeography of the Red-backed Fairy-wren (Malurus melanocephalus).Crossref | GoogleScholarGoogle Scholar | 19087188PubMed |
Legge, S., Murphy, S., Heathcote, J., Flaxman, E., Augusteyn, J., and Crossman, M. (2008). The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildlife Research 35, 33–43.
| The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Murphy, S., Kingswood, R., Maher, B., and Swan, D. (2011). EcoFire: restoring the biodiversity values of the Kimberley region by managing fire. Ecological Management & Restoration 12, 84–92.
| EcoFire: restoring the biodiversity values of the Kimberley region by managing fire.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Webb, T., Swan, D., Maher, B., Lawler, P., Smith, J., and Tuft, K. (2013). EcoFire 2000–2012 fire pattern analysis, Central and North Kimberley. Australian Wildlife Conservancy, Perth.
Lewis, D. (2002). ‘Slower Than the Eye Can See: Environmental Change in Northern Australia’s Cattle Lands – A Case Study from the Victoria River District, Northern Territory.’ (Tropical Savannas CRC: Darwin.)
Lewis, M. (2007). Foraging responses of the endangered Gouldian Finch to temporal differences in seed availability in north Australian savanna grasslands. In ‘Temporal Dimensions in Landscape Ecology: Wildlife Responses to Variable Resources’. (Eds J. A. Bissonette and I. Storch.) pp. 218–235. (Springer: New York.)
Liedloff, A. C., and Cook, G. D. (2007). Modelling the effects of rainfall variability and fire on tree populations in an Australian tropical savanna with the FLAMES simulation model. Ecological Modelling 201, 269–282.
| Modelling the effects of rainfall variability and fire on tree populations in an Australian tropical savanna with the FLAMES simulation model.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, D. B., MacGregor, C., Wood, J. T., Cunningham, R. B., Crane, M., Michael, D., Montague-Drake, R., Brown, D., Fortescue, M., Dexter, N., Hudson, M., and Gill, A. M. (2009). What factors influenced rapid post-fire site re-occupancy? A case study of the endangered Eastern Bristlebird in eastern Australia. International Journal of Wildland Fire 18, 84–95.
| What factors influenced rapid post-fire site re-occupancy? A case study of the endangered Eastern Bristlebird in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lonsdale, W., and Braithwaite, R. W. (1991). Assessing the effects of fire on vegetation in tropical savannas. Australian Journal of Ecology 16, 363–374.
| Assessing the effects of fire on vegetation in tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Malan, D. (1998). Summer grassland cover on cattle farms in KwaZulu-Natal: does it limit nesting habitat for Helmeted Guineafowl? South African Journal of Wildlife Research 28, 105–109.
Martin, T. G., Kuhnert, P. M., Mengersen, K., and Possingham, H. P. (2005). The power of expert opinion in ecological models using Bayesian methods: impact of grazing on birds. Ecological Applications 15, 266–280.
| The power of expert opinion in ecological models using Bayesian methods: impact of grazing on birds.Crossref | GoogleScholarGoogle Scholar |
Maute, K. (2011). Variation in the health of tropical finches in relation to conservation status, season and land tenure. Ph.D. Thesis, University of Wollongong, NSW.
Mawson, P. R., and Long, J. L. (1994). Size and age parameters of nest trees used by four species of parrot and one species of cockatoo in south-west Australia. Emu 94, 149–155.
| Size and age parameters of nest trees used by four species of parrot and one species of cockatoo in south-west Australia.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N. L. (1981). Mammals of the Phanerozoic south-west Kimberley, Western Australia: biogeography and recent changes. Journal of Biogeography 8, 263–280.
| Mammals of the Phanerozoic south-west Kimberley, Western Australia: biogeography and recent changes.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N. L., and Belbin, L. (1991). Kimberley rainforest communities: reserve recommendations and management implications. In ‘Kimberley Rainforests of Australia’. (Eds N. L. McKenzie, R. B. Johnston and P. G. Kendrick.) pp. 453–480. (Surrey Beatty and Sons: Sydney.)
McQuade, C. V., Arthur, J. T., and Butterworth, I. J. (1996). Climate and hydrology. In ‘Landscape and Vegetation Ecology of the Kakadu Region’. (Eds C. M. Finlayson and I. von Oertzen.) pp. 17–35. (Kluwer Academic Publishers: Dordrecht, the Netherlands.)
Mills, M. S. L. (2004). Bird community responses to savanna fires: should managers be concerned? South African Journal of Wildlife Research 34, 1–11.
Moorcroft, H., Ignjic, E., Cowell, S., Goonack, J., Mangolomara, S., Oobagooma, J., Karadada, R., Williams, D., and Waina, N. (2012). Conservation planning in a cross-cultural context: the Wunambal Gaambera Healthy Country Project in the Kimberley, Western Australia. Ecological Management & Restoration 13, 16–25.
| Conservation planning in a cross-cultural context: the Wunambal Gaambera Healthy Country Project in the Kimberley, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Morton, S. R., and Brennan, K. G. (1991). Birds. In ‘Monsoonal Australia: Landscape Ecology and Man in the Northern Lowlands’. (Eds C. D. Haynes, M. G. Ridpath and M. A. J. Williams.) pp. 133–149. (Balkema: Rotterdam, the Netherlands.)
