Bárcena Volcano, 1952: a 60-year report on the repopulation of San Benedicto Island, Mexico, with a review of the ecological impacts of disastrous events
Bayard H. BrattstromHorned Lizard Ranch, PO Box 166, Wikieup, AZ 85360, USA. Email: bayard@hughes.net
Pacific Conservation Biology 21(1) 38-59 https://doi.org/10.1071/PC14903
Submitted: 2 May 2014 Accepted: 28 June 2014 Published: 1 May 2015
Abstract
Long-term ecological studies are desirable, but rare. I here present data from a 60-year study on the repopulation of San Benedicto Island following a volcano eruption in 1952. Bárcena Volcano appeared on 1 August 1952 on San Benedicto Island, Revillagigedo Islands, Mexico. Within 20 min, the entire island was engulfed in a cloud of ash and pumice, which covered all the plants, killed an estimated 20 000 sea birds within hours and caused the subsequent extinction of an endemic race of rock wren (Salpinctes obsoleta exsul). The results of studies on revegetation and repopulation of the island for the first 10 years after the volcanic eruption were summarised by Brattstrom in 1963. This report extends the studies to 2012. The distribution of the land crab (Aegecarcinus planatus) has increased on the island. By 1971 the crab occurred only over the northern one-eighth of the island, but by 1978 it could be found on one-third of the island. No studies on its distribution have been made since then. Total sea bird populations steadily increased up to 1971 and then rapidly declined, though these changes in numbers are largely due to a fluctuation in the populations of the masked booby (Sula dactylatra). The changes in the booby population may have been due to reproductive and feeding success or to immigration and emigration. The decline in the shearwater (Puffinus ssp.) populations are largely due to erosion and destruction of their burrows; their numbers did not increase until 2000. The formation of a large lava delta created a new habitat, which permitted the establishment of a species of sea bird new to the island, the red-footed booby (Sula sula). Numerous non-resident waifs or stray birds have been observed on the island but most have not become established. The exception is the Laysan albatross (Phoebastria immutabilis), breeding at present in low (3–712) numbers. The original flora consisted of 10 species. The volcano caused four species to become extinct, two re-established themselves, and two species new to the island arrived. There have been marked erosional changes, and the accidental introduction of exotic plants may dramatically alter the vegetation of the island.
Additional keywords: ecological disasters, island ecology, Revillagigedo Islands, sea birds.
Introduction
The Islas Revillagigedo consist of four volcanic oceanic islands (Socorro, Clarion, Roca Partida and San Benedicto) rising independently from the ocean floor (about 4 km deep) along the Clarion Fracture Zone, some 483 km west of Mexico and 354 km south of the tip of Baja California, Mexico (Fig. 1). San Benedicto, the youngest of the four islands, is 6.4 km long and 3.2 km wide and consists of several old coalescent and partially eroded volcanic cones. The boundary of the island has at least one discontinuous submerged Pleistocene erosion shelf.
|
On the morning of 1 August 1952, a new volcano, Bárcena, appeared in a large valley on the southern portion of San Benedicto Island (Fig. 2). The initial eruption of Bárcena occurred at 0745 hours, 1 August 1952 (Fig. 3). Within 20 min a dark cloud of ash had spread over the entire island. By 2 August, the pyroclastic volcanic cone had developed to a height of 335 m above sea level, but cone formation ceased during mid-September to early November. Activity resumed, and two domes of irregular blocks of lava formed in Bárcena crater during November and early December. On 8 December, lava issuing from the base of the volcano formed a delta, which extended nearly 0.8 km out to sea by the end of February 1953 (Fig. 4). Calculation (Richards 1959) indicated that 3200 m2 of tephra and lava were produced during the eruption of Bárcena. No further activity except sulfataric steaming occurred after February 1953. The volcano had the highest index of explosiveness of any oceanic volcano in the eastern Pacific Ocean (Richards 1959). The entire island was quickly covered with a cloud of tephra, ash, and pumice, which killed an estimated 20 000 sea birds within hours. Thus, for the second time an island laboratory appeared for the study of the repopulation and revegetation of an island. Krakatau in 1883 was the first such laboratory, and the new island of Surtsey (1963) off the coast of Iceland was the third. Investigations on newer island volcanos as well as on old volcanic islands – Motmot Island, New Guinea, 1968; Deception Island, Antarctica, 1967; Ritter Island, 1888, New Guinea; Long Island, New Guinea, 1700s; Bogoslof Island, Bering Sea, 1976 (Ball and Glucksman 1975; Bassot and Ball 1972; Diamond 1974; Fridriksson 1975; Simkin and Fiske 1983, 2003; Thornton 2000) – have contributed greatly to our understanding of extinction, survival, colonisation and repopulations of islands. The eruption of Bárcena differed from these other islands in that San Benedicto is a true oceanic island, the original biota of the island was a depauperate one and, unlike Krakatau and others where complete destruction of the biota occurred, only a portion of the terrestrial biota of San Benedicto was destroyed.

|

|

|
Numerous scientific expeditions have visited San Benedicto Island (Richards and Brattstrom 1959). The most detailed collections and observations were obtained by the California Academy of Sciences in 1905 and 1925. The pre-Bárcena biota of San Benedicto included no terrestrial mammals, reptiles, amphibians or freshwater fish. However, ten species of plants (herbs and low shrubs), several species of sea birds (boobies, frigate birds, shearwaters and tropic birds) and an endemic race of rock wren (Salpinctes obsoletus exsul) did live on the island. The terrestrial invertebrate fauna was poorly known save for the land crab, Aegecarcinus planatus. The marine intertidal fauna was typical for a subtropical island of the eastern Pacific (Brattstrom, 1990). The marine fish fauna was poorly described (Richards and Brattstrom 1959; Brattstrom 1990).
The geological and biological aspects of Bárcena Volcano, its destructive effects and changes occurring after volcanic quiescence have been the basis of numerous long-term studies by a large team of scientists. Richards (1959, 1965, 1966) described the geology of San Benedicto Island, including a description of the origin of the volcano and subsequent erosional changes. The birds, reptiles and cactus (of the Islas Revillagigedo) have been described, as well as the initial effects of the volcanic eruption on the animals and plants (Brattstrom 1953, 1955, 1990; Brattstrom and Howell 1956). Brattstrom (1963) described the revegetation and repopulation of the island 10 years after the eruption. Long-term ecological studies are desirable but rare (Buden 2000; Collins 2001; Fryxell et al. 1998; Holmes and Sherry 2001; Marshall 1988; Shultz et al. 2012). This report summarises the results of studies by Brattstrom and others through 60 years of the history of Bárcena.
Effect of Bárcena on the biota
The primary destructive force of Bárcena Volcano was the dense pumice dust, tephra and gas that covered the entire island, including a 0.5–1.0-m deposit of ash and pumice on the extreme north end of the island and on many of the small islets about the island. Many of the birds were killed when they refused to leave nests or were unable to fly due to the effects of the gas and dust (Cook et al. 1981; Pollack 1981; Hayward et al. 1982; Stolzenburg 2011). In November 1953, many skeletons of birds (some on nests with eggs) were exposed as ash was removed by erosion. Small numbers of the endemic rock wren survived the volcanic activity, as a few were seen by Lewis Wayne Walker in December 1952 (pers. comm.). However, none have been seen since December 1952 and they are assumed to be extinct. The eruption apparently destroyed all above-ground parts of all plants. There are early records (Townsend 1890; Kaeding 1905) of common ravens (Corvus corax) being common on San Benedicto and a predator of eggs and young of seabirds (Pitman and Ballance 2002). Brattstrom and Howell (1956) considered that the occasional ravens seen around the island were visitors or waifs from the population on Clarion Island far to the west. Only a few ravens have been seen about the islands in the 60 years since the eruption of Bárcena and none are known to breed there.
The intertidal regions of the island in 1953 were scoured by the large amounts of floating or partly submerged pumice and dust, and the water was extremely turbid. Repopulation, up to 1961, of these regions by marine algae, invertebrates and fish has been described by Brattstrom (1963). No subsequent marine studies have been done.
