Fairy-wrens and their relatives (Maluridae) as model organisms in evolutionary ecology: the scientific legacy of Ian Rowley and Eleanor Russell
Katherine L. Buchanan A B D and Andrew Cockburn CA School of Life and Environmental Sciences, Deakin University, Geelong, Vic. 3216, Australia.
B Editor – Emu: AUSTRAL ORNITHOLOGY.
C Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, ACT 0200, Australia.
D Corresponding author. Email: kate.buchanan@deakin.edu.au
Emu 113(3) i-vii https://doi.org/10.1071/MUv113n3_ED
Published: 15 August 2013
The ornithologist, publisher and entrepreneur John Gould was the first to hint at the truly remarkable complexity of the behaviour of fairy-wrens (Malurus spp.) (Gould 1840–1848). In his published lithograph of Superb Fairy-wrens (Malurus cyaneus) he shows three adults attending a nest (Fig. 1). Two brilliantly coloured males are both carrying food to the nest, watched by a single dull brown female. However, the males are being duped, for the chick poking out of the nest is not their own offspring, but a bronze-cuckoo (Chalcites sp.) brood-parasite. This early lithograph encompasses much of the fascination that has endured with this group as well as virtually all the themes addressed in this special issue on the family of fairy-wrens Maluridae: sexual signalling, cooperative care of nestlings and the evolutionary paradox that male fairy-wrens often care for young that are not their own.

|
Most accounts of Malurus fairy-wrens by early European naturalists alluded to the possibility that they were cooperative breeders (Boland and Cockburn 2002), the social system in which more than two birds share the task of raising a brood of young. Indeed, the fact that fairy-wrens are cooperative breeders seems to have been common knowledge decades before the behaviour was supposedly ‘discovered’ by Alexander Skutch (Rowley 1957, Skutch 1961), the great student of neotropical ornithology. In hindsight, it is easy to see why cooperative breeding, which is now known to occur in ~10% of bird species (Cockburn 2006) and is particularly prevalent in the Australian avifauna, was so obvious in fairy-wrens. They typically build nests close to the ground, and some species adapt well to gardens, so their nests are easy to find and often easily observed by amateurs and professionals alike. In addition, and in contrast to most cooperatively breeding species where males and females are essentially monomorphic (Stacey and Koenig 1990), the pronounced sexual dimorphism in fairy-wrens and frequent attendance at the nest of two breeding-plumaged bright males, makes cooperative behaviour straightforward to detect. However, although cooperative breeding was identified, it attracted little attention for much of the twentieth century, nor was there any real conceptual explanation as to why males and females in these species were so strikingly different in plumage.
We owe the formal documentation of cooperative breeding in fairy-wrens to a technique that is now so commonplace, that it seems astonishing that it was not attempted in cooperative species until the 1950s. This revolutionary method involved attaching unique combinations of coloured plastic rings to the legs of birds so they could be individually identified (Rowley 1957; Bradley and Bradley 1958). It was CSIRO scientist Ian Rowley who developed this method with Malurus as a side project while working on rabbit control in Canberra. He used colour-ringing of the birds near the CSIRO Gungahlin laboratories to analyse their behaviour in unprecedented detail, culminating in one of the most influential papers (Rowley 1964) ever published in Emu. Indeed, that paper is currently the most highly cited paper from the entire archive of Emu – Austral Ornithology. Ian was then directed by CSIRO to investigate the effect that crows and ravens (Corvus spp.) have on sheep. This similarly distinguished work of his clarified the taxonomy and ecology of the Australian Corvus resulting in recognition of two further Australian corvid species: Little (C. mellori) and Forest Ravens (C. tasmanicus). However, he found that he often caught groups of White-winged Choughs (Corcorax melanoramphos) in his corvid traps, enabling him to conduct another classic study on cooperative breeding in this very different species (Rowley 1978). This research experience placed him at the forefront of attempts to understand cooperative breeding and at a time when the behaviour had attracted new interest worldwide. Not only had several long-term field studies commenced, but cooperative behaviour had assumed centre stage as a ‘problem’ in the newly founded discipline of behavioural ecology. That endeavour had rejected the naïve arguments proposed by Alexander Skutch and many others that cooperative breeding was a form of reproductive restraint that operated for the good of the species (Skutch 1961). New ideas concerning the benefits of investing in kin were starting to take hold.