Mott, J. J., and Andrew, M. H. (1985). The effect of fire on the population dynamics of native grasses in tropical savannas of north-west Australia. Proceedings of the Ecological Society of Australia 13, 231–239.
Murphy, B. P., and Bowman, D. M. J. S. (2007). The interdependence of fire, grass, kangaroos and Australian Aborigines: a case study from central Arnhem land, northern Australia. Journal of Biogeography 34, 237–250.
| The interdependence of fire, grass, kangaroos and Australian Aborigines: a case study from central Arnhem land, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Murphy, S. A., and Legge, S. (2007). The gradual loss and episodic creation of Palm Cockatoo (Probosciger aterrimus) nest-trees in a fire- and cyclone-prone habitat. Emu 107, 1–6.
| The gradual loss and episodic creation of Palm Cockatoo (Probosciger aterrimus) nest-trees in a fire- and cyclone-prone habitat.Crossref | GoogleScholarGoogle Scholar |
Murphy, B. P., and Russell-Smith, J. (2010). Fire severity in a northern Australian savanna landscape: the importance of time since previous fire. International Journal of Wildland Fire 19, 46–51.
| Fire severity in a northern Australian savanna landscape: the importance of time since previous fire.Crossref | GoogleScholarGoogle Scholar |
Murphy, S. A., Legge, S. M., Heathcote, J., and Mulder, E. (2010). The effects of early and late-season fires on mortality, dispersal, physiology and breeding of Red-backed Fairy-wrens (Malurus melanocephalus). Wildlife Research 37, 145–155.
| The effects of early and late-season fires on mortality, dispersal, physiology and breeding of Red-backed Fairy-wrens (Malurus melanocephalus).Crossref | GoogleScholarGoogle Scholar |
Murray, P. F., and Vickers-Rich, P. (2004). ‘Magnificent Mihirungs: The Colossal Flightless Birds of the Australian Dreamtime.’ (Indiana University Press: Bloomington, IN.)
Neidjie, B., Davis, D., and Fox, A. (1986). ‘Australia’s Kakadu Man, Bill Neidjie.’ (Resource Managers: Darwin.)
Nicholson, E., Lill, A., and Andersen, A. (2006). Do tropical savanna skink assemblages show a short-term response to low-intensity fire? Wildlife Research 33, 331–338.
| Do tropical savanna skink assemblages show a short-term response to low-intensity fire?Crossref | GoogleScholarGoogle Scholar |
Noske, R. A. (1992). The status and ecology of the White-throated Grasswren Amytornis woodwardi. Emu 92, 39–51.
| The status and ecology of the White-throated Grasswren Amytornis woodwardi.Crossref | GoogleScholarGoogle Scholar |
Noske, R. A., and Franklin, D. C. (1999). Breeding seasons of land birds in the Australian monsoonal tropics: diverse responses to a highly seasonal environment. Australian Biologist 12, 72–90.
Oakwood, M. (2000). Reproduction and demography of the Northern Quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48, 519–539.
| Reproduction and demography of the Northern Quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Orgeas, J., and Andersen, A. N. (2001). Fire and biodiversity: responses of grass-layer beetles to experimental fire regimes in an Australian tropical savanna. Journal of Applied Ecology 38, 49–62.
| Fire and biodiversity: responses of grass-layer beetles to experimental fire regimes in an Australian tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Pardon, L. G., Brook, B. W., Griffiths, A. D., and Braithwaite, R. W. (2003). Determinants of survival for the Northern Brown Bandicoot under a landscape-scale fire experiment. Journal of Animal Ecology 72, 106–115.
| Determinants of survival for the Northern Brown Bandicoot under a landscape-scale fire experiment.Crossref | GoogleScholarGoogle Scholar |
Parker, T. A., and Willis, E. O. (1997). Notes on three tiny grassland flycatchers, with comments on the disappearance of South American fire-diversified savannas. Ornithological Monographs 48, 549–555.
| Notes on three tiny grassland flycatchers, with comments on the disappearance of South American fire-diversified savannas.Crossref | GoogleScholarGoogle Scholar |
Parr, C. L., and Andersen, A. N. (2006). Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20, 1610–1619.
| Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm.Crossref | GoogleScholarGoogle Scholar | 17181796PubMed |
Parr, C. L., and Chown, S. L. (2003). Burning issues for conservation: a critique of faunal fire research in southern Africa. Austral Ecology 28, 384–395.
| Burning issues for conservation: a critique of faunal fire research in southern Africa.Crossref | GoogleScholarGoogle Scholar |
Parr, C. L., Woinarski, J. C. Z., and Pienaar, D. J. (2009). Cornerstones of biodiversity conservation? Comparing the management effectiveness of Kruger and Kakadu National Parks, two key savanna reserves. Biodiversity and Conservation 18, 3643–3662.
| Cornerstones of biodiversity conservation? Comparing the management effectiveness of Kruger and Kakadu National Parks, two key savanna reserves.Crossref | GoogleScholarGoogle Scholar |
Perry, J., Fisher, A., and Palmer, C. (2011a). Status and habitat of the Carpentarian Grasswren (Amytornis dorotheae) in the Northern Territory. Emu 111, 155–161.