Materials and methods
Six trips were made to San Benedicto Island by ship (March 1953, November 1953, April 1955, November 1971, April 1978, and April 1981). I have spent over 915 h on one or more of the four islands of the Revillagigedo Archepelago, of which 270 were on or about San Benedicto. Between August 1952 and August 1961 many photoreconnaissance flights were made of the islands using planes of the United States Navy. Detailed low-altitude photographs, some being stereo pairs, taken on these flights clearly show plant and bird distribution. I was on two of these flights (October 1954, August 1961). Observations and photographs were also provided to me by many other investigators during 1952–2012. Each of my trips to the island consisted of landing north of the Lava Delta, traversing the delta, climbing the eastern slope of Bárceno and Herrara Craters, then north to the northern tip of the island (Fig. 4). The return trip descended to the eastern beach between the two larger craters. Erosion on the island made hiking extremely difficult and dangerous by 1978, so I was unable to reach the northern end of the island. Robert Pitman was able to do so in 1978, through 2000, and made population counts of birds (Pitman and Ballance 2002). Although it is possible to observe 80% of the island from the crest of Bárcena, Pitman’s observations provided important data on canyons not visible from the volcano or ship. In 1981, the visit to the island was limited and erosion much more extensive. I was able to traverse the lava flow, and Drs Joe Jehl and Reid Moran ascended Herrera Crater and made population counts of birds and took photographs. On each of my trips the island was also circumnavigated by ship as close as safely possible, and the vertical cliffs were examined with binoculars. In this way, almost every portion of the island was observed.
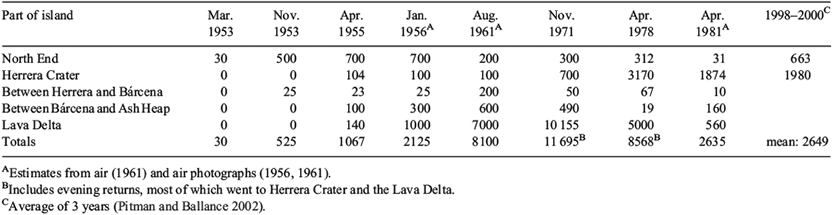
Bird numbers (see Table 1; Jehl and Parkes 1982a; Pitman and Ballance 2002 for scientific names) were determined by actual count during the walk around the island and from counts as the ship circumnavigated the island. Since many of the birds were at sea during the day, these birds also had to be counted. On many trips we were in the vicinity of the island for several days doing geological and oceanographic work aboard ship. It soon became apparent after several early evening circumnavigation trips that most of the sea birds returned to the island from the south and south-east. Therefore, the ship was anchored in the late afternoon and evening to the north-east of the Lava Delta (Albatross Beach of Pitman and Ballance 2002) and actual counts of incoming birds were made on tally clickers as they approached the islands (often two or more observers would count, each doing one or more species). Counts made on 2–3 successive days did not vary significantly. These counts were supplemented by aerial photographs during 1952–61 and by photographs and observations made by others on other trips. I have not been on the island since 1981, other researchers (Pitman and Ballance 2002) did not necessarily circumnavigate the island nor make evening returning bird counts so their numbers are necessarily lower than mine. The number of burrowing shearwaters (Puffinus ssp.) was estimated from evening counts or the number of active burrows observed on the island (multiplied by two as a pair of birds share a single burrow). Shearwater distribution was plotted from the distribution of the burrows. While others made bird counts (Table 1) they may have not surveyed the island as well as Pitman or I. So counts made by Brattstrom and complete survey data in Pitman and Ballance (2002) are used. Only Brattstrom and Pitman surveys are similar.

|
Plant distribution was mapped by observation, photographs and Google maps (2013). Crab distribution up to 1978 was done by mapping active burrows and live crabs. Because of erosion, access and interests by others, no crab distribution data after 1978 are available.
Results
Repopulation of San Benedicto
Invertebrates, fish and marine algae
The repopulation studies of marine algae, invertebrates and fish by 1961 have been summarised elsewhere (Brattstrom 1963). Unfortunately, no subsequent studies have been made on these organisms.
Little has been done on the terrestrial invertebrates of San Benedicto Island before or after the eruption of Bárcena. Insects collected by the expeditions of the California Academy of Sciences and other expeditions have been described by numerous authors (see Richards and Brattstrom 1959 for a bibliography and Brattstrom 1990; Palacios-Vargas et al. 1982; Brattstrom 2015). None of these studies covered San Benedicto. In 1925, Hanna (1926) noted ‘clouds of grasshoppers … ground beetles, carrion, and hippoboscid flies‘. Flies were common on the island in 1953, and various entomologists collected many winged insects in 1953 and 1955. These insects are currently under study. In 1971, two species of grasshoppers, hippoboscid and muscid flies, ants, and honeybees were observed. Pitman (pers. comm.) saw the above plus two species of spiders in 1978. I saw only grasshoppers and two species of flies in 1981. However, time and terrain did not allow extensive collecting or population estimates of insects to be made on any of the trips.
Land crabs
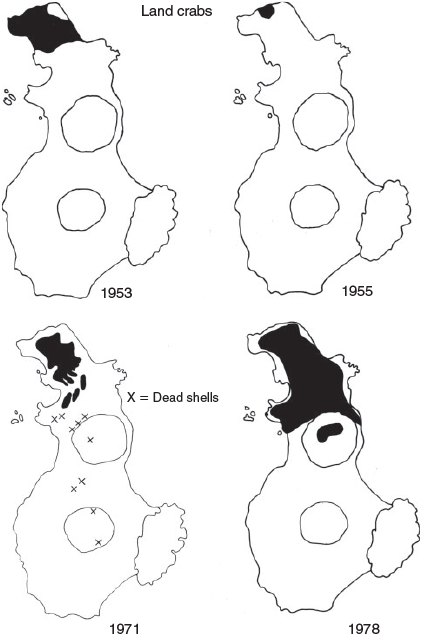
Land crabs, Aegecarcinus plantus, are common on Clarion, Socorro and San Benedicto Islands. They live in holes and crevices, and migrate to the sea only to breed. Although the total population on San Benedicto prior to Bárcena is unknown, their distribution probably extended over the entire island associated with the wide distribution of plants (Fig. 5).
|
Undoubtedly, many crabs were killed when they and their burrows were covered with pumice, ash and dust. Toxic gases and dust also may have killed many by interfering with respiration. Some could possibly have survived in holes or crevices, at the northern end of the island, where the ash was only a few feet deep. Sea birds eat these crabs but it is unlikely that birds carried living crabs the long distance from Socorro to San Benedicto. Thus, repopulation of the island by land crabs probably resulted from the reproduction of those that survived the volcano or by recruitment of young crabs from the sea.
In March 1953, two live and several dead land crabs were observed on the north edge of the crater rim of Bárcena. The dead crabs had been torn apart, indicating that birds had dropped them there. In November 1953, land crabs were fairly common at the extreme northern end of the island (Fig. 5), but by 1955 crabs were found in only a small portion of the previous range. This reduction in the area occupied by the crabs may have been due to: (1) a depletion of food plants, (2) predation by the increasing bird population, and/or (3) destruction of burrows and crabs by ash reworked by wind and rain.
By 1971, land crab distribution had increased to include the northern one-eighth of the island (Fig. 5). In 1978, Robert Pitman (pers. comm.) observed land crabs all over the north end of the island as well as in the center of Herrara Crater, but in no other locations. Thus the crabs occurred on about one-third of the island by 1978 (Fig. 5). No other distribution data have been published by subsequent observers.
Birds
The most obvious inhabitants of San Benedicto Island are sea birds. Prior to the Bárcena eruption, eight seabird species and one passerine species were resident (Table 1).
Photographs taken during the expeditions of the California Academy of Sciences in 1905 (by Slevin) and 1925 (by Hanna) showed that most of the area on top of San Benedicto was occupied by birds. Frigate birds (Fregata ssp.) were the most common of the pre-Bárcena sea birds, making up 95% of the avifauna (Jehl and Parkes 1982a). Density estimates from photographs taken by Slevin and Hanna of San Benedico Island were 1–3 frigate birds per square metre in 1903 and 1–5 frigate birds per square metre in 1925. Hanna (pers. comm.), who was with me on the trip in November 1953, estimated that the sea bird population in November 1953 was only 1% of the 1925 population. Based on skeletal counts and Hanna’s photographs and remarks, the pre-Bárcena sea bird population was estimated to be about 20 000 birds, most of which were killed by the volcano in the first minutes or hours after the initial explosion, as determined by skeletons buried by the ash, and later exposed by erosion. Some skeletons of adult birds were found on or next to eggs and young (see photographs in Brattstrom and Howell 1956; Brattstrom 1963; also see Southern and Southern 1982).
The only resident passerine bird was the endemic race of rock wren, Salpinctes obsoletus exsul. As a result of the volcanic action this subspecies is now extinct.