Ian then moved to the CSIRO laboratories in Western Australia to examine the effect of the most-familiar Australian cockatoo, the Galah (Eolophus roseicapillus), on agriculture. Once again, this led to more classic work (Rowley 1990), but also brought Ian into close contact with a new and diverse group of fairy-wrens. Eventually, he conducted work on five of the Western Australian Malurus species (Rowley et al. 1988, 1991; Rowley 1993; Rowley and Russell 1995, 2002), initiating a long-term study on the Splendid Fairy-wren (Malurus splendens), and collaborating with Dick and Molly Brown on a separate long-term study of the Red-winged Fairy-wren (M. elegans) (Rowley et al. 1988). Ian’s wife Eleanor Russell, who had achieved a considerable reputation as a marsupial biologist (Russell 1982, 1984), became increasingly involved in this work. She brought analytical rigour that led to two major papers summarising the demography of these species (Russell and Rowley 1993, 2000). The couple (Fig. 2) also elegantly compared the malurids in a wonderful monograph in the Oxford Bird Families series (Rowley and Russell 1997).
Eleanor was also the first to appreciate the significance of the massive advances in avian systematics to ecology that came from the application of DNA–DNA hybridisation studies by Charles Sibley and Jon Ahlquist (Sibley and Ahlquist 1985). This work rearranged much of the avian phylogenetic tree, but arguably the most radical changes lay in our understanding of the evolution of Australian songbirds (passerines). They had been thought to have originated in a series of invasions from Asia (e.g. Mayr 1944). Instead, Sibley and Ahlquist (1985; see also Christidis and Schodde 1991; Barker et al. 2002, 2004; Ericson et al. 2002) showed that the Australo-Papuan region is now known to have been the place of origin of the advanced songbirds (the oscines). This is despite a major radiation of oscines (the Passerida) having diversified outside the region after one lineage of Australo-Papuan passerines dispersed out of it (Barker et al. 2004). Eleanor demonstrated that, although the older lineages of Australian songbirds were commonly cooperative breeders, the Passerida that had secondarily re-invaded Australia never bred cooperatively (Russell 1989). Indeed, other older lineages of Australo-Papuan core Corvoidea songbirds dispersed and radiated in the rest of the world and they have often taken their cooperative habits with them (Cockburn 2003, 2006). Hence, we need to take phylogeny, as well as ecology into account when explaining cooperative breeding. This insight has been confirmed repeatedly, but is still resistant to a coherent explanation (Cockburn 2003; Cockburn and Russell 2011).
While working in Western Australia, Ian worked closely with Mike and Lesley Brooker, who used studies of fairy-wrens to make a series of novel contributions to conservation biology, exploiting the ravages of fire at some study sites. This strand of work included important papers on habitat fragmentation (Brooker and Brooker 2001, 2003). One paper of Ian’s that attracted particular attention in the conservation literature was based on the observation that as a consequence of limited dispersal, breeding pairs of fairy-wrens were often close relatives (Rowley et al. 1986). However, the offspring of such incestuous pairing suffered no detectable disadvantage, compared to those produced by pairs where the parents were unrelated. This provided conservation biologists with the tantalising possibility that if there were few deleterious effects of incest in a natural population when inbreeding was naturally common, the prognosis for the preservation of small, vulnerable populations could be improved by using controlled inbreeding to purge deleterious alleles from those populations. However, the reliability of the results from fairy-wrens was questioned, because females have several males to choose from on their territory. This prompted an exploration of the Malurus breeding system using molecular techniques to ascribe paternity and dissect the parentage in Splendid Fairy-wrens. This produced one of the most surprising results in all behavioural ecology – most fairy-wren nestlings are unrelated to any of the males that care for them (Dunn et al. 1995), because most fertilisations are gained by extra-group males living on other territories (Brooker et al. 1990), inbreeding not being the explanation at all.