| Status and habitat of the Carpentarian Grasswren (Amytornis dorotheae) in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Perry, J. J., Kutt, A. S., Garnett, S. T., Crowley, G. M., Vanderduys, E. P., and Perkins, G. C. (2011b). Changes in the avifauna of Cape York Peninsula over a period of 9 years: the relative effects of fire, vegetation type and climate. Emu 111, 120–131.
| Changes in the avifauna of Cape York Peninsula over a period of 9 years: the relative effects of fire, vegetation type and climate.Crossref | GoogleScholarGoogle Scholar |
Petty, A. M., and Werner, P. A. (2010). How many buffalo does it take to change a savanna? A response to Bowman et al. (2008). Journal of Biogeography 37, 193–195.
| How many buffalo does it take to change a savanna? A response to Bowman et al. (2008).Crossref | GoogleScholarGoogle Scholar |
Petty, A. M., Alderson, J., Muller, R., Scheibe, O., Wilson, K., and Winderlich, S. (2007). Kakadu National Park, Arnhem Land Plateau fire management plan. Parks Australia, Jabiru, NT.
Preece, N. (2002). Aboriginal fire in monsoonal Australia from historical accounts. Journal of Biogeography 29, 321–336.
| Aboriginal fire in monsoonal Australia from historical accounts.Crossref | GoogleScholarGoogle Scholar |
Price, O. (1998). Conservation of frugivorous birds and monsoon rainforest patches in the Northern Territory. Ph.D. Thesis, Australian National University, Canberra.
Price, O., and Bowman, D. M. J. S. (1994). Fire-stick forestry: a matrix model in support of skilful fire management of Callitris intratropica R.T.Baker by north Australian Aborigines. Journal of Biogeography 21, 573–580.
| Fire-stick forestry: a matrix model in support of skilful fire management of Callitris intratropica R.T.Baker by north Australian Aborigines.Crossref | GoogleScholarGoogle Scholar |
Price, O., Woinarski, J. C. Z., and Robinson, D. (1999). Very large area requirements for frugivorous birds in monsoon rainforests of the Northern Territory, Australia. Biological Conservation 91, 169–180.
| Very large area requirements for frugivorous birds in monsoon rainforests of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Price, O. F., Edwards, A., Connors, G., Woinarski, J. C. Z., Ryan, G., Turner, A., and Russell-Smith, J. (2005). Fire heterogeneity, Kakadu National Park, 1980–2000. Wildlife Research 32, 425–433.
| Fire heterogeneity, Kakadu National Park, 1980–2000.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J. (2012). Threatened mammals become more predatory after small-scale prescribed fires in a high-rainfall rocky savanna. Austral Ecology 37, 926–935.
| Threatened mammals become more predatory after small-scale prescribed fires in a high-rainfall rocky savanna.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., Grice, A. C., Abbott, B. N., Nicholas, D. M., and Whiteman, L. (2008). Impacts of changed fire regimes on tropical riparian vegetation invaded by an exotic vine. Austral Ecology 33, 151–167.
| Impacts of changed fire regimes on tropical riparian vegetation invaded by an exotic vine.Crossref | GoogleScholarGoogle Scholar |
Reside, A. E., VanDerWal, J., Kutt, A., Watson, I., and Williams, S. (2012). Fire regime shifts affect bird species distributions. Diversity & Distributions 18, 213–225.
| Fire regime shifts affect bird species distributions.Crossref | GoogleScholarGoogle Scholar |
Rossiter, N., Setterfield, S., Douglas, M., and Hutley, L. (2003). Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia. Diversity & Distributions 9, 169–176.
| Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Rossiter-Rachor, N. A., Setterfield, S. A., Douglas, M. M., Hutley, L. B., Cook, G. D., and Schmidt, S. (2009). Invasive Andropogon gayanus (Gamba Grass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecological Applications 19, 1546–1560.
| Invasive Andropogon gayanus (Gamba Grass) is an ecosystem transformer of nitrogen relations in Australian savanna.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MnjvVSgsA%3D%3D&md5=7c4d7a4e99ee0fd8439065fbb00a3cadCAS | 19769102PubMed |
Russell-Smith, J. (1991). Classification, species richness and environmental relations of monsoon rain forest in northern Australia. Journal of Vegetation Science 2, 259–278.
| Classification, species richness and environmental relations of monsoon rain forest in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J. (2006). Recruitment dynamics of the long-lived obligate seeders Callitris intratropica (Cupressaceae) and Petraeomyrtus punicea (Myrtaceae). Australian Journal of Botany 54, 479–485.
| Recruitment dynamics of the long-lived obligate seeders Callitris intratropica (Cupressaceae) and Petraeomyrtus punicea (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., and Edwards, A. (2006). Seasonality and fire severity in savanna landscapes of monsoonal northern Australia. International Journal of Wildland Fire 15, 541–550.
| Seasonality and fire severity in savanna landscapes of monsoonal northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., and Stanton, P. (2002). Fire regimes and fire management of rainforest communities across northern Australia. In ‘Flammable Australia: The Fire Regimes and Biodiversity of a Continent’. (Eds R. A. Bradstock, J. E. Williams and A. M. Gill.) pp. 329–350. (Cambridge University Press: Cambridge, UK.)
Russell-Smith, J., McKenzie, N. L., and Woinarski, J. C. Z. (1992). Conserving vulnerable habitat in northern and northwestern Australia: the rainforest archipelago. In ‘Conservation and Development Issues in Northern Australia’. (Eds I. Moffatt and A. Webb.) pp. 63–68. (Australian National University, North Australia Research Unit: Darwin.)