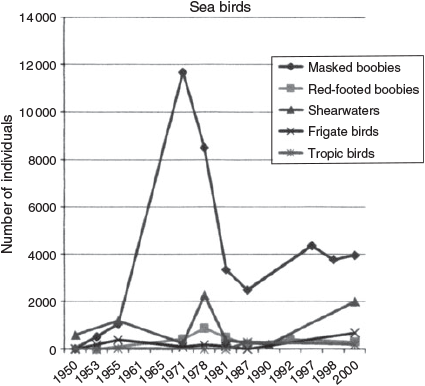
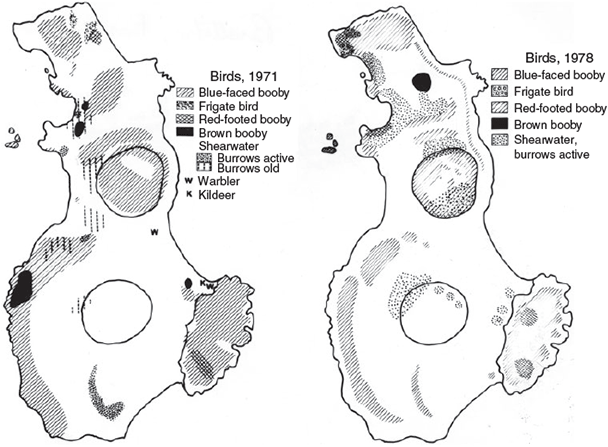
The total number of birds of each species observed on the post-Bárcena trips by me and other researchers (see review in Pitman and Ballance 2002) is listed in Table 1. The total numbers of masked boobies occupying various regions of the island are given in Table 2. The area distributions of all species of birds on the island during the history of the investigations are shown in Fig. 6. While Table 1 gives bird numbers reported by a number of researchers, Figs 7 and 8 include Brattstrom’s personal observations plus numbers from complete surveys reported only in Pitman and Ballance (2002) as only Brattstrom’s and Pitman’s survey techniques are similar.
|

|
|
|
After the eruption there was an increase in the numbers of sea birds and the area of the island occupied by them (Table 1, Fig. 6). In March 1953, birds occurred only on the bare vertical cliffs at the north end of the island. By November of that year, frigate birds were found in other regions at the north end of the island in areas where Bárcena ash had been removed (Figs 8, 9) and boobies were found in ash-covered areas not subject to winds. By 1955, the birds occupied much of the island in limited numbers. This was especially true of the shearwaters, which had begun to dig burrows in Bárcena ash on top of the volcano as well as in areas of their old centre of concentration called the Ash Heap. In addition, a few birds, mostly boobies, had begun to roost on the bare lava delta. By 1961, the number of birds roosting and nesting on the lava delta had risen to 7000, and by 1971 the population there had increased to 10 155. By 1981, the number of birds on the Lava Delta and the south end of the island was reduced and by 2000 no birds roosted or nested on the south end of the island, nor the Lava Delta. Erosion of Bárcena probably caused shearwaters to leave. Only by 1998–2000 did shearwaters start burrowing in northern parts of the island. Why birds left the Lava Delta is unknown. Laysan albatross started to roost and breed on the sandy area north of the Lava Delta (Albatross Beach) in 1987 (Podolsky 1990; Pitman and Ballance 2002).
|
Of the several species of birds on San Benedicto, the frigate birds were the most abundant before the eruption. After the eruption, the number of masked boobies far exceeded the number of frigate birds (Fig. 8). The low frigate bird population may be due to the absence of adequate sticks for nesting material, lack of food or a slow rate of reproduction. The other species of boobies also had low population numbers compared with the masked booby. One of the probable contributing factors to the absence of red-footed boobies prior to the formation of the Lava Delta was the lack of shrubs on the island, which could provide protected nesting sites. Brattstrom and Howell (1956) noted that on Clarion Island, 364 km to the west, masked boobies nested on the ground while red-footed boobies built their stick nests in bushes or on cliffs. The lava delta on San Benedicto provided an equivalent habitat, and red-footed boobies were breeding there in 1971 (although two birds were seen there as early as November 1953). The rugged lava flow with its diverse topography provided both wind-protected areas for nesting and allowed more nests in a limited area. Sometime in the late 1980s, all species of booby left the Lava Delta. While there were still several hundred Red-footed Boobies nesting on the Lava Delta in 1978, there were a larger number (400) nesting on the northern part of the island and a small number (67) in the valley between Bárcena and Herrera. Many of the red-footed boobies on the northern part of the island were on steep cliffs, while those in the valley were on the edges of the cliff-like gullies caused by the extensive erosion. Until 1955, most of the masked boobies occupied the north end of the island (Table 2); by 1961, most of them were nesting on the Lava Delta. Masked boobies had largely left the Lava Delta by 1978, and a large number occurred in Herrera Crater (Table 2). Brown boobies were found primarily on the northern part of the island. Frigate birds had left their main nesting area (Frigate Arroyo) in the north-central portion of the island (Fig. 9). The reasons for specific nest site selection and changing of nesting area from year to year is not clear (see Clark et al. 1983 and Duffy 1984, for a discussion).
While the shearwaters had begun to dig their burrows in the Ash Heap (by 1953) and on Bárcena (by 1955), the extensive erosion of the soft pumice volcano crater had destroyed almost all of their burrows by 1971. No shearwaters were seen, even during an early evening boat reconnaissance around the island when daytime-feeding shearwaters were expected to return to the island. The population estimate in Table 1 was based on the number of undestroyed burrows. Thermal winds on the island created large dust storms, contributing greatly to the elimination of shearwater burrows in 1971. By 1978, the hard crust of the Bárcena ash was mostly gone. The exposed softer material apparently made it easier for shearwaters to burrow and extensive burrows again appeared on Bárcena Crater, in Herrera Crater and in the central part of the island. By 1978 and 1981, there were a few burrows just above the Lava Delta where the original lava had extruded from the volcano. The extensive erosion seen in 1981 may have further damaged these burrows as few shearwaters were seen in the vicinity (Table 1). Shearwaters left the south part of the island in the late 1980s. Santaella and Sada (1991) report shearwaters on the north part of the island in November 1990. Pitman and Ballance (2002) also report shearwaters in the north part of the island in 1998 and 2000.
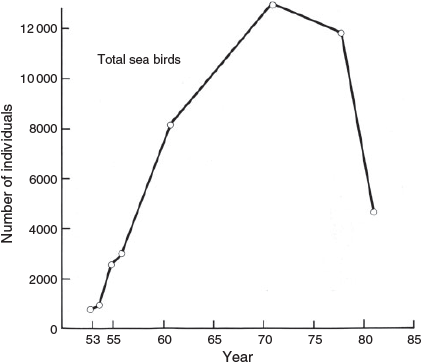
The total population of sea birds on San Benedicto increased steadily from 1953 to 1971 (Fig. 7), due almost entirely to the increase in the numbers of masked boobies (Fig. 8). By 1978, there was a decline in total sea birds and in the number of masked boobies. This decline was even more marked by 1981. By 1988 through to 2000, numbers of masked boobies had levelled off at 3000–4000. Shearwaters increased by 2000, and the sea birds maintained steady but low (under 500) population numbers (Figs 7, 8). A reduction in the total area of the island utilised by the sea birds also occurred with the decline in bird numbers (Fig. 6).
Non-resident waifs or stray birds have been observed in small numbers on San Benedicto (Table 1). Twenty-five species of birds (two from nearby Socorro) have been seen on San Benedicto after the eruption of the volcano (Brattstrom and Howell 1956; Howell and Webb 1990, 1992a, 1992b; Jehl and Parkes 1982a, 1982b, 1983; Pitman and Ballance 2002). These include: brown pelican (Pelicanus occidentalis), red-tailed tropic bird (Phaethon rubricauda), brown noddy (Anous stolidus), great blue heron (Ardea herodias), Sabines gull (Kema sabini), Socorro red-tailed hawk (Buteo jamaiccensis socorroensis), osprey (Phandion haliaetus), peregrine falcon (Falcon peregrinus), American kestrel (Falco sparverius), Socorro dove (Zenaida grayson), mourning dove (Zenaida macroura), short-eared owl (Asio flammeus), white-throated swift (Aeronautes saxatalis), barn swallow (Hirundo rustica), northern rough-winged swallow (Stelgidopteryx serripennis), violet-green swallow (Tachycineta thalassina), tree swallow (Tachycineta bicolor), barn swallow (Hirundo restica), northern mockingbird (Mimus polyglottos), bay-breasted warbler (Dendroica castanea), killdeer (Charadrius vociferous), American redstart (Setophaga ruticilla), curlew sp. (Numenious sp.), yellow-rumped warbler (Dendroica coronata) and common raven (Corvus corax).