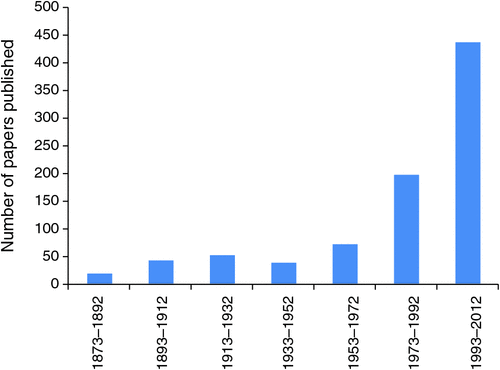
Alongside these events in the late 1980s, Andrew Cockburn’s group at the Australian National University and Steve Pruett-Jones’ group at the University of Chicago began work on fairy-wrens, both initially prompted by an interest in cooperative breeding. The remarkable discovery of infidelity gave new impetus to their research. Indeed, 9 of the 11 contributions to this special issue come from authors who have had an association with either the Cockburn or the Pruett-Jones laboratories at some time. In the 1980s there was disapproval among some Australian ornithologists of the prospect of these young upstarts working on a group of birds that someone else had already ‘claimed’. The exceptions to this disapproval were Ian and Eleanor, who greeted the newcomers and their students and postdoctoral fellows with enthusiasm, encouragement, and an eagerness to share the benefits of their considerable experience. The groundwork that they had established in documenting the behaviour and ecology of malurids was surely fundamental in allowing the development of subsequent research and the establishment of this group as a model system for study in many areas (Fig. 3).
|
It is clear from the contents of this special issue that extra-group parentage continues to fascinate and perplex researchers working on Malurus species. Fairy-wrens provide a remarkable opportunity to study the phenomenon of sexual selection, one of the other predominant problems of behavioural ecology. We know that in many species female preference for males with extravagant traits can lead to strange but wonderful evolutionary trajectories, manifested in the Australo-Papuan avifauna most extravagantly in groups such as birds-of-paradise (Passeriformes : Paradisaeidae). In birds-of-paradise and other taxa with exaggerated male plumage it is often the case that the only resource females obtain from males is the DNA in the sperm required to fertilise their eggs. However, in many species all females often prefer to mate with exactly the same male, suggesting that they detect subtle differences in male quality that might improve the quality of their own offspring. The theoretical difficulty posed by this behaviour arises because strong female preference should fix any beneficial genetic variants in the population, eroding any differences in quality among males, and eliminating the benefits of this mate choice. Female choice for exaggerated displays or plumage should therefore result in a population of males that don’t differ in these traits – but this is not observed. This problem has been called the paradox of the lek (Kirkpatrick and Ryan 1991), after the lek mating system in which males aggregate to display to females. The true lekking birds are often denizens of remote rainforest, and are extremely difficult to study in the field. When it became clear that extra-pair mating was common among birds, it was initially hoped that such mating systems would provide an alternative way of studying the paradox of the lek. However, it soon became clear that there are several explanations for extra-pair mating, many of which bear little resemblance to the unanimous preference exhibited by true lekking birds (Griffith and Immler 2009). Fairy-wrens allow us to overcome both these difficulties, because female preferences are unanimous, extra-pair mating is universal rather than conditional, and at least some of the species are very easy to study (Cockburn et al. 2013).
Although adaptive explanations can be produced for the occurrence of extra-pair matings, this mating system also raises many other conceptual difficulties. Most important, the issue of male care becomes much more difficult to understand, because the males that diligently feed young on their own territory, investing considerable time and effort, will often be caring for nestlings to which they are unrelated. Why males tolerate repeated cuckoldry remains an outstanding question.