Russell-Smith, J., Ryan, P. G., Klessa, D., Waight, G., and Harwood, R. (1998). Fire regimes, fire-sensitive vegetation and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia. Journal of Applied Ecology 35, 829–846.
| Fire regimes, fire-sensitive vegetation and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Ryan, P. G., and Cheal, D. C. (2002). Fire regimes and the conservation of sandstone heath in monsoonal northern Australia: frequency, interval, patchiness. Biological Conservation 104, 91–106.
| Fire regimes and the conservation of sandstone heath in monsoonal northern Australia: frequency, interval, patchiness.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Whitehead, P. J., Cook, G. D., and Hoare, J. L. (2003a). Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996. Ecological Monographs 73, 349–375.
| Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Yates, C. P., Edwards, A., Allan, G. E., Cook, G. D., Cooke, P., Craig, R., Heath, B., and Smith, R. (2003b). Contemporary fire regimes of northern Australia: change since Aboriginal occupancy, challenges for sustainable management. International Journal of Wildland Fire 12, 283–297.
| Contemporary fire regimes of northern Australia: change since Aboriginal occupancy, challenges for sustainable management.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Stanton, P. J., Whitehead, P. J., and Edwards, A. (2004a). Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes. Journal of Biogeography 31, 1293–1303.
| Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: I. Successional processes.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Stanton, P. J., Whitehead, P. J., and Edwards, A. (2004b). Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: II. Rates of landscape change. Journal of Biogeography 31, 1305–1316.
| Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: II. Rates of landscape change.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Yates, C. P., Whitehead, P., Smith, R., Craig, R., Allan, G., Thackway, R., Frakes, I., Cridland, S., Meyer, C. P., and Gill, A. M. (2007). Bushfires ‘down under’: patterns and implications of Australian landscape burning. International Journal of Wildland Fire 16, 361–377.
| Bushfires ‘down under’: patterns and implications of Australian landscape burning.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Whitehead, P. J., and Cooke, P. (Eds) (2009). ‘Culture, Ecology and Economy of Fire Management in Northern Australia: Rekindling the Wurrk Tradition.’ (CSIRO Publishing: Melbourne.)
Russell-Smith, J., Yates, C. P., Brock, C., and Westcott, V. (2010). Fire regimes and interval-sensitive vegetation in semi-arid Gregory National Park, Northern Territory. Australian Journal of Botany 58, 300–317.
Russell-Smith, J., Edwards, A. C., and Price, O. F. (2012a). Simplifying the savanna: the trajectory of fire-sensitive vegetation mosaics in northern Australia. Journal of Biogeography 39, 1303–1317.
| Simplifying the savanna: the trajectory of fire-sensitive vegetation mosaics in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith, J., Gardener, M., Brock, C., Brennan, K., Yates, C. P., and Grace, B. (2012b). Fire persistence traits can be used to predict vegetation responses to changing fire regimes at expansive landscape scales—an Australian example. Journal of Biogeography 39, 1657–1668.
| Fire persistence traits can be used to predict vegetation responses to changing fire regimes at expansive landscape scales—an Australian example.Crossref | GoogleScholarGoogle Scholar |
Setterfield, S. A., Rossiter-Rachor, N. A., Hutley, L. B., Douglas, M. M., and Williams, R. J. (2010). Turning up the heat: the impacts of Andropogon gayanus (Gamba Grass) invasion on fire behaviour in northern Australia. Diversity & Distributions 16, 854–861.
| Turning up the heat: the impacts of Andropogon gayanus (Gamba Grass) invasion on fire behaviour in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Sharp, B. R., and Bowman, D. M. J. S. (2004). Net woody vegetation increase confined to seasonally inundated lowlands in an Australian tropical savanna, Victoria River District, Northern Territory. Austral Ecology 29, 667–683.
| Net woody vegetation increase confined to seasonally inundated lowlands in an Australian tropical savanna, Victoria River District, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Skroblin, A., and Legge, S. (2010). The distribution and status of the western subspecies of the Purple-crowned Fairy-wren (Malurus coronatus coronatus). Emu 110, 339–347.
| The distribution and status of the western subspecies of the Purple-crowned Fairy-wren (Malurus coronatus coronatus).Crossref | GoogleScholarGoogle Scholar |
Skroblin, A., and Legge, S. (2012). Influence of fine-scale habitat requirements and riparian degradation on the distribution of the Purple-crowned Fairy-wren (Malurus coronatus coronatus). Austral Ecology 37, 874–884.
| Influence of fine-scale habitat requirements and riparian degradation on the distribution of the Purple-crowned Fairy-wren (Malurus coronatus coronatus).Crossref | GoogleScholarGoogle Scholar |
Skroblin, A., and Legge, S. (2013). Conservation of the patchily distributed and declining Purple-crowned Fairy-wren (Malurus coronatus coronatus) across a vast landscape: the need for a collaborative landscape-scale approach. PLoS ONE 8, e64942.
| Conservation of the patchily distributed and declining Purple-crowned Fairy-wren (Malurus coronatus coronatus) across a vast landscape: the need for a collaborative landscape-scale approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsFemtrw%3D&md5=5304752c2301656861c98a346d52f466CAS | 23734229PubMed |
Skroblin, A., Lanfear, R., Cockburn, A., and Legge, S. (2012). Inferring population connectivity across the range of the Purple-crowned Fairy-wren (Malurus coronatus) from mitochondrial DNA and morphology: implications for conservation management. Australian Journal of Zoology 60, 199–209.
| Inferring population connectivity across the range of the Purple-crowned Fairy-wren (Malurus coronatus) from mitochondrial DNA and morphology: implications for conservation management.Crossref | GoogleScholarGoogle Scholar |
Specht, R. L. (1958). The geographical relationships of the flora of Arnhem Land. In ‘Records of the American-Australian Scientific Expedition to Arnhem Land. 3. Botany and Plant Ecology’. (Eds R. L. Specht and C. P. Mountford.) pp. 415–478. (Melbourne University Press: Melbourne.)
Stocker, G. C. (1966). Effects of fire on vegetation in the Northern Territory. Australian Forestry 30, 223–230.
| Effects of fire on vegetation in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Taylor, J. A., and Dunlop, C. R. (1985). Plant communities of the wet-dry tropics of Australia: the Alligator Rivers region, Northern Territory. Proceedings of the Ecological Society of Australia 13, 83–128.
Taylor, J. A., and Tulloch, D. (1985). Rainfall in the wet-dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983 inclusive. Australian Journal of Ecology 10, 281–295.
| Rainfall in the wet-dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983 inclusive.Crossref | GoogleScholarGoogle Scholar |
Taylor, R., Woinarski, J., and Chatto, R. (2003). Hollow use by vertebrates in the Top End of the Northern Territory. Australian Zoologist 32, 462–476.
Telfer, W. R., and Garde, M. J. (2006). Indigenous knowledge of rock kangaroo ecology in western Arnhem Land Australia. Human Ecology 34, 379–406.
| Indigenous knowledge of rock kangaroo ecology in western Arnhem Land Australia.Crossref | GoogleScholarGoogle Scholar |
Thackway, R., and Cresswell, I. D. (Eds.) (1995). An interim biogeographic regionalisation for Australia: a framework for establishing the national system of reserves, Version 4.0. Australian Nature Conservation Agency, Canberra.
Toon, A., Hughes, J. M., and Joseph, L. (2010). Multilocus analysis of honeyeaters (Aves : Meliphagidae) highlights spatio-temporal heterogeneity in the influence of biogeographical barriers in the Australian monsoonal zone. Molecular Ecology 19, 2980–2994.
| Multilocus analysis of honeyeaters (Aves : Meliphagidae) highlights spatio-temporal heterogeneity in the influence of biogeographical barriers in the Australian monsoonal zone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyktb3F&md5=06715fbb8405d2a1e630b5b1be9b6cfcCAS | 20609078PubMed |
Trainor, C. R., and Woinarski, J. C. Z. (1994). Responses of lizards to three experimental fires in the savanna forests of Kakadu National Park. Wildlife Research 21, 131–148.
| Responses of lizards to three experimental fires in the savanna forests of Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
Valentine, L. E., Schwarzkopf, L., Johnson, C. N., and Grice, A. C. (2007). Burning season influences the response of bird assemblages to fire in tropical savannas. Biological Conservation 137, 90–101.
| Burning season influences the response of bird assemblages to fire in tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Valentine, L. E., Schwarzkopf, L., and Johnson, C. N. (2012). Effects of a short fire-return interval on resources and assemblage structure of birds in a tropical savanna. Austral Ecology 37, 23–34.
| Effects of a short fire-return interval on resources and assemblage structure of birds in a tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Van Langevelde, F., Van De Vijver, C. A. D. M., Kumar, L., Van De Koppel, J., De Ridder, N., Van Andel, J., Skidmore, A. K., Hearne, J. W., Stroosnijder, L., Bond, W. J., Prins, H. H. T., and Rietkerk, M. (2003). Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84, 337–350.
| Effects of fire and herbivory on the stability of savanna ecosystems.Crossref | GoogleScholarGoogle Scholar |
van Wilgen, B. W., and Biggs, H. C. (2011). A critical assessment of adaptive ecosystem management in a large savanna protected area in South Africa. Biological Conservation 144, 1179–1187.
| A critical assessment of adaptive ecosystem management in a large savanna protected area in South Africa.Crossref | GoogleScholarGoogle Scholar |
van Wilgen, B. W., Govender, N., Biggs, H. C., Ntsala, D., and Funda, X. N. (2004). Response of savanna fire regimes to changing fire-management policies in a large African national park. Conservation Biology 18, 1533–1540.
| Response of savanna fire regimes to changing fire-management policies in a large African national park.Crossref | GoogleScholarGoogle Scholar |
van Wilgen, B. W., Govender, N., and Biggs, H. C. (2007). The contribution of fire research to fire management: a critical review of a long-term experiment in the Kruger National Park, South Africa. International Journal of Wildland Fire 16, 519–530.
| The contribution of fire research to fire management: a critical review of a long-term experiment in the Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar |
Vigilante, T., and Bowman, D. M. J. S. (2004a). Effects of fire history on the structure and floristic composition of woody vegetation around Kalumburu, North Kimberley, Australia: a landscape-scale natural experiment. Australian Journal of Botany 52, 381–404.
| Effects of fire history on the structure and floristic composition of woody vegetation around Kalumburu, North Kimberley, Australia: a landscape-scale natural experiment.Crossref | GoogleScholarGoogle Scholar |
Vigilante, T., and Bowman, D. M. J. S. (2004b). Effects of individual fire events on the flower production of fruit-bearing tree species, with reference to Aboriginal people’s management and use, at Kalumburu, North Kimberley, Australia. Australian Journal of Botany 52, 405–415.
| Effects of individual fire events on the flower production of fruit-bearing tree species, with reference to Aboriginal people’s management and use, at Kalumburu, North Kimberley, Australia.Crossref | GoogleScholarGoogle Scholar |
Watkinson, A., Lonsdale, W., and Andrew, M. H. (1989). Modelling the population dynamics of an annual plant Sorghum intrans in the Wet-Dry tropics. Journal of Ecology 77, 162–181.
| Modelling the population dynamics of an annual plant Sorghum intrans in the Wet-Dry tropics.Crossref | GoogleScholarGoogle Scholar |
Watson, S. J., Rick, S., Nimmo, D. G., Kelly, L. T., Haslem, A., Clarke, M. F., and Bennett, A. F. (2012a). Effects of time since fire on birds: how informative are generalized fire response curves for conservation management? Ecological Applications 22, 685–696.
| Effects of time since fire on birds: how informative are generalized fire response curves for conservation management?Crossref | GoogleScholarGoogle Scholar | 22611864PubMed |
Watson, S. J., Taylor, R. S., Nimmo, D. G., Kelly, L. T., Clarke, M. F., and Bennett, A. F. (2012b). The influence of unburnt patches and distance from refuges on post-fire bird communities. Animal Conservation 15, 499–507.
| The influence of unburnt patches and distance from refuges on post-fire bird communities.Crossref | GoogleScholarGoogle Scholar |
Werner, P. A., and Prior, L. D. (2007). Tree-piping termites and growth and survival of host trees in savanna woodland of north Australia. Journal of Tropical Ecology 23, 611–622.
| Tree-piping termites and growth and survival of host trees in savanna woodland of north Australia.Crossref | GoogleScholarGoogle Scholar |
Whelan, R. J., Rodgerson, L., Dickman, C. R., and Sutherland, E. F. (2002). Critical life cycles of plants and animals: developing a process-based understanding of population changes in fire-prone environments. In ‘Flammable Australia: The Fire Regimes and Biodiversity of a Continent’. (Eds R. A. Bradstock, J. E. Williams and A. M. Gill.) pp. 94–124. (Cambridge University Press: Cambridge, UK.)
Whitehead, P. J., and Saalfeld, K. (2000). Nesting phenology of Magpie Geese (Anseranas semipalmata) in monsoonal northern Australia: responses to antecedent rainfall. Journal of Zoology 251, 495–508.
| Nesting phenology of Magpie Geese (Anseranas semipalmata) in monsoonal northern Australia: responses to antecedent rainfall.Crossref | GoogleScholarGoogle Scholar |
Whitehead, P. J., Bowman, D. M. J. S., and Tidemann, S. C. (1992). Biogeographic patterns, environmental correlates and conservation of avifauna in the Northern Territory, Australia. Journal of Biogeography 19, 151–161.
| Biogeographic patterns, environmental correlates and conservation of avifauna in the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Whitehead, P. J., Bowman, D. M. J. S., Preece, N., Fraser, F., and Cooke, P. (2003). Customary use of fire by indigenous peoples in northern Australia: its contemporary role in savanna management. International Journal of Wildland Fire 12, 415–425.
| Customary use of fire by indigenous peoples in northern Australia: its contemporary role in savanna management.Crossref | GoogleScholarGoogle Scholar |
Whitehead, P. J., Purdon, P., Cooke, P. M., Russell-Smith, J., and Sutton, S. (2009). The West Arnhem land Fire Abatement (WALFA) project: the institutional environment and its implications. In ‘Culture, Ecology and Economy of Fire Management in Northern Australia: Rekindling the Wurrk Tradition’. (Eds J. Russell-Smith, P. J. Whitehead and P. Cooke.) pp. 287–312. (CSIRO Publishing: Melbourne.)
Williams, M. A. J. (1991). Evolution of the landscape. In ‘Monsoonal Australia: Landscape, Ecology and Man in the Northern Lowlands’. (Eds C. D. Haynes, M. G. Ridpath and M. A. J. Williams.) pp. 5–17. (Balkema Press: Rotterdam.)
Williams, R. J., Cook, G. D., Gill, A. M., and Moore, P. H. R. (1999). Fire regimes, fire intensity and tree survival in a tropical savanna in northern Australia. Australian Journal of Ecology 24, 50–59.
| Fire regimes, fire intensity and tree survival in a tropical savanna in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, R. J., Gill, A. M., and Moore, P. H. R. (2003a). Fire behavior. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 33–46. (Springer-Verlag: New York.)
Williams, R. J., Muller, W. J., Wahren, C.-H., Setterfield, S. A., and Cusack, J. (2003b). Vegetation. In ‘Fire in Tropical Savannas: The Kapalga Experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams.) pp. 79–106. (Springer-Verlag: New York.)