The barn swallows, redstart and warblers were probably lost birds or post-breeding migrants as they were seen only on November trips. The northern mockingbird has recently invaded Socorro Island and may have replaced the near-extinct Socorro mockingbird (Mimoides grayson) there (Jehl and Parkes 1982a). None of these birds have become established on the island, though the red-tailed tropic bird has been seen in prebreeding behavior on the island and may now be a coloniser (Pitman and Ballance 2002). The raven was reported on San Benedicto Island by Townsend (1890) and Kaeding (1905). Brattstrom and Howell (1956) considered the few ravens occasionally seen on San Benedicto as strays from Clarion.
Terrestrial plants
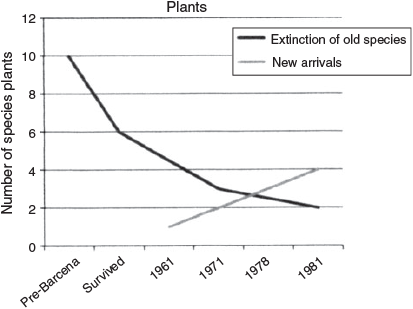
Photographs taken by Joseph R. Slevin in 1905 and G. Dallas Hanna in 1925 showed that San Benedicto was almost completely covered with low vegetation (Fig. 2). The upper slopes, the top of the Ash Heap and some of the steep rocky cliffs were devoid of vegetation. The angiosperm flora consisted of 10 species (Table 3), and the dominant plants were the two grasses, Eragrostis diversifolia and Cenchrus myosuroides, and the Euphorbia species.

|
The Bárcena ash fall covered the entire island with 0.6–335 m of ash. Photographs of the island, including those of the northern end, showed no plants visible in 1952 and early 1953 (Fig. 10). By November 1953, sufficient wind and water erosion had occurred to remove the Bárcena ash from the steeper slopes and gullies. It was at these locations that plants managed to sprout from old roots, underground stems or seeds (Zobel et al. 1992). No plants grew where there was any Bárcena ash; all were growing on pre-Bárcena ‘soil’. Many dead stalks of pre-Bárcena plants seen in the eroded areas were being used by frigate birds for nests. By 1955, however, Perityle was growing in Bárcena ash in Herrera Crater – perhaps sufficient leaching of the ash had occurred in this area. The most common post-Bárcena plants in 1955 were Euphorbia and Perityle. On the 1957 trip, Richards (1959, 1965) noted fewer plants than on the 1955 trip. Erosion of Bárcena ash and the pre-Bárcena surface by rain and wind had apparently removed a large portion of the plants, which had resprouted and were well established in 1953 and 1955. By August 1961, the revegetation on the north end of the island had been greatly curtailed (Fig. 10); however, two new patches of plants were seen from the air. One consisted of a low group of grass-like plants covering an area of ~9.2 m2 at the southern end of the Ash Heap. It was impossible to determine whether these plants represented roots or seeds covered and dormant until 1961 or whether they represented a new invasion. All previous air photographs of this portion of the island were re-examined and all showed that the region concerned was covered with ash until at least 1957. This region was also an area of heavy burrowing activity by shearwaters which could have uncovered or brought to the surface the seeds or roots of plants. The most likely explanation is that these plants sprouted from seeds or old roots after the Bárcena ash had been removed by erosion and burrowing activity of birds between 1957 and 1961.
Heavy erosion of the outer edges of the Lava Delta occurred between 1957 and 1971. Waves had carried large rocks shoreward, forming two large rock barriers north and south of the Lava Delta. Eroded pumice and ash from Bárcena filled in the area behind these rock barriers to form two large beaches. There are only two other beaches on the island (most of the perimeter of the island consists of vertical cliffs with no beaches or, at most, rocky ones that are awash at high tide with heavy seas. These two beaches (one east of the canyon between Bárcena and Herrera, the other south-east of the Ash Heap) are subjected to waves and seem unlikely places for the establishment of new immigrants to the island. The first invasion on the new beaches north and south of the Lava Delta had occurred by August 1961. From the air, clumps of beach morning glory (Ipomoea pes-caprae) were seen on the pumice beach just north of the Lava Delta. Since the beach did not exist prior to Bárcena, these plants did not resprout from old plants. This species is known for its ability to establish itself on oceanic islands. This patch persists to 2013 (Google Maps) and is where the Laysan albatross started roosting and breeding in 1987 and the beach was renamed Albatross Beach by Pitman and Ballance (2002).
By 1971, the vegetation of San Benedicto Island had not only recovered from the low level of 1961 but had extended over almost all of the northern end of the island (Fig. 10). However, three further species had become extinct during these 10 years (Table 3). The earlier arrival (the beach morning glory) now occurred on ~80% of the beach north of the Lava Delta. In addition, another species new to this portion of the island (Perityle) was found on the north beach in 1971. Dr Charles Hougue (pers. comm.) observed what appeared to be beach morning glory on the beach south of the Lava Delta in June 1977. This was the first record of plants on that beach.
By 1978, the entire northern end of the island and Herrera Crater, were covered by plants (Eragrostis and Perityle). The plants on the south and west portions of the Ash Heap still persisted (Fig. 10). On the beach north of the Lava Delta, Ipomoea and a single Perityle plant persisted (the latter in poor condition, probably due to salt spray and erosion of Bárcena). The morning glory had begun to invade the lava flow to a distance of ~4 m. No plants were seen on the south beach, thus the Ipomoea seen there in 1977 by Hogue had been eliminated. This may have been largely due to the beach being covered by pumice eroding from Bárcena. While erosion of the south beach continues with Bárcena ash filling it in, Google Maps of the island in 2013 show a small green patch, presumably of Ipomoea, persisting between erosion channels.
Euphorbia had apparently been eliminated from the island by 1971. However, leafy branches of this plant were observed in a red-footed booby nest on the Lava Delta although it was not growing there. Robert Pitman (pers. comm.) observed that this species was present and established on the north end of the island by 1978. While blue-faced boobies have bare nests, red-footed boobies and frigate birds use plant material to make their nests.
Euphorbia may have been brought in by these birds from nearby Socorro but could, of course, have sprouted from old roots or seeds.
There are now extensive nutrients (bird droppings) on the Lava Delta and it is slowly being covered by leached Bárcena ash eroding down from the east face of Bárcena. This area is thus ripe for the establishment of plants brought into this area by sea birds.
By 1981, the erosion of Bárcena had eliminated the small clump of Perityle that was seen on the south-west flank of the volcano in 1971 and 1978, and the area occupied by the grass south of the Ash Heap was more extensive (Fig. 10). Google Maps for 2012 and 2013 shows no plants on the south or south-west part of Bárcena. The morning glory on the beach north of the Lava Delta covered about 70% of the beach and had invaded the Lava Delta in many places (Fig. 10, Google Maps). The northern part of the island was almost entirely covered with plants except for a few ridges that were still covered with Bárcena ash (Fig. 10). Perityle also occurred on many of the vertical and near-vertical cliffs although it had been eliminated from the beach north of the Lava Delta.
Table 3 and Figs 10 and 11 summarise the changes in the terrestrial flora of San Benedicto Island. Of the 10 original species, six survived Bárcena, and four became extinct. Two of these re-established themselves and two new species arrived on the island, resulting in two of the original 10 species now considered to be extinct on the island. While Perityle never became extinct on the north end of the island, I consider the establishment of this species on the North Beach in 1971 as a new invasion of the island, since it never before occurred in this area and the beach did not even exist there until sometime before 1961.
|
Discussion
Brattstrom (1963) summarised 14 biological impacts and aftermaths of such destructive events as fires, volcanoes, deforestation, drought and radiation. These generalisations are expanded below and emended with ideas from McIntire (2004), Novak et al. (2011), Parker and Wiens (2005), Platt and Connell (2003), Turner (2010), Walker and del Moral (2003), and new studies on such disasters as:
-
Volcanos (Krakatau, San Benedicto, Parícutin, Surtsey, Mount Saint Helens and others) (Eggler 1948; Duellman 1954; Burt 1961; Thorarinsson 1964; Ball and Glucksman 1975; Ball and Johnson 1976; Hayward et al. 1982; Simkin and Fiske 1983; Andersen and MacMahon 1985, 1986; Wood and Del Moral 1987; Thornton et al. 1988, 1990; Compton et al. 1994; Bush et al.1995; Thornton 2000; Whittaker et al. 1998; Lawrence and Ripple 2000; Bishop 2002; Winchester 2003; Dale et al. 2005a, 2005b; Lindig-Cisneros et al. 2006; O’Shea and Cook 2006; del Moral 2010; Munoz and Hewlett 2011; Pedersen et al. 2012).