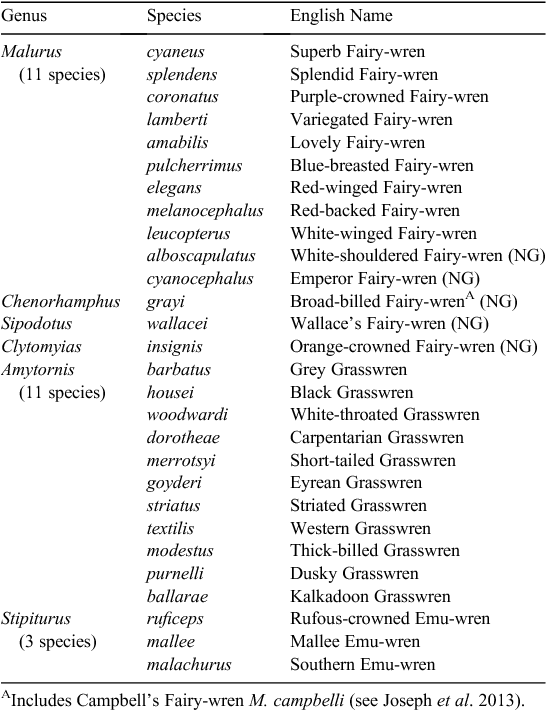
Another problem arose as work on Malurus expanded. Variety, they say is the spice of life, but in Malurus it appears to make it difficult to draw any generally applicable conclusions. For example, there is evidence in one species that cooperative breeding is based on a shortfall of females (Pruett-Jones and Lewis 1990). But this sits uncomfortably with the observation across species that non-breeding females are most common in the species where helpers are most prevalent (Margraf and Cockburn 2013). Similarly, extra-pair mating makes sense for populations where incestuous pairing is common (Brooker et al. 1990), but the conclusion that this exists as an adaptive response to minimise inbreeding is undermined when extra-pair mating is most common where incestuous pairing is almost absent (Cockburn et al. 2013). Most scandalous, and bucking the trend, there is even a monogamous fairy-wren, the Purple-crowned Fairy-wren (M. coronatus) (Kingma et al. 2009). Throughout this special issue, papers highlight again and again this tremendous diversity within the family and demonstrate how study of current diversity across closely related species can yield fundamental insights into evolution and ecology. Across the family some genera contain many species, whereas others are monotypic having just one present-day species (Table 1). A now solid phylogenetic framework (reviewed by Joseph et al. 2013) allows the comparison of closely related and more distantly related species. By comparing phenotypic traits against that phylogenetic framework, it is possible to infer the key evolutionary drivers of change within the group. This approach can help us to understand the current diversity in variables, such as mating system or plumage colouration. Owing to the investment made by researchers in this group over the last 45 years, the Maluridae has provided tremendous insight, not only into the ecological and behavioural diversity of its member species, but also into the fundamental processes driving avian evolution.
|
In the first paper of the issue, Joseph et al. (2013) review how phylogenetic and phylogeographic analyses of the Maluridae have helped pioneer the integration of ecological and molecular data to achieve an understanding of the evolutionary history of a family. In this paper the authors review the group as a whole and what we can deduce about the evolutionary origins of its diversity, particularly in plumage colours and patterns seen today. Studies from this group demonstrate the importance of considering the sampling strategy, at the nucleotide, individual and species levels for inferring phylogenetic relationships (Joseph et al. 2013). Such studies also contribute to our understanding of the rates of evolution within both the nuclear and mitochondrial genomes and what this might mean for speciation processes. Joseph et al. review the molecular evidence within and between each of the three major malurid taxonomic tribes and infer historic patterns of taxonomic divergence from which we can infer past distributions. Their review helps explain the origins of the diversity seen within Maluridae, clarifies evolutionary patterns and encourages future development of interdisciplinary approaches making use of molecular data that can enhance our understanding of evolutionary processes, especially selection and genotype–environment interactions.
Understanding the relationships between species within Maluridae is crucial if we are to understand whether the traits we see in present day species are ancestral (evolved early in the history of the group), or derived (have appeared more recently). Using the well documented relationships among species it is now possible to ask whether the strikingly high levels of infidelity seen in some species are a derived. Two research groups use this approach to ask what factors have driven variation in mating systems (Cockburn et al. 2013) and sperm competition (Rowe and Pruett-Jones 2013) within the Maluridae. Cockburn et al. (2013) review the origins of the high levels of promiscuity seen in many (although not all) species. They examine the theoretical problems these data raise and suggest hypotheses to explain high levels of infidelity. Rowe and Pruett-Jones (2013) also tackle the evolutionary origin of this extraordinary state of affairs, concluding that increased sperm competition and intense sexual selection are derived traits not present in ancestral malurids. Both papers demonstrate the importance of the Maluridae for the study of how mating systems evolve and the drivers of sexual selection.
Diversity between species also lies at the heart of the studies reviewed by Langmore (2013) documenting the prevalence of nest parasitism of Maluridae members by cuckoos. This diversity allows for quantification of factors promoting nest parasitism, as well as the study of nest defence mechanisms. Langmore discusses the host–parasite evolutionary arms race, which has given rise to a situation where fairy-wrens are unable to discriminate the eggs of the brood parasite’s eggs from their own, but are able to discriminate and reject the chicks (Langmore 2013). Little is known about how the movements of cuckoos might underlie the highly variable rates of nest parasitism at some sites (Langmore 2013).
If the Maluridae are recognised for their variable, and often high levels of mate infidelity, they are also iconic symbols of bright plumage colouration, mostly in breeding males. However, within the group, plumage colours and patterns as with other phenotypic traits, show high levels of inter-specific variability. The factors controlling the bright plumage colours of male Malurus fairy-wrens are discussed by Peters et al. (2013). From the mechanistic angle they discuss how the colours are produced and the effect of the endocrine system on signal production. The relationship between age and the production of breeding plumage is discussed – a relationship that sees considerable variability within Malurus sp. Although the moult into bright breeding plumage is hypothesised to be costly to males, the nature of this cost and therefore the reasons underlying this inter-specific variability are still poorly understood. Peters et al. (2013) highlight the untested role of male–male competition in the evolution of the bright, gaudy colours seen in this group, as well as the potential role of condition in controlling the quality of the signal. In a complementary paper, Karubian (2013) addresses the factors underlying selection on female plumage traits in Malurus. Within the group, females are mostly drab in comparison to males, however the authors conclude that both plumage and bill colour in females are the product of selective processes that are independent of those acting on males. This paper certainly highlights the potential for demonstrating how selection is acting through sexual differences in morphological traits both at the intra- and inter-specific level.
Inter-specific variation is further considered by Greig et al. (2013), who examine variation in song structure in relation to levels of sperm competition, but within the phylogenetic framework of relationships within Maluridae. In particular, the authors have sought to quantify the effects of the strength of sexual selection on both song structure and complexity. Interestingly, they also report strong effects of latitude on song structure. Changes in song structure are associated with a transition between temperate and tropical climates, which appears to be related to factors other than habitat per se. Similarly, vocal variation at the intra-specific level is examined on a finer scale by Kleindorfer et al. (2013), using empirical field data. In their study, they test whether two subspecies of Superb Fairy-wrens differ in either their song structure or their response to playback of songs. The finding that these two subspecies (one on an island and the other on nearby mainland) do differ in both vocal performance and response suggests an important role for bioacoustics in the speciation process within the group. Of course, singing is not just the job of male fairy wrens, as females also participate in vocalisations. The third paper in this special issue addressing vocal behaviour in the group focuses on duetting in the Red-backed Fairy-wren (Dowling and Webster 2013). On balance, the authors conclude that duetting probably serves, at least in part, as a territorial defence function. The authors do acknowledge that the behaviour may also serve to coordinate the pair in a species having a moderately high rate of promiscuity. Making sure your partner is singing from the same song sheet seems an adaptive strategy for maximising your fitness!
Just as with other ecological traits, there is tremendous variation between different Malurid species in their vulnerability. Skroblin and Murphy (2013) review the conservation status of malurids and report on how effective conservation management strategies have been applied to the species. Some members of the family are so numerous that they are common back yard birds, even in urban areas. Other more restricted species (particularly some grasswrens, Amytornis spp., and emu-wrens, Stipiturus spp.) are threatened by habitat loss, owing to numerous complex and interacting environmental factors, a critical one being fire. However, this paper highlights the fundamental contribution of malurids to understanding how species respond to threatening processes and therefore how effective management strategies may be developed to limit species’ declines.
Together, the papers in this special issue demonstrate that the importance of the Maluridae as a model family lies in its diversity, whether ecological, phylogenetic or biogeographic. Part of the reason for this lies in the investment made by early researchers, and in particular the Rowleys, who documented the basic species’ ecology and demonstrated the variability within the family. This special issue would not exist without their extraordinary research and kindness, and we dedicate it to Ian and Eleanor, with profound admiration and gratitude. Future work will without doubt maintain the Maluridae as a key group for the study of avian evolution, given the historical investment in documenting key ecological characteristics across the group. Despite this investment, many unanswered questions remain. In particular, explaining the adaptive basis for the variation in mating systems seen across the group remains a question for future investigation. We hope that this special issue will bring such questions into sharper focus and promote another generation of research leading to their resolution. In that way, the legacy of Ian Rowley and Eleanor Russell will endure long into the future.