Williams, R. J., Woinarski, J. C. Z., and Andersen, A. N. (2003c). Fire experiments in northern Australia: lessons for ecology, management and biodiversity conservation. International Journal of Wildland Fire 12, 391–402.
| Fire experiments in northern Australia: lessons for ecology, management and biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Williams, R. J., Bradstock, R. A., Cary, G. J., Enright, N. J., Gill, A. M., Liedloff, A. C., Lucas, C., Whelan, R. J., Andersen, A. N., Bowman, D. M. J. S., Clarke, P. J., Cook, G. D., Hennessy, K. J., and York, A. (2009). Interactions between climate change, fire regimes and biodiversity in Australia – a preliminary assessment. Report to the Department of Climate Change and Department of the Environment, Water, Heritage and the Arts, Canberra. CSIRO, Darwin. Available at http://www.climatechange.gov.au/sites/climatechange/files/documents/04_2013/20100630-climate-fire-biodiversity-PDF.pdf [Verified 21 July 2013]
Winter, J. W., Dillewaard, H. A., Williams, S. E., and Bolitho, E. E. (2004). Possums and gliders of north Queensland: distribution and conservation status. In ‘The Biology of Australian Possums and Gliders’. (Eds R. L. Goldingay and S. M. Jackson.) pp. 26–50. (Surrey Beatty & Sons: Sydney.)
Woinarski, J. C. Z. (1990). Effects of fire on bird communities of tropical woodlands and open forests in northern Australia. Australian Journal of Ecology 15, 1–22.
| Effects of fire on bird communities of tropical woodlands and open forests in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z. (1993). A cut-and-paste community: birds of monsoon rainforests in Kakadu National Park, Northern Territory. Emu 93, 100–120.
| A cut-and-paste community: birds of monsoon rainforests in Kakadu National Park, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z. (1999). Fire and Australian birds: a review. In ‘Australia’s Biodiversity – Responses to Fire: Plants, Birds and Invertebrates’. Biodiversity Technical Paper number 1. pp. 55–111. (Environment Australia: Canberra.)
Woinarski, J. C. Z. (2004). The forest fauna of the Northern Territory: knowledge, conservation and management. In ‘Conservation of Australia’s Forest Fauna’. 2nd edn. (Ed. D. Lunney). pp. 36–55. (Royal Zoological Society of New South Wales: Sydney.)
Woinarski, J. C. Z., and Ash, A. J. (2002). Responses of vertebrates to pastoralism, military land use and landscape position in an Australian tropical savanna. Austral Ecology 27, 311–323.
| Responses of vertebrates to pastoralism, military land use and landscape position in an Australian tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Fisher, A. (1995a). Wildlife of Lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 1. Variation in vertebrate species composition across the environmental range occupied by Lancewood vegetation in the Northern Territory. Wildlife Research 22, 379–411.
| Wildlife of Lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 1. Variation in vertebrate species composition across the environmental range occupied by Lancewood vegetation in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Fisher, A. (1995b). Wildlife of Lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 2. Comparisons with other environments of the region (Acacia woodlands, Eucalyptus savanna woodlands and monsoon rainforests). Wildlife Research 22, 413–443.
| Wildlife of Lancewood (Acacia shirleyi) thickets and woodlands in northern Australia. 2. Comparisons with other environments of the region (Acacia woodlands, Eucalyptus savanna woodlands and monsoon rainforests).Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Fisher, A. (2003). Conservation and the maintenance of biodiversity in the rangelands. Rangeland Journal 25, 157–171.
| Conservation and the maintenance of biodiversity in the rangelands.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Recher, H. F. (1997). Impact and response: a review of the effects of fire on the Australian avifauna. Pacific Conservation Biology 3, 183–205.
Woinarski, J. C. Z., and Tidemann, S. C. (1991). The bird fauna of a deciduous woodland in the wet-dry tropics of northern Australia. Wildlife Research 18, 479–500.
| The bird fauna of a deciduous woodland in the wet-dry tropics of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., and Westaway, J. (2008). Hollow formation in the Eucalyptus miniata – E. tetrodonta open forests and savanna woodlands of tropical northern Australia. Final report to Land and Water Australia. Department of Natural Resources, Environment, The Arts and Sport, Darwin.
Woinarski, J. C. Z., Recher, H. F., and Majer, J. (1997). Vertebrates of eucalypt formations. In ‘Eucalypt Ecology: Individuals to Ecosystems’. (Eds J. E. Williams and J. C. Z. Woinarski.) pp. 303–341. (Cambridge University Press: Cambridge, UK.)
Woinarski, J. C. Z., Fisher, A., and Milne, D. (1999a). Distribution patterns of vertebrates in relation to an extensive rainfall gradient and soil variation in the tropical savannas of the Northern Territory, Australia. Journal of Tropical Ecology 15, 381–398.
| Distribution patterns of vertebrates in relation to an extensive rainfall gradient and soil variation in the tropical savannas of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Brock, C., Fisher, A., Milne, D., and Oliver, B. (1999b). Response of birds and reptiles to fire regimes on pastoral land in the Victoria River District, Northern Territory. Rangeland Journal 21, 24–38.
| Response of birds and reptiles to fire regimes on pastoral land in the Victoria River District, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Brock, C., Armstrong, M., Hempel, C., Cheal, D., and Brennan, K. (2000a). Bird distribution in riparian vegetation of an Australian tropical savanna: a broad-scale survey and analysis of a distributional data base. Journal of Biogeography 27, 843–868.
| Bird distribution in riparian vegetation of an Australian tropical savanna: a broad-scale survey and analysis of a distributional data base.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Franklin, D., and Connors, G. (2000b). Thinking honeyeater: nectar maps for the Northern Territory, Australia, showing spatial and temporal variation in nectar availability. Pacific Conservation Biology 6, 61–80.
Woinarski, J. C. Z., Milne, D. J., and Wanganeen, G. (2001). Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia. Austral Ecology 26, 360–370.
| Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Risler, J., and Kean, L. (2004a). The response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| The response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Armstrong, M., Price, O., McCartney, J., Griffiths, T., and Fisher, A. (2004b). The terrestrial vertebrate fauna of Litchfield National Park, Northern Territory: monitoring over a 6-year period and response to fire history. Wildlife Research 31, 587–596.
| The terrestrial vertebrate fauna of Litchfield National Park, Northern Territory: monitoring over a 6-year period and response to fire history.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Williams, R. J., Price, O., and Rankmore, B. (2005). Landscapes without boundaries: wildlife and their environments in northern Australia. Wildlife Research 32, 377–388.
| Landscapes without boundaries: wildlife and their environments in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Hempel, C., Cowie, I., Brennan, K., Kerrigan, R., Leach, G., and Russell-Smith, J. (2006). Distributional patterns of plant species endemic to the Northern Territory, Australia. Australian Journal of Botany 54, 627–640.
| Distributional patterns of plant species endemic to the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., Mackey, B., Nix, H., and Traill, B. (2007a). ‘The Nature of Northern Australia: Its Natural Values, Ecological Processes and Future Prospects.’ (ANU Press: Canberra.)
Woinarski, J., Pavey, C., Kerrigan, R., Cowie, I., and Ward, S. (2007b). ‘Lost From Our Landscape: Threatened Species of the Northern Territory.’ (NT Government Printer: Darwin.)
Woinarski, J., Hokkanen, R., Milne, D., and Armstrong, M. (2008). ‘Long-term Vertebrate Fauna Monitoring. Rio Tinto Alcan Gove Rehabilitation Monitoring.’ (NT Department of Natural Resources, Environment and The Arts: Darwin.)
Woinarski, J. C. Z., Russell-Smith, J., Andersen, A., and Brennan, K. (2009a). Fire management and biodiversity of the western Arnhem Land Plateau. In ‘Culture, Ecology and Economy of Fire Management in Northern Australia: Rekindling the Wurrk Tradition’. (Eds J. Russell-Smith, P. J. Whitehead and P. Cooke.) pp. 201–228. (CSIRO Publishing: Melbourne.)
Woinarski, J. C. Z., Rankmore, B., Hill, B., Griffiths, A. D., Stewart, A., and Grace, B. (2009b). Fauna assemblages in regrowth vegetation in tropical open forests of the Northern Territory, Australia. Wildlife Research 36, 675–690.
| Fauna assemblages in regrowth vegetation in tropical open forests of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Armstrong, M., Brennan, K., Fisher, A., Griffiths, A. D., Hill, B., Milne, D. J., Palmer, C., Ward, S., Watson, M., Winderlich, S., and Young, S. (2010). Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37, 116–126.
| Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Legge, S., Fitzsimons, J. A., Traill, B. J., Burbidge, A. A., Fisher, A., Firth, R. S. C., Gordon, I. J., Griffiths, A. D., Johnson, C. N., McKenzie, N. L., Palmer, C., Radford, I., Rankmore, B., Ritchie, E. G., Ward, S., and Ziembicki, M. (2011). The disappearing mammal fauna of northern Australia: context, cause and response. Conservation Letters 4, 192–201.
| The disappearing mammal fauna of northern Australia: context, cause and response.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Fisher, A., Armstrong, M., Brennan, K., Griffiths, A. D., Hill, B., Low Choy, J., Milne, D., Stewart, A., Young, S., Ward, S., Winderlich, S., and Ziembicki, M. (2012). Monitoring indicates greater resilience for birds than for mammals in Kakadu National Park, northern Australia. Wildlife Research 39, 397–407.
| Monitoring indicates greater resilience for birds than for mammals in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Green, J., Fisher, A., Ensbey, M., and Mackey, B. (2013). The effectiveness of conservation reserves: land tenure impacts upon biodiversity across extensive natural landscapes in the tropical savannas of the Northern Territory, Australia. Land 2, 20–36.
| The effectiveness of conservation reserves: land tenure impacts upon biodiversity across extensive natural landscapes in the tropical savannas of the Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Yates, C. P., Edwards, A. C., and Russell-Smith, J. (2008). Big fires and their ecological impacts in Australian savannas: size and frequency matters. International Journal of Wildland Fire 17, 768–781.
| Big fires and their ecological impacts in Australian savannas: size and frequency matters.Crossref | GoogleScholarGoogle Scholar |
Yibarbuk, D., Whitehead, P. J., Russell-Smith, J., Jackson, D., Godjuwa, C., Fisher, A., Cooke, P., Choquenot, D., and Bowman, D. M. J. S. (2001). Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: a tradition of ecosystem management. Journal of Biogeography 28, 325–343.
| Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: a tradition of ecosystem management.Crossref | GoogleScholarGoogle Scholar |
Ziembicki, M. (2009). Ecology and movements of the Australian Bustard Ardeotis australis in a dynamic landscape. Ph.D. Thesis, University of Adelaide.