Hendra virus: what do we know?
Isabel M. R. Hess A E , Peter D. Massey B , Belinda Walker C , Deborah J. Middleton D and Therese M. Wright CA NSW Public Health Officer Training Program, NSW Department of Health
B Population Health, Hunter New England Local Health District
C Biosecurity Unit, NSW Department of Trade and Investment, Regional Infrastructure and Services
D Australian Animal Health Laboratory, Commonwealth Scientific and Industrial Research Organisation
E Corresponding author. Email: ihess@doh.health.nsw.gov.au
NSW Public Health Bulletin 22(6) 118-122 https://doi.org/10.1071/NB10077
Published: 25 July 2011
Abstract
Hendra virus infection is an emerging infectious disease that is not well understood. Most cases of Hendra virus infection have occurred in Queensland, with one case in a horse in NSW. Hendra virus infection has a high mortality rate in horses and humans and as cases could occur anywhere in Australia it is important to be ready for prompt action should an outbreak occur in NSW. This paper: reviews the current knowledge on Hendra virus infection including methods for preventing the disease; explains the animal health and human health response for an outbreak within NSW; and discusses possible future avenues for post-exposure prophylaxis and prevention by vaccination.
Hendra virus infection is an emerging infectious disease in horses and humans and, due to its high mortality rate, is attracting extensive media and public interest.
Hendra virus was first described in September 1994 during an outbreak in Hendra, Brisbane. 1 This outbreak involved 18 horses and two humans (a horse trainer and a stable hand). One person and 14 horses died from what was a mystery disease. A novel equine virus belonging to the family Paramyxoviridae was isolated; 2 first named equine morbillivirus, the novel virus was later renamed Hendra virus. 3
In August 1994 at Mackay in north Queensland, a person caring for two ill horses and then assisting in their autopsies developed aseptic meningitis. 4 He recovered fully but developed severe encephalitis 13 months later and died. Retrospectively he and the horses were found to have been infected with Hendra virus. There were no known links between the two outbreaks.
This paper reviews the existing knowledge about the virus, outlines the planned outbreak response in NSW, and discusses possible future avenues for post-exposure prophylaxis and prevention by vaccination.
Methods
A literature review was conducted using Ovid Medline and Google Scholar. The search terms used were ‘Hendra’, ‘equine morbillivirus’, ‘paramyxoviridae’ and ‘horse’. Article abstracts were reviewed and the articles which met the selection criteria (English-language, Australian or international studies, peer-reviewed empirical or descriptive literature, published since 1994) were retrieved. The reference lists of these articles were searched for further appropriate articles which were located using Ovid Medline.
Results of literature review
Outbreaks of disease
Fourteen outbreaks of Hendra virus infection have occurred to March 2011: 13 in Queensland and one in Murwillumbah, northern NSW. Five of these outbreaks involved humans with four deaths among a total of seven human cases. 1 , 4 – 6
Australian Flying-foxes
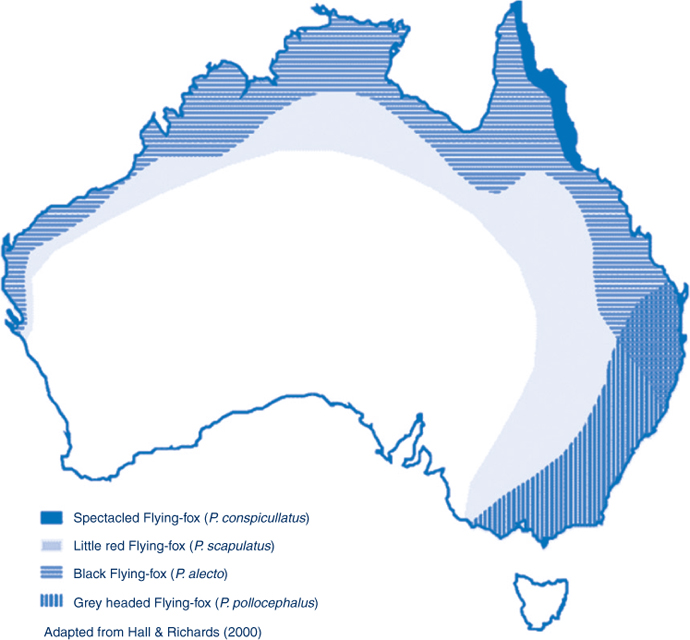
All four species of Australian Flying-foxes (Pteropus spp., also called fruit bats) have been found to have serological evidence of previous exposure to Hendra virus. The virus was also detected in uterine fluid and foetal tissue, confirming Flying-foxes as the natural host. 7
Australian Flying-foxes live along much of the Australian coast (Figure 1). Hendra virus infection could theoretically occur anywhere in Australia where there are Flying-foxes. 8 Frequent horse movements between states and territories mean that cases could also occur outside the geographic distribution of the Flying-fox populations.