-
Lava flows (Britten 1956; Drake and Mueller-Dombois 1993; Yamaguchi 1993; Thornton 2000).
-
Atomic blasts or radiation leaks (Platt and Mohrbracher 1959; Platt 1961; Shields and Wells 1962; Rickard and Beatley 1965; Turner and Gist 1965; Eden 2004; Planes et al. 2005).
-
Land slides (Flaccus 1959; Restrepo and Vitousek 2001; Velázquez and Gomez-Sal 2007).
-
Fires (Horton and Kraebel 1955; Sweeney 1956; Hjerpe et al. 2001; Schulte and Mladenoff 2005; Freeman et al. 2007).
-
Deforestation, logging, wind blow-downs, slash and burn (Rabor 1959; Bury 1983; Sakai 1988; Wilkie and Finn 1990; Rebertus et al. 1997; Carlton and Bazzaz 1998; Robinson 1998; Sinton et al. 2000; Kramer et al. 2001; Webb and Scanga 2001; Manolis et al. 2002; Penteriani et al. 2002; Brook et al. 2003; Schulte and Mladenoff 2005; Burris and Haney 2005; Wunderle et al. 2006; Rios-López and Aide 2007; Wardle et al. 2008; Brooks and Stouffer 2010; Pike et al. 2011; Belote et al. 2012).
-
Drought (Blair 1957; Tobin et al. 1999; Beard et al. 2005).
-
El Niño (=ENSO) (Gibbs et al. 1984, 1987; Hodder and Graybill 1985; Gibbs and Grant 1987; Curry and Stoleson 1988; Anderson 1989; Schreiber and Schreiber 1989; Cruz and Cruz 1990; Wilson 2005; Polis et al. 1997; Jaksic and Lazo 1999; Grant et al. 2000; Jehl et al. 2002; Fuller et al. 2004; Velarde et al. 2004; Charrette et al. 2006; Devney et al. 2009).
-
Hurricanes (Boucher et al. 1990; Dunning 1991; Roth 1992; Rivera-Milán 1995; Wunderle 1995; Faaborg and Arendt 1995; Latta et al. 1995; Asner and Goldstein 1997; Censky et al. 1998; Vandermeer et al. 1998; Frangi and Lugo 1998; Mabry et al. 1998; Hunter and Forkner 1999; Sullivan et al. 1999; Sherman et al. 2001; Walker et al. 2003; Boose et al. 2004; Ostertag et al. 2005; Pavelka and Behie 2005; Piou et al. 2006; Hawley 2006; Spiller and Schoener 2007; Watts and Byrd 2007; Knapp and Valeri 2008; Yaukey 2008; Vandecar 2011; McKnight 2012; Ramírez-Barajas et al. 2012; Plotkin et al. 2013).
-
Tsunamis (Wikramanayake et al. 2006).
-
Ski slopes (Titus and Landau 2003).
-
Earthquakes, seismic settlements (Gill et al. 2004; Lai et al. 2007).
-
Off-road vehicles (Brattstrom and Bondello 1983, 1997).
-
Mining, mining debris, dumps, waste sites (Wray et al. 1982; Manner et al. 1984; Skousen et al. 1990; Hopkins et al. 1997; Schreiber et al. 2001; Thompson and Thompson 2007; Baasch et al. 2010; Graves et al. 2010; Jorgenson et al. 2010; Stauffer et al. 2011).
-
Agriculture (Holl 1999; Knops and Tilman 2000; Martin et al. 2004).
-
Roads (Laurance 2004; Clark et al. 2010).
-
Moving sand dunes (Belsky and Amundson 1986).
-
Post-glacier melting (Tisdale et al. 1966).
-
Grazing (Day and Detling 1990; Sirotnak and Huntly 2000; Browning and Archer 2011).
-
Climate change (Kingsford 2011).
-
Oil spills (Belsky 1982; Wilson et al. 2004; Iverson and Esler 2010).
-
Winds, dust-bowls, Santa Anas, mistrals, siroccós.
-
Floods (McCarley 1959; Quanstrom 1966; Burger and Shisler 1980: Walker and Chapin 1986; Knopf and Sedgwick 1987; Friedman et al. 1996; Knutson and Klaas 1997; Seigel et al. 1998; Andersen et al. 2000; Benke et al. 2000; Tucker et al. 2001; Chamberlain and Leopold 2003; Jenkins and Boulton 2003).
-
Insect outbreaks (beetles, gypsy moths) (Logan et al. 2010; Koenig et al. 2011).
-
Disease outbreaks (Fonnesbeck and Dodd 2003; plus extensive literature on amphibian diseases).
-
Tornados (Prather and Smith 2003; McGlinn et al. 2010).
-
Wind turbines, transmission lines (Niemi and Hanowski 1984; Grodsky et al. 2011).
-
Meteors and asteroids (Fraser and Sues 1994; Officer and Page 1996).
These generalisations are as follows:
-
Repopulation or reinvasion is rapid. If there has been partial destruction (as in the case of San Benedicto), reproductive rates can usually allow for rapid intrinsic repopulation. If the area is surrounded by normal vegetation (as in instances of lava flow, atomic blast, fires, land slides) the reinvasion is rapid. If the area is in a location of high population density, repopulation from without (in the case of Krakatau) or reinvasion (in the case of atomic blasts, fires and land slides) will be rapid. If the site is distant from a source, reinvasion, but not necessarily intrinsic population growth, may be slow (San Benedicto).
-
Islands with complete or partial destruction appear to repopulate more slowly than terrestrial areas (lava flows, fires, Parícutin, atomic blasts) but this appears to be largely a matter of (a) amount of surviving intrinsic population, and (b) rate of chance dispersal to the island.
-
Species with good methods of dispersal invade areas faster than those with poor dispersal mechanisms, and species with high reproductive rates or with certain reproductive attributes (for instance, viviparity) repopulate faster than those without.
-
Reinvasion and repopulation may follow a pattern similar to the primary succession in the area (for example, primary soil-forming plants, Parícutin), or there may be peculiar jumps in the succession due to (a) chance survival of some forms, (b) population pressure outside the area, (c) peculiar dispersal patterns or mechanisms, or (d) special advantages given to certain species by the catastrophe (for instance, change in soil texture, type, moisture).
-
Species dominant in an area before the catastrophe may be dominant subsequently, or another species or group (or a species new to the area) may become dominant. Which obtains depends on, among other things, the limiting factors of the organism (that is, soil moisture, habitat availability, exposure, and so forth), number and type of surviving forms, and differences in dispersal or reproductive rates, or chance.
-
Weather and erosion factors, as well as competition, food, and behaviour may markedly alter the success of a species in its reinvasion or repopulation (Furness and Barrett 1991; McNab 1994).
-
The population size of a species may be reduced (due to limited survival, as on an oceanic island, or by isolation, as between lava flows). This may make the population ripe for genetic-drift mechanisms and hence lead to rapid evolution in such situations and areas.
-
The major effect of the catastrophe may not be the event itself. Even though the event be spectacular and destructive, the side effects or after-effects may be more critical to the survival of both individual and species (fires, hurricanes, absence of food, lack of water, change in erosion pattern, radiation after-effects) and to reinvasion and repopulation.
-
A major contributing effect of some of these events is the creation of new land (as in the cases of San Benedicto, Krakatau, and Hawaii) or the alteration of the physical or chemical character of the land (Parícutin, San Benedicto, fire, landslides, hurricanes or atomic blasts). Colonisation on new land may be fast and include exotics (Santos et al. 2011).
-
After the catastrophe, species may occupy new habitats or new types of habitats. The creation of new habitats often allows species not formerly present to become established in the area.
-
Organisms that have special powers of locomotion (insects, birds, large mammals) usually suffer heavy population losses by their ‘refusal’ to leave home areas or territories or by their confusion and inability to comprehend the event.
-
Unless a surviving animal has extended powers of flight (birds, insects), food supply may be one of the more critical limiting factors to animals.
-
Human factors related to the event (for example, planting and building, as in Parícutin and Hawai‘i) may increase the opportunity and decrease the time for reinvasion and repopulation.
-
The effect of the catastrophe depends upon its size and nature.
-
Changes in behaviour and ecology (especially feeding) may occur after the event.
-
Changes in soil chemistry (nutrients and antigrowth chemicals) may affect survival, recovery, and aid or deter new exotics (Green 1998; Shiels 2006; Meyer and Cowie 2010).
-
Postdisturbance management and restoration may be planned for the better but often the worse occurs (Chapman 1963; Jones and Kress 2012; Spear et al. 2012).
-
Weather, especially rainfall, temperature, seasonality events, or the event itself may affect physiology, reproduction, stress (in its short- and long-term effects) (Schreiber and Chovan 1986; Reichert et al. 2012).
-
Plants may survive the event, but die later due to the lack of water, nutrients, herbivore damage, and stress. Signals of stress (Christmann and Grill 2013) may affect plants that were unaffected by the event itself.
-
Some arrivals to the site may be soon after the event; others may not arrive until years or decades later (Denslow et al. 2009; Jones 2010a, 2010b).
-
The absence of pollinators will prevent some surviving species from reproducing and new species from becoming established.
-
After the event, animals may leave the area or move about the area in search of food, shelter, or reproductive sites. Some may return to the area, some may not.
-
The events or local weather events may make damaged plants more susceptible to insectivory, herbivory, and parasites, and they may not be able to produce protective chemical defences.
-
Some impacts of the event are immediate or short while others may last for centuries (Jones 2010a, 2010b).
-
The event may change recovery from previous disastrous events in the same area.
-
These events ‘… can negatively or positively affect social, economic and environmental assets’ (of humans). ‘Few cause major losses of human and homes, but when they do they make banner headlines in the world’s media’ (Gill et al. 2013).
-
Large islands will have more invaders earlier than small islands; but small continental sites will have new invaders more quickly than large sites. Edges will be invaded first unless the invader can fly.
-
The rate of extinction versus new arrivals may or may never come to equilibrium.
-
Exotic new invaders may do more damage than the event itself (Blackburn et al. 2004; Davis et al. 2008; Meyer and Cowie 2010).
-
Human visitors, even researchers and managers, to the area may have an impact on breeding birds or import exotic spores or seeds (Beale and Monaghan 2005).
-
If an endemic species occurs on the site (especially on an island), small post-event population numbers can lead to inbreeding aiding ‘founders effect’ evolutionary change or extinction (Frankham 1998).
-
Invaders or colonisers may bring in parasites or seeds, which may have beneficial or harmful effects on the native biota.
-
Aerial plankton (seeds, spores, insects, spiders), whether windborne or from the stratosphere, may bring early immigrants to the site (Edwards and Sugg 1993).
-
The topography of the area destroyed will impact the kind and rate of recovery (Cox and Cox 1977; Eason et al. 2012).
-
Survivors of the event may control revegetation and repopulation more than invaders (Plotkin et al. 2013).
-
Most impacts of these events are local. Some (hurricanes, fires, volcanic ash) may cover several States at most. Rarely are the impacts country- or world-wide (exceptions include volcanic ash in the stratosphere for years (Robock and Matson 1983), and speculation about world-wide effects of asteroids on dinosaurs, but not birds, mammals, crocodilians, snakes, lizards and amphibians).
The eruption on San Benedicto stands out in the following ways:
-
The island is far from sources of new invasions and thus reinvasion is slow, although nearby islands may be a source for some plants.
-
Frigate birds were more common on the island prior to the appearance of the volcano, but masked boobies became more numerous following its appearance.
-
Weather and erosion markedly affected the survival and repopulation of plants and may have been more destructive than the volcano itself.
-
Elimination of shearwater burrows by Bárcena and subsequent covers or erosion caused the major decline in shearwater numbers. Subsequent exposure and pre-Bárcena ash, then more erosion caused a rise and then a fall in shearwater numbers in 1978–81. Later (1988–2000) increases in shearwaters resulted from their finding new burrowing sites on the north part of the island.
-
New land and new landforms (Lava Delta and the barrier-protected beaches) aided in the establishment of a species of seabird new to the island and allowed for the establishment of new plant invaders (Stolzenburg 2011).
-
Tourism, fishermen and casual visitors slightly impact the island today, but provide a potential for the accidental introduction of exotics.
The question arises as to whether the increased sea bird population on San Benedicto is intrinsic or extrinsic. The reproductive strategies of tropical island sea birds are characterised by a seasonal or continuous breeding, large egg size, low clutch size, long fledging period, long postfledging feeding and low adult mortality (Dorward 1962; Diamond 1973; Nelson 1975; Dodson and Fitzgerald 1980; Schreiber and Burger 2003). This results in high survival of young and a long adult life at a slow, but stable reproductive rate consistent with food availability (low in tropical seas) and feeding strategies and energetics (Spendelow 1985), all conducive to a slow steady increase in the bird population (Hunt et al. 1986). This may be the reason for the steady population numbers of the frigate bird, tropic bird, and brown and red-footed booby populations on San Benedicto (Carmona et al. 1995). It cannot be the case for the rapid rise and subsequent decline seen in the masked booby populations (see discussion in Fontaine 2013). Little is known about interisland movements of sea birds in the eastern Pacific. Yet Anderson and Ricklefs (1987) show that masked boobies forage widely. At no time were there large populations of sea birds on the other islands of the Revillagigedo group, nor were anything but small populations of some of the Revillagigedo species ever observed on the Baja California peninsula, Alijos Rocks or Cedros Islands. The population increase of masked boobies on San Benedicto does seem to require some immigration of these boobies from elsewhere. However, the nearest potential sources of these birds would most likely be Clipperton Island, Cocos and the Galápagos (Mills 1998), both far south of the Revillagigedos (Ehrhardt 1971; Nelson 1968; Harris 1974; Anderson 1993).
With the subsequent decline in masked booby population numbers, the area occupied by sea birds also declined. The reason for the decline in the booby population has not been determined. Unexpectedly, there also was no breeding of boobies on Socorro in 1978 or 1981. There have always been no more than several hundred boobies nesting on the extensive lava flows and islets on and about that large island. Clarion Island to the west has never had more than several hundred sea birds. Roca Partida is so small that it provides room for only a few dozen birds. The general impression was that there were fewer fish (especially the surface fish upon which boobies feed) about the islands in 1978 than ever before. In 1978 and 1981, no flying fish were seen (they had always been numerous), and few fish or fish larvae came about the lights on the boat at night while at anchor. On previous trips flying fish (Cypselurus), half-beaks (Hyporhamphus), jacks (Caranx), larval fish and invertebrates were abundant about the boat at night. Further, in 1978 and 1981 there were few commercial tuna boats around the islands, and sport-fishing boats reported by radio that fish were rare. Intertidal and subtidal fish seemed abundant, but sea birds seldom feed on these fish (Brattstrom 1998). Evening fly-in counts suggest that sea birds fly long distances and fish at greater depths for food (Lewis 1973; Haney 1985; Anderson and Ricklefs 1987; Seki and Harrison 1989; Pitman and Ballance 1990; Anderson 1993; Spear et al. 1995; Le Corre 1997; Brattstrom 1998; Castillo-Guerrero and Mellink 2006). Perhaps there has been some oceanographic change, perhaps preceding or related to El Níño events, or the effect of numerous hurricanes that cross over the island each year (about one-third of all Eastern Pacific hurricanes recorded, 1851–2010, passed over the archipelago). Data from NOAA, which lowered the productivity about the islands, hence causing the decline in the masked booby population (Gibbs et al. 1984; Schreiber and Schreiber 1984; Halpern et al. 1983) in any event, masked booby populations levelled off and then increased slightly by 2002. Hurricane impacts (Duffy 1984) may have caused boobies to leave the Lava Delta. It is also possible that Brattstrom overestimated, and subsequent researchers underestimated (by not doing evening flying counts), this possible sampling error, combined with the seasonality of visits by researchers (Craig 1996; Douglas 1998; Norvell et al. 2003), and years of no visits by researchers (Bart and Earnst 2002; Thompson 2002), combined with El Níño and hurricane impacts may have affected the masked booby populations more than those of other species. Changes in shearwater populations (Fig. 8) are explained in part by erosion and exposure of burrow sites (Pitman and Ballance 2002).
Future of the island
There have been marked erosional changes on San Benedicto during the last 60 years. The soft volcanic pumice and ash has been extensively eroded so that the cone of Bárcena and other areas of ash are now heavily furrowed by erosion channels 40–50 m deep. The volcanic ash of the eastern side of the cone of Bárcena has washed down onto the lava flow and has filled in almost one-third of this delta. The guano deposits of the sea birds seem to have stabilised in the ash and, of course, added nutrients to both the land and the sea. This area should provide a suitable habitat for further plant growth.
The erosion from the cone has also formed the beaches north and south of the lava delta to such an extent that they have increased their size some 10-fold since 1961. Erosion of the outer fingers of the lava delta has destroyed the protective coves (an area previously heavily occupied by marine algae and fish). Lava boulders have been deposited as a barrier along the outer edge of both beaches, thus protecting them from subsequent marine erosion.
In 1971, a camping site of stranded fishermen was found north of the Lava Delta and in 1978 and 1981 there was a considerable amount of garbage (from fishing and sport boats) on the wave-swept beach east of Bárcena. In March 1988, Pitman and Ballance (2002) found much of the northern part of the island burned and exploded, presumably due to military ordinance on the island. Sport fishing boats inadvertently catch and drown unknown numbers of young boobies and frigate birds with their trolling gear (Pitman and Ballance 2002). Fishermen, sight-seeing visitors and scientists may accidentally bring outside organisms to the island. As yet, no rats, mice or cats have been introduced on the island. These animals could be disastrous for the seabird populations. The four islands of the Revillagigedos were designated in 1984 as a Biosphere Reserve for conservation purposes. It is hoped that the Mexican Government will restrict access to San Benedicto so that the casual introduction of plants and animals will not occur and this natural laboratory can be preserved and utilised for the ongoing study of repopulation and revegetation of such a unique island and event.
Acknowledgements
I have elsewhere (Richards and Brattstrom 1959; Brattstrom 1963) expressed my gratitude to the many who helped with the early investigations of the islands. The 1971 expedition was sponsored by the Janss Foundation on the R/V Searcher, and I especially thank Ed Janss for a most stimulating and rewarding trip. The 1978 trip was sponsored by the Carnegie Museum and Hubbs/Sea World Research Institute aboard the R/V Sea World under the joint leadership of Drs Joe Jehl and Kenneth Parkes. Tom and Dorothy Hawthorn of San Diego, California sponsored the 1981 trip aboard the M/V Tom Cat, with additional support from the Carnegie Museum. I thank those individuals and their institutions for their support. Dr Charles Hogue of the Los Angeles County Museum provided photographs and observations from his 1977 trip, and Dr George Lindsay of the California Academy of Sciences provided photographs of his 1971 trip to the islands. I thank Dr Thomas Howell, Dr Joe Jehl, the late Dr Reid Moran and Dr Charles Hogue for identifications of specimens. Mr Robert Pittman provided observations he made on the northern end of the island in 1978. Paula McKenzie, Mark Zolle and Mary Shipman did the drawings.
This paper was originally part of a 1981 American Association for the Advancement of Science Symposium on the ecological effects of Mount St Helens and other volcanoes. It was reviewed, approved for publication and edited for press to be published by Freeman Press. Suddenly in autumn 1985 the Symposium was to be published by the University of California Press and all zoological and non-Mount St Helens papers from the Symposium were unceremoniously returned to their authors! I have not been on San Benedicto since 1981, but I have brought the paper up to date largely through the papers and personal communications of Robert Pitman and others. I also mapped current vegetation from Google Maps on the Internet.
References
Andersen, D. C., and MacMahon, J. (1985). The effects of catastrophic ecosystem disturbance: the residual mammals at Mount St. Helens. Journal of Mammalogy 66, 581–589.| The effects of catastrophic ecosystem disturbance: the residual mammals at Mount St. Helens.Crossref | GoogleScholarGoogle Scholar |
Andersen, D. C., and MacMahon, J. A. (1986). An assessment of ground-nest depredation in a catastrophically disturbed region, Mount St. Helens, Washington. The Auk 103, 622–626.
Andersen, D. C., Wilson, K. R., Miller, M. S., and Falk, M. (2000). Movement patterns of riparian small mammals during predictable floodplain inundation Journal of Mammalogy 81, 1087–1099.
| Movement patterns of riparian small mammals during predictable floodplain inundationCrossref | GoogleScholarGoogle Scholar |
Anderson, D. J. (1989). Differential responses of boobies and other seabirds in the Galápagos to the 1986–87 El Niño Southern Oscillation event. Marine Ecology Progress Series 52, 209–216.
| Differential responses of boobies and other seabirds in the Galápagos to the 1986–87 El Niño Southern Oscillation event.Crossref | GoogleScholarGoogle Scholar |
Anderson, D. J. (1993). Sula dactylatra – masked booby. Birds of America 73, 2–14.
Anderson, D. J., and Ricklefs, R. E. (1987). Radio-tracking masked and blue-footed boobies. (Sula spp.) in the Galàpagos Islands. National Geographic Research 3, 152–163.
Asner, G. P., and Goldstein, G. (1997). Correlating stem biomechanical properties of Hawaiian canopy trees with hurricane wind damage. Biotropica 29, 145–150.
| Correlating stem biomechanical properties of Hawaiian canopy trees with hurricane wind damage.Crossref | GoogleScholarGoogle Scholar |
Baasch, A., Tischew, S., and Bruelheide, H. (2010). Twelve years of succession on sandy substrates in a post-mining landscape: a Markov chain analysis. Ecological Applications 20, 1136–1147.
| Twelve years of succession on sandy substrates in a post-mining landscape: a Markov chain analysis.Crossref | GoogleScholarGoogle Scholar | 20597296PubMed |
Ball, E., and Glucksman, J. (1975). Biological colonization of Motmot, a recently-created tropical island. Proceedings of the Royal Society of London. Series B, Biological Sciences 190, 421–442.
| Biological colonization of Motmot, a recently-created tropical island.Crossref | GoogleScholarGoogle Scholar |
Ball, E., and Johnson, R. W. (1976). Volcanic history of Long Island, Papua New Guinea. In ‘Volcanism in Australia‘. (Ed. R. W. Johnson.) (Elsevier Scientific Publishing Co.: Amsterdam.)
Bart, J., and Earnst, S. (2002). Double sampling to estimate density and population trends in birds. The Auk 119, 36–45.
| Double sampling to estimate density and population trends in birds.Crossref | GoogleScholarGoogle Scholar |
Bassot, J. M., and Ball, E. (1972). Biological colonization of recently created islands in Lake Wisdom, Long Island, Papau New Guinea, with observations on the fauna of the lake. Papua New Guinea Science Society Proceedings 23, 26–35.
Beale, C. M., and Monaghan, P. (2005). Modeling the effects of limiting the number of visitors on failure rates of seabird nests. Conservation Biology 19, 2015–2019.
| Modeling the effects of limiting the number of visitors on failure rates of seabird nests.Crossref | GoogleScholarGoogle Scholar |
Beard, K. H., Vogt, K. A., Vogt, D. J., Scatena, F. N., Covich, A. D., Sigurdardottir, R., Siccama, T. G., and Croul, T. A. (2005). Structural and functional responses of a sub-tropical forest to 10 years of hurricanes and droughts. Ecological Monographs 75, 345–361.
| Structural and functional responses of a sub-tropical forest to 10 years of hurricanes and droughts.Crossref | GoogleScholarGoogle Scholar |
Belote, R. T., Jones, R. H., and Wieboldt, T. F. (2012). Compositional stability and diversity of vascular plant communities following logging disturbance in Appalachian forests. Ecological Applications 22, 502–516.
| Compositional stability and diversity of vascular plant communities following logging disturbance in Appalachian forests.Crossref | GoogleScholarGoogle Scholar | 22611850PubMed |
Belsky, J. (1982). Diesel oil spill in a sub-alpine meadow: 9 years of recovery. Canadian Journal of Botany 60, 906–910.
| Diesel oil spill in a sub-alpine meadow: 9 years of recovery.Crossref | GoogleScholarGoogle Scholar |
Belsky, A. J., and Amundson, R. G. (1986). Sixty years of successional history behind a moving sand dune near Olduvai Gorge, Tanzania. Biotropica 18, 231–235.
| Sixty years of successional history behind a moving sand dune near Olduvai Gorge, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Benke, A. C., Chaubey, I., Ward, G. M., and Dunn, E. R. (2000). Flood pulse dynamics of an unregulated river floodplain in the southeastern U.S. coastal plain. Ecology 81, 2730–2741.
| Flood pulse dynamics of an unregulated river floodplain in the southeastern U.S. coastal plain.Crossref | GoogleScholarGoogle Scholar |
Bishop, J. G. (2002). Early primary succession on Mount St. Helens: impact of insect herbivores on colonizing lupines. Ecology 83, 191–202.
| Early primary succession on Mount St. Helens: impact of insect herbivores on colonizing lupines.Crossref | GoogleScholarGoogle Scholar |
Blackburn, T. M., Cassey, P., Duncan, R. P., Evans, K. L., and Gaston, K. J. (2004). Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958.
| Avian extinction and mammalian introductions on oceanic islands.Crossref | GoogleScholarGoogle Scholar | 15448269PubMed |
Blair, W. J. (1957). Changes in vertebrate populations under conditions of drought. Cold Spring Harbor Symposium on Quantative Biology XXII, pp. 273–275.