References
Barker, F. K., Barrowclough, G. F., and Groth, J. G. (2002). A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proceedings of the Royal Society of London – B. Biological Sciences 269, 295–308.| A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data.Crossref | GoogleScholarGoogle Scholar |
Barker, F. K., Cibois, A., Schikler, P., Feinstein, J., and Cracraft, J. (2004). Phylogeny and diversification of the largest avian radiation. Proceedings of the National Academy of Sciences of the United States of America 101, 11040–11045.
| Phylogeny and diversification of the largest avian radiation.Crossref | GoogleScholarGoogle Scholar |
Boland, C. R. J., and Cockburn, A. (2002). Short sketches from the long history of cooperative breeding in Australian birds. Emu 102, 9–17.
| Short sketches from the long history of cooperative breeding in Australian birds.Crossref | GoogleScholarGoogle Scholar |
Bradley, E., and Bradley, J. (1958). Notes on the behaviours and plumage of colour-ringed Blue Wrens. Emu 58, 313–326.
| Notes on the behaviours and plumage of colour-ringed Blue Wrens.Crossref | GoogleScholarGoogle Scholar |
Brooker, M., and Brooker, L. (2001). Breeding biology, reproductive success and survival of Blue-breasted Fairy-wrens in fragmented habitat in the Western Australian wheatbelt. Wildlife Research 28, 205–214.
| Breeding biology, reproductive success and survival of Blue-breasted Fairy-wrens in fragmented habitat in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Brooker, M., and Brooker, L. (2003). Brood parasitism by Horsfield’s Bronze-Cuckoo in a fragmented agricultural landscape in Western Australia. Emu 103, 357–361.
| Brood parasitism by Horsfield’s Bronze-Cuckoo in a fragmented agricultural landscape in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Brooker, M. G., Rowley, I., Adams, M., and Baverstock, P. R. (1990). Promiscuity: an inbreeding avoidance mechanism in a socially monogamous species? Behavioral Ecology and Sociobiology 26, 191–200.
Christidis, L., and Schodde, R. (1991). Relationships of Australo-Papuan songbirds: protein evidence. Ibis 133, 277–285.
| Relationships of Australo-Papuan songbirds: protein evidence.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A. (2003). Cooperative breeding in oscine passerines: does sociality inhibit speciation? Proceedings of the Royal Society of London – B. Biological Sciences 270, 2207–2214.
| Cooperative breeding in oscine passerines: does sociality inhibit speciation?Crossref | GoogleScholarGoogle Scholar |
Cockburn, A. (2006). Prevalence of different modes of parental care in birds. Proceedings of the Royal Society of London – B. Biological Sciences 273, 1375–1383.
| Prevalence of different modes of parental care in birds.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A., and Russell, A. F. (2011). Cooperative breeding: a question of climate? Current Biology 21, R195–R197.
| Cooperative breeding: a question of climate?Crossref | GoogleScholarGoogle Scholar |
Cockburn, A., Brouwer, L., Double, M. C., Margraf, N., and van de Pol, M. (2013). Evolutionary origins and persistence of infidelity in Malurus: the least faithful birds. Emu 113, 208–217.
| Evolutionary origins and persistence of infidelity in Malurus: the least faithful birds.Crossref | GoogleScholarGoogle Scholar |
Dowling, J., and Webster, M. S. (2013). The form and function of duets and choruses in Red-backed Fairy-wrens. Emu 113, 282–293.
| The form and function of duets and choruses in Red-backed Fairy-wrens.Crossref | GoogleScholarGoogle Scholar |
Dunn, P. O., Cockburn, A., and Mulder, R. A. (1995). Fairy-wren helpers often care for young to which they are unrelated. Proceedings of the Royal Society of London – B. Biological Sciences 259, 339–343.
| Fairy-wren helpers often care for young to which they are unrelated.Crossref | GoogleScholarGoogle Scholar |
Ericson, P. G. P., Christidis, L., Cooper, A., Irestedt, M., Jackson, J., Johansson, U. S., and Norman, J. A. (2002). A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proceedings of the Royal Society of London – B. Biological Sciences 269, 235–241.
| A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens.Crossref | GoogleScholarGoogle Scholar |
Gould, J. (1840–1848) ‘The Birds of Australia Vol. III.’ (Taylor: London.) [36 parts in seven volumes].
Greig, E. I., Price, J. J., and Pruett-Jones, S. (2013). Song evolution in Maluridae: influences of natural and sexual selection on acoustic structure. Emu 113, 270–281.
| Song evolution in Maluridae: influences of natural and sexual selection on acoustic structure.Crossref | GoogleScholarGoogle Scholar |
Griffith, S. C., and Immler, S. (2009). Female infidelity and genetic compatibility in birds: the role of the genetically loaded raffle in understanding the function of extrapair paternity. Journal of Avian Biology 40, 97–101.
| Female infidelity and genetic compatibility in birds: the role of the genetically loaded raffle in understanding the function of extrapair paternity.Crossref | GoogleScholarGoogle Scholar |
Joseph, L., Edwards, S. V., and McLean, A. J. (2013). The Maluridae: inferring avian biology and evolutionary history from DNA sequences. Emu 113, 195–207.
| The Maluridae: inferring avian biology and evolutionary history from DNA sequences.Crossref | GoogleScholarGoogle Scholar |
Karubian, J. (2013). Female ornamentation in Malurus fairy-wrens: a hidden evolutionary gem for understanding female perspectives on social and sexual selection. Emu 113, 248–258.
| Female ornamentation in Malurus fairy-wrens: a hidden evolutionary gem for understanding female perspectives on social and sexual selection.Crossref | GoogleScholarGoogle Scholar |
Kingma, S. A., Hall, M. L., Segelbacher, G., and Peters, A. (2009). Radical loss of an extreme extra-pair mating system. BMC Ecology 9, 15.
| Radical loss of an extreme extra-pair mating system.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, M., and Ryan, M. J. (1991). The evolution of mating preferences and the paradox of the lek. Nature 350, 33–38.
| The evolution of mating preferences and the paradox of the lek.Crossref | GoogleScholarGoogle Scholar |
Kleindorfer, S., Evans, C., Mihailova, M., Colombelli-Négrel, D., Hoi, H., Griggio, M., Mahr, K., and Robertson, J. (2013). When subspecies matter: resident Superb Fairy-wrens (Malurus cyaneus) distinguish the sex and subspecies of intruding birds. Emu 113, 259–269.
| When subspecies matter: resident Superb Fairy-wrens (Malurus cyaneus) distinguish the sex and subspecies of intruding birds.Crossref | GoogleScholarGoogle Scholar |
Langmore, N. E. (2013). Fairy-wrens as a model system for studying cuckoo–host coevolution. Emu 113, 302–308.
| Fairy-wrens as a model system for studying cuckoo–host coevolution.Crossref | GoogleScholarGoogle Scholar |
Margraf, N., and Cockburn, A. (2013). Helping behaviour and parental care in fairy-wrens (Malurus). Emu 113, 294–301.
| Helping behaviour and parental care in fairy-wrens (Malurus).Crossref | GoogleScholarGoogle Scholar |
Mayr, E. (1944). Timor and the colonization of Australia by birds. Emu 44, 113–130.
| Timor and the colonization of Australia by birds.Crossref | GoogleScholarGoogle Scholar |
Peters, A., Kingma, S. A., and Delhey, K. (2013). Seasonal male plumage as a multi-component sexual signal: insights and opportunities. Emu 113, 232–247.
| Seasonal male plumage as a multi-component sexual signal: insights and opportunities.Crossref | GoogleScholarGoogle Scholar |
Pruett-Jones, S. G., and Lewis, M. J. (1990). Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens. Nature 348, 541–542.
| Sex ratio and habitat limitation promote delayed dispersal in superb fairy-wrens.Crossref | GoogleScholarGoogle Scholar |
Rowe, M., and Pruett-Jones, S. (2013). Extra-pair paternity, sperm competition and their evolutionary consequences in the Maluridae. Emu 113, 218–231.
| Extra-pair paternity, sperm competition and their evolutionary consequences in the Maluridae.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1957). Cooperative feeding of young by superb blue wrens. Emu 57, 356–357.
| Cooperative feeding of young by superb blue wrens.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1964). The life history of the superb blue wren. Emu 64, 251–297.
| The life history of the superb blue wren.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1978). Communal activities among white-winged choughs Corcorax melanorhamphos. Ibis 120, 178–197.
| Communal activities among white-winged choughs Corcorax melanorhamphos.Crossref | GoogleScholarGoogle Scholar |
Rowley, I. (1990) ‘Behavioural Ecology of the Galah Eolophus roseicapillus in the Wheatbelt of Western Australia.’ (Surrey Beatty & Sons: Sydney, NSW.)
Rowley, I. (1993). The purple-crowned fairy-wren Malurus coronatus. 1. History, distribution and present status. Emu 93, 220–234.
| The purple-crowned fairy-wren Malurus coronatus. 1. History, distribution and present status.Crossref | GoogleScholarGoogle Scholar |
Rowley, I., and Russell, E. (1995). The breeding biology of the White-winged Fairy-wren Malurus leucopterus leuconotus in a Western-Australian coastal heathland. Emu 95, 175–184.
| The breeding biology of the White-winged Fairy-wren Malurus leucopterus leuconotus in a Western-Australian coastal heathland.Crossref | GoogleScholarGoogle Scholar |
Rowley, I., and Russell, E. (1997) ‘Fairy-wrens and Grasswrens: Maluridae.’ (Oxford University Press: Oxford, UK.)
Rowley, I., and Russell, E. (2002). A population study of the Blue-breasted Fairy-wren, Malurus pulcherrimus, at Dryandra, Western Australia. Emu 102, 127–135.
| A population study of the Blue-breasted Fairy-wren, Malurus pulcherrimus, at Dryandra, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rowley, I., Russell, E., and Brooker, M. (1986). Inbreeding: benefits may outweigh costs. Animal Behaviour 34, 939–941.
| Inbreeding: benefits may outweigh costs.Crossref | GoogleScholarGoogle Scholar |
Rowley, I., Russell, E., Brown, R., and Brown, M. (1988). The ecology and breeding biology of the Red-winged Fairy-wren Malurus elegans. Emu 88, 161–176.
| The ecology and breeding biology of the Red-winged Fairy-wren Malurus elegans.Crossref | GoogleScholarGoogle Scholar |
Rowley, I., Brooker, M., and Russell, E. (1991). The breeding biology of the Splendid Fairy-wren Malurus splendens – the significance of multiple broods. Emu 91, 197–221.
| The breeding biology of the Splendid Fairy-wren Malurus splendens – the significance of multiple broods.Crossref | GoogleScholarGoogle Scholar |
Russell, E. M. (1982). Patterns of parental care and parental investment in marsupials. Biological Reviews of the Cambridge Philosophical Society 57, 423–486.
| Patterns of parental care and parental investment in marsupials.Crossref | GoogleScholarGoogle Scholar |
Russell, E. M. (1984). Social behaviour and social organization of maruspials. Mammal Review 14, 101–154.
| Social behaviour and social organization of maruspials.Crossref | GoogleScholarGoogle Scholar |
Russell, E. M. (1989). Co-operative breeding: a Gondwanan perspective. Emu 89, 61–62.
| Co-operative breeding: a Gondwanan perspective.Crossref | GoogleScholarGoogle Scholar |
Russell, E. M., and Rowley, I. (1993). Demography of the cooperatively breeding Splendid Fairy-wren, Malurus splendens (Maluridae). Australian Journal of Zoology 41, 475–505.
| Demography of the cooperatively breeding Splendid Fairy-wren, Malurus splendens (Maluridae).Crossref | GoogleScholarGoogle Scholar |
Russell, E., and Rowley, I. (2000). Demography and social organisation of the Red-winged Fairy-wren, Malurus elegans. Australian Journal of Zoology 48, 161–200.
| Demography and social organisation of the Red-winged Fairy-wren, Malurus elegans.Crossref | GoogleScholarGoogle Scholar |
Sibley, C. G., and Ahlquist, J. E. (1985). The phylogeny and classification of the Australo-Papuan Australian birds. Emu 85, 1–14.
| The phylogeny and classification of the Australo-Papuan Australian birds.Crossref | GoogleScholarGoogle Scholar |
Skroblin, A., and Murphy, S. A. (2013). The conservation status of Australian malurids and their value as models in understanding land-management issues. Emu 113, 309–318.
| The conservation status of Australian malurids and their value as models in understanding land-management issues.Crossref | GoogleScholarGoogle Scholar |
Skutch, A. F. (1961). Helpers among birds. Condor 63, 198–226.
| Helpers among birds.Crossref | GoogleScholarGoogle Scholar |
Stacey, P. B., and Koenig, W. D. (Eds) (1990) ‘Cooperative Breeding in Birds: Long-Term Studies of Ecology and Behaviour.’ (Cambridge University Press: Cambridge, UK.)