Transmission between species
It is not yet clear how the virus spills over from Flying-foxes to horses. Research suggests the most likely route is ingestion by horses of pasture or feed contaminated with the urine, faeces, saliva or birthing products from infected Flying-foxes. 9 , 10 The risk of transmission to horses was found to be increased during Flying-fox reproductive periods and at times when the colonies were undergoing nutritional stress. 9 Spill-over to horses seems to be a rare event; over 2000 horses were tested in Queensland in 1995 after the first two outbreaks of Hendra virus, and none had serological evidence of previous infection. 11
Transmission of Hendra virus between horses appears to be more likely in horses kept in close proximity in stables; however, companion horses in paddocks have also been infected on three occasions. 10 Hendra virus may survive on fomites for short periods of time, assisting spread between horses. Further, infected horses can excrete viral RNA through nasal discharge prior to the onset of clinical signs. 12
Extensive serological testing of people in contact with known infected horses and humans showed negative results, indicating that the virus is not easily transmitted to humans. 13 Direct physical contact with the secretions or body fluids (such as nasal discharge or blood) from an infected or dead horse appears to be necessary.
To date, there is no evidence of human-to-human, human-to-horse or Flying-fox-to-human 14 transmission of Hendra virus. Other species do not seem to be affected, however, experimentally, cats, guinea pigs and pigs can be infected. 15 , 16
Clinical features and management
Horses
Infection in horses usually causes acute onset of respiratory and/or neurological signs, but signs can be variable. The first signs include fever, tachycardia, discomfort, weight shifting and depression (lethargy, unresponsiveness). There may be laboured breathing and frothy nasal discharge. The rapid deterioration of the horse’s condition is considered an important sign in determining the likelihood of Hendra virus infection. Most cases are fatal, 10 with a mortality rate greater than 70%. 17 Horses are considered potentially infectious from 72 hours prior to the onset of disease until their death and the safe disposal of their carcass. 10
Humans
Hendra virus disease in humans is characterised by influenza-like illness, which can progress to severe pneumonia and death, or encephalitis including symptoms such as headache, high fever and drowsiness. 18 Further, Hendra virus can lead to encephalitis following a symptom-free period after an initial illness. 4 , 19 The incubation period has been estimated at 5–21 days and the human case fatality rate is over 50%. 18 Current treatment for Hendra virus is supportive and includes intravenous therapy and mechanical ventilation. Antiviral therapy has not been effective.
Laboratory testing
In acute cases of disease, Hendra virus genome is readily detected by quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) assays of blood or urine samples, nasal or oropharyngeal swabs or tissue samples collected at post-mortem. However, samples taken very early during the incubation period may test negative by qRT-PCR and it may be necessary to repeat the sampling to exclude transmission. Hendra virus qRT-PCR testing is available: in NSW for horses at the Elizabeth MacArthur Agricultural Institute (NSW Department of Primary Industries); in Victoria for horses and humans at the Australian Animal Health Laboratory in Geelong (Commonwealth Scientific and Industrial Research Organisation) and; in Queensland for humans at Queensland Health Forensic and Scientific Services.
Serological tests such as an indirect enzyme-linked immunosorbent assay (ELISA) are methods for screening sera of humans and horses for the presence of antibodies to Hendra virus, and a virus neutralisation test may be used for antibody detection in any species. The diagnostic sensitivity of the ELISA is not well established. ELISA testing for horses is available at the Elizabeth MacArthur Agricultural Institute and the Australian Animal Health Laboratory for both horses and humans.
In order to exclude Hendra virus infection in humans, qRT-PCR is performed for symptomatic contacts. For asymptomatic contacts serological testing is performed at baseline and at 3 and 6 weeks after last exposure (earlier if symptomatic). Results from initial testing are usually available within 24–48 hours.
Prevention of infection
Infection of horses can be prevented by minimising contact with Flying-foxes and their excretions. 17 , 19 Feed and water troughs should be placed under cover and away from trees where Flying-foxes might feed or roost and horse feed that might attract them (such as apples, carrots or anything sweet) should be avoided. As loss of habitat contributes to nutritional stress of Flying-foxes, removal of fruit trees on horse paddocks is not recommended; 9 however access beneath trees while flowering and fruiting should be prevented.
Flying-foxes are a protected species so culling is inappropriate and is unlikely to be a feasible or effective prevention strategy. 9 , 20 Habitat loss and alteration, roost disturbance, urbanisation and being hunted stress Flying-fox colonies, which may magnify the problem. 9
To prevent infection in humans it is important to control viral spread from diseased horses, including horses that are incubating Hendra virus but not yet showing clinical signs. People most at risk include horse owners, veterinary personnel, horse dentists, farriers and any other persons in close contact with horses. The routine use of personal protective equipment by horse health workers handling/contacting horses that may have been exposed to fluids from Flying-foxes will minimise the risk of exposure. Horse handlers should always apply general good hygiene practices such as covering cuts and abrasions, especially on arms and hands, and hand washing after handling a horse. 17
When examining ill horses, an initial risk assessment should be conducted for the likelihood of Hendra virus to avoid unnecessary exposure and to instigate appropriate infection control procedures as described in Guidelines for veterinarians handling potential Hendra virus infection in horses. 10
The outbreak response in NSW
Hendra virus infection in horses is a notifiable disease in NSW. 17 The Department of Primary Industries notifies the NSW Department of Health of any highly suspect or confirmed cases. Hendra virus infection in humans is currently not notifiable in NSW.
Animal health response
When Hendra virus infection is suspected in a horse, a Livestock Health and Pest Authority or Department of Primary Industries inspector must be notified. An experienced veterinary officer conducts a risk assessment and classifies the case according to the likelihood of Hendra virus:
-
Not/Unlikely – priority green
-
Possible – priority amber
-
Likely – priority red.
All priority amber and red cases are promptly investigated. The horse should be isolated and handling minimised. If the horse is deceased, post-mortem examination is not recommended due to the high risk of viral exposure but, as a minimum, blood and nasal swabs should be collected. The Department of Primary Industries provides advice on the dispatch of samples. Horses shown to have been infected but surviving the infection are euthanased as they may pose an ongoing risk to humans. The property where the horse case/s is/are located is quarantined and other properties that may require risk assessment and quarantine are identified.
Public health response
The public health response involves close collaboration of the NSW Department of Health with the local health services where the horse case has occurred. The response follows the Hendra Virus National Guidelines for Public Health Units. 21 These Guidelines were developed by public health and veterinary experts in Australia, including people with direct experience in the management of Hendra outbreaks. An outbreak control team undertakes a prompt epidemiological investigation to identify all persons at risk of Hendra virus infection and to minimise any further exposure. The infection risk will be assessed for all people who may have been exposed to infected horses or people; in some cases this can be a large number. 5 For all people at risk, information is provided and testing is arranged on a case by case basis. If possible, infectious disease specialists should be involved in patient management. Close follow up of all people tested is important and may be managed by local general practitioners.
Hendra virus investigations can generate intense media interest and may evoke fear amongst the public. Open and transparent communication is therefore important. An experienced spokesperson should be nominated and all enquiries referred to them. Community meetings with public health professionals and animal health specialists may be necessary to inform the public on risks and outbreak management.
Rural areas
Hendra virus infection may be more likely in rural and peri-urban areas due to the greater prevalence of both Flying-foxes and horses. Further, the larger geographical distances and smaller social distances 22 mean that people are often closely connected locally but are far from services. A collaborative approach across the public health network will be needed to implement the required response.
Future treatment and vaccination
There are presently no licensed therapeutics available to treat infection caused by Hendra virus. However, a neutralising human monoclonal antibody that recognises the Hendra virus G surface glycoprotein has recently been extensively characterised. Administration of the antibody early after exposure to Nipah virus has prevented the development of significant clinical disease in laboratory animals 23 and is under review for Hendra infections in humans.
A potential mechanism for controlling the disease is equine vaccination. The most promising approaches to Hendra virus vaccine development are based on the expression of the F and/or G envelope glycoproteins through recombinant canarypox viruses or recombinant Hendra G as subunit vaccines; candidate vaccines are currently being formulated for immunogenicity and efficacy studies in horses. Preliminary work suggests that both approaches will provide protection from Hendra virus disease and significantly reduce the viral loads in vaccinated animals, 24 but the necessity of completing efficacy studies at a physical containment level 4 facility means that progress is both costly and slow.
Conclusion
Hendra virus infection is an emerging infectious disease that is still not well understood. Further research is needed to determine what factors cause the virus to spill over from Flying-foxes to horses to humans. Diagnosis in horses is difficult due to the variable signs and symptoms and people at risk can be unwittingly exposed to infected horses. As there is no effective treatment or vaccination for humans good hygiene practices and appropriate personal protective equipment are therefore important to prevent disease transmission.
References
[1] Selvey LA, Wells RM, McCormack JG, Ansford AJ, Murray K, Rogers RJ, et al. Infection of humans and horses by a newly described morbillivirus. Med J Aust 1995; 162 642–5.| 1:STN:280:DyaK2Mzis12qsg%3D%3D&md5=0ca9c827bb5079ccafd77bbab725d98aCAS |
[2] Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, et al. A morbillivirus that caused fatal disease in horses and humans. Science 1995; 268 94–7.
| A morbillivirus that caused fatal disease in horses and humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkvV2ksLw%3D&md5=2a76fecaed15de826358f2455e32de56CAS |
[3] Paterson DL, Murray PK, McCormack JG. Zoonotic disease in Australia caused by a novel member of the paramyxoviridae. Clin Infect Dis 1998; 27 112–8.
| Zoonotic disease in Australia caused by a novel member of the paramyxoviridae.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czktVSltg%3D%3D&md5=3f7050aad0050049d27128adb83bec7eCAS |
[4] O’Sullivan JD, Allworth AM, Paterson DL, Snow TM, Boots R, Gleeson LJ, et al. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 1997; 349 93–5.
| Fatal encephalitis due to novel paramyxovirus transmitted from horses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7kvFKisQ%3D%3D&md5=313b9dc33ce412c92b1dc77cad24af8cCAS |
[5] Playford EG, McCall B, Smith G, Slinko V, Allen G, Smith I, et al. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg Infect Dis 2010; 16 219–23.
[6] AusVet (Nigel Perkins). September 2009. Progress audit of Biosecurity Queensland response activities at Cawarral in August 2009. Available from: http://www.dpi.qld.gov.au/documents/Biosecurity_EmergencyResponse/HendraVirus-Cawarral-Perkins-Sep2009.pdf (Cited 31 August 2010.)
[7] Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 2000; 81 1927–32.
| 1:CAS:528:DC%2BD3cXlsFOhtLo%3D&md5=7c528d9cca1f89c66a4eba39e10a78abCAS |
[8] Department of Primary Industry and Resources. South Australia; May 2010. Flying-foxes and Hendra virus – advice for horse owners. Available from: http://outernode.pir.sa.gov.au/__data/assets/pdf_file/0004/132889/hendra.pdf (Cited 31 August 2010.)
[9] Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc Biol Sci 2008; 275(1636): 861–9.
[10] Biosecurity Queensland. Guidelines for veterinarians handling potential Hendra virus infection in horses, version 4.0. Department of Employment, Economic Development and Innovation, Queensland Government; May 2010. Available from: http://www.dpi.qld.gov.au/4790_13371.htm (Cited 8 September 2010.)
[11] Ward MP, Black PF, Childs AJ, Baldock FC, Webster WR, Rodwell BJ, et al. Negative findings from serological studies of equine morbillivirus in the Queensland horse population. Aust Vet J 1996; 74 241–3.
| Negative findings from serological studies of equine morbillivirus in the Queensland horse population.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FkvFeisA%3D%3D&md5=dc7b66242d313b51fffdff882b173985CAS |
[12] Middleton D. Initial experimental characterisation of HeV (Redland Bay 2008) infection in horses. CSIRO Australian Animal Health Laboratory (AAHL); 2009. Available from: http://www.dpi.qld.gov.au/documents/Biosecurity_GeneralAnimalHealthPestsAndDiseases/HeV-Initial-experimental-characterisation.pdf (Cited 5 September 2010.)
[13] McCormack JG, Allworth AM, Selvey LA, Selleck PW. Transmissibility from horses to humans of a novel paramyxovirus, equine morbillivirus (EMV). J Infect 1999; 38 22–3.
| Transmissibility from horses to humans of a novel paramyxovirus, equine morbillivirus (EMV).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7ovV2isg%3D%3D&md5=17876968ab1dc93e2cbd2ed5fa89740cCAS |
[14] Selvey L, Taylor R, Arklay A, Gerrard J. Screening of bat carers for antibodies to equine morbillivirus. Commun Dis Intell 1996; 20 477–8.
[15] Hooper PT, Westbury HA, Russell GM. The lesions of experimental equine morbillivirus disease in cats and guinea pigs. Vet Pathol 1997; 34 323–9.
| The lesions of experimental equine morbillivirus disease in cats and guinea pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szot1ymsg%3D%3D&md5=378eb7dd8ce3b06b7dbafc6f753506c4CAS |
[16] Li M, Embury-Hyatt C, Weingartl HM. Experimental inoculation study indicates swine as a potential host for Hendra virus. Vet Res 2010; 41 33
| Experimental inoculation study indicates swine as a potential host for Hendra virus.Crossref | GoogleScholarGoogle Scholar |
[17] Robinson S, Walker B. Hendra virus. NSW Department of Industry and Investment, Primefact 970, February 2010. Available from: http://www.dpi.nsw.gov.au/agriculture/livestock/horses/health/general/hendra-virus/hendra (Cited 8 September 2010.)
[18] Queensland Health. Hendra Virus Infection fact sheet. April 2010. Available from: http://access.health.qld.gov.au/hid/InfectionsandParasites/ViralInfections/hendraVirusInfection_fs.pdf (Cited 10 September 2010.)
[19] Tan CT, Goh KJ, Wong KT, Sarji SA, Chua KB, Chew NK, et al. Relapsed and late-onset Nipah encephalitis. Ann Neurol 2002; 51 703–8.
| Relapsed and late-onset Nipah encephalitis.Crossref | GoogleScholarGoogle Scholar |
[20] Biosecurity Queensland. Flying foxes and Hendra virus – information for the community. Department of Employment, Economic Development and Innovation, Queensland Government; May 2010. Available from: http://www.dpi.qld.gov.au/documents/Biosecurity_GeneralAnimalHealthPestsAndDiseases/Flying-foxes-and-Hendra-virus.pdf (Cited 8 September 2010.)
[21] Communicable Diseases Network Australia. Hendra Virus National Guidelines for Public Health Units. Available from: http://www.commcarelink.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-hendra.htm (Cited 19 May 2011.)
[22] Wakerman J. Rural and remote public health in Australia: building on our strengths. Aust J Rural Health 2008; 16 52–5.
| Rural and remote public health in Australia: building on our strengths.Crossref | GoogleScholarGoogle Scholar |
[23] Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog 2009; 5 e1000642
| A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection.Crossref | GoogleScholarGoogle Scholar |
[24] McEachern JA, Bingham J, Crameri GS, Green DJ, Hancock TJ, Middleton D, et al. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine 2008; 26 3842–52.
| A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotlCrsbg%3D&md5=a2608ddd554faa4544e40e2b19bf81deCAS |