Boose, E. R., Serrano, M. I., and Foster, D. R. (2004). Landscape and regional impacts of hurricanes in Puerto Rico. Ecological Monographs 74, 335–352.
| Landscape and regional impacts of hurricanes in Puerto Rico.Crossref | GoogleScholarGoogle Scholar |
Boucher, D., Vandemeer, J., Yih, K., and Zamora, N. (1990). Contrasting hurricane damage in tropical rain forest and pine forest. Ecology 71, 2022–2023.
| Contrasting hurricane damage in tropical rain forest and pine forest.Crossref | GoogleScholarGoogle Scholar |
Brattstrom, B. H. (1953). The cactus of the Revillagigedo Islands, Mexico. Cactus and Succulent Journal 25, 181–182.
| The cactus of the Revillagigedo Islands, Mexico.Crossref | GoogleScholarGoogle Scholar |
Brattstrom, B. H. (1955). Notes on the herpetology of the Revillagigedo Islands, Mexico. American Midland Naturalist 54, 219–229.
| Notes on the herpetology of the Revillagigedo Islands, Mexico.Crossref | GoogleScholarGoogle Scholar |
Brattstrom, B. H. (1963). Bárcena Volcano, 1952: its effect on the fauna and flora of San Benedicto Island, Mexico. In ‘Pacific Basin Biography’. (Ed. J. L. Grisset.) pp. 499–524. (Bishop Museum Press: Honolulu, HI.)
Brattstrom, B. H. (1990). Biogeography of the Islas Revillagigedo, Mexico. Journal of Biogeography 17, 177–183.
| Biogeography of the Islas Revillagigedo, Mexico.Crossref | GoogleScholarGoogle Scholar |
Brattstrom, B. H. (1998). Strategies of predator attacks on the schooling fish, Selar crumenophthalmus in Academy Bay, Socorro Island, Islas Revillagigedo, Mexico. Bulletin of the Southern California Academy of Sciences 97, 76–81.
Brattstrom, B. H. (2015). Food Webs and Feeding Habits on the Islas Revillagigedo, Mexico. Pacific Science 69, .
Brattstrom, B. H., and Bondello, M. C. (1983). The effects of off-road vehicle sounds on desert vertebrates. In ‘Enviromental impacts of off-road vehicles’. (Eds R. G. Webb, and H. G. Wilshire.) pp. 167–207. (Springer Verlag: Berlin.)
Brattstrom, B. H., and Bondello, M. C. (1997). Natural sounds and man-made noise in the desert. In ‘The California Desert; a Introduction to Natural Resources and Man’s Impact’. (Eds J. Lating and P. G. Rowlands.) pp. 437–465. (J. Lating Books: Riverside, CA.)
Brattstrom, B. H., and Howell, T. R. (1956). The birds of the Revillagigedo Islands, Mexico. The Condor 58, 107–120.
| The birds of the Revillagigedo Islands, Mexico.Crossref | GoogleScholarGoogle Scholar |
Britten, E. J. (1956). The effect of the volcanic eruption of 1955 on plant life in Hawaii. Botanical Society of America, Pacific Section. Abstracts.
Brook, B. W., Sodhi, N. S., and Ng, P. K. L. (2003). Catastrophic extinctions follow deforestation in Singapore. Nature 424, 420–426.
| Catastrophic extinctions follow deforestation in Singapore.Crossref | GoogleScholarGoogle Scholar | 12879068PubMed |
Brooks, M. E., and Stouffer, P. C. (2010). Effects of Hurricane Katrina and salvage logging on Bachman’s sparrow. The Condor 112, 744–753.
| Effects of Hurricane Katrina and salvage logging on Bachman’s sparrow.Crossref | GoogleScholarGoogle Scholar |
Browning, D. M., and Archer, S. R. (2011). Protection from livestock fails to deter shrub proliferation in a desert landscape with a history of heavy grazing. Ecological Applications 21, 1629–1642.
| Protection from livestock fails to deter shrub proliferation in a desert landscape with a history of heavy grazing.Crossref | GoogleScholarGoogle Scholar | 21830707PubMed |
Buden, D. W. (2000). A comparison of 1983 and 1994 bird surveys of Pohnpei, Federated States of Micronesia. The Wilson Bulletin 112, 403–410.
| A comparison of 1983 and 1994 bird surveys of Pohnpei, Federated States of Micronesia.Crossref | GoogleScholarGoogle Scholar |
Burger, J., and Shisler, J. (1980). Colony and nest site selection in laughing gulls in response to tidal flooding. The Condor 82, 251–258.
| Colony and nest site selection in laughing gulls in response to tidal flooding.Crossref | GoogleScholarGoogle Scholar |
Burris, J. M., and Haney, A. W. (2005). Bird communities after blowdown in a late-successional Great Lakes spruce–fir forest. The Wilson Bulletin 117, 341–352.
| Bird communities after blowdown in a late-successional Great Lakes spruce–fir forest.Crossref | GoogleScholarGoogle Scholar |
Burt, W. H. (1961). Some effects of Volcán Paricutin on vertebrates. Museum of Zoology, University of Michigan, Occasional Paper No. 620.
Bury, R. B. (1983). Differences in amphibian populations in logged and old growth redwood forest. Northwest Science 57, 167–178.
Bush, M. B., Wittaker, R. J., and Partomihardjo, T. (1995). Colonization and succession on Krakatau: an analysis of the guild of vining plants. Biotropica 27, 355–372.
| Colonization and succession on Krakatau: an analysis of the guild of vining plants.Crossref | GoogleScholarGoogle Scholar |
Carlton, G. C., and Bazzaz, F. A. (1998). Resource congruence and forest regeneration following an experimental hurricane blowdown. Ecology 79, 1305–1319.
| Resource congruence and forest regeneration following an experimental hurricane blowdown.Crossref | GoogleScholarGoogle Scholar |
Carmona, R., Guzmán, J., and Elorduy, J. F. (1995). Hatching, growth and mortality of magnificent frigetbird chicks in southern Baja California. The Wilson Bulletin 107, 328–337.
Castillo-Guerrero, J. A., and Mellink, E. (2006). Maximum diving depth in fledging blue-footed boobies: skill development and transition to independence. The Wilson Journal of Ornithology 118, 527–531.
| Maximum diving depth in fledging blue-footed boobies: skill development and transition to independence.Crossref | GoogleScholarGoogle Scholar |
Censky, E. J., Hodge, K., and Dudley, J. (1998). Over-water dispersal of lizards due to hurricanes. Nature 395, 556.
| Over-water dispersal of lizards due to hurricanes.Crossref | GoogleScholarGoogle Scholar |
Chamberlain, M. J., and Leopold, B. D. (2003). Effects of a flood on relative abundance and diversity of small mammals in a regenerating bottomland hardwood forest. The Southwestern Naturalist 48, 306–309.
| Effects of a flood on relative abundance and diversity of small mammals in a regenerating bottomland hardwood forest.Crossref | GoogleScholarGoogle Scholar |
Chapman, G. A. (1963). Mission Bay – a review of previous studies and the status of the sport fishery. California Fish and Game 49, 30–43.
Charrette, N. A., Cleary, D. F. R., and Moore, A. D. (2006). Range-restricted, specialist Bornean butterflies are less likely to recover from ENSO-induced disturbance. Ecology 87, 2330–2337.
| Range-restricted, specialist Bornean butterflies are less likely to recover from ENSO-induced disturbance.Crossref | GoogleScholarGoogle Scholar | 16995633PubMed |
Christmann, A., and Grill, E. (2013). Electric defense. Nature 500, 404–405.
| Electric defense.Crossref | GoogleScholarGoogle Scholar | 23969452PubMed |
Clark, L., Ricklefs, R. E., and Schreiber, R. W. (1983). Nest-site selection by the red-tailed tropicbird. The Auk 100, 953–959.
Clark, R. W., Brown, W. S., Stechert, R., and Zamudio, K. R. (2010). Roads, interrupted dispersal and genetic diversity in timber rattlesnakes. Conservation Biology 24, 1059–1069.
| Roads, interrupted dispersal and genetic diversity in timber rattlesnakes.Crossref | GoogleScholarGoogle Scholar | 20151984PubMed |
Collins, S. L. (2001). Long-term research and the dynamics of bird populations and communities. The Auk 118, 583–588.
| Long-term research and the dynamics of bird populations and communities.Crossref | GoogleScholarGoogle Scholar |
Compton, S. G., Ross, S. J., and Thorton, I. W. B. (1994). Pollinator limitation of fig tree reproduction on the island of Anak Krakatau (Indonesia). Journal of Mammalogy 26, 180–186.
Cook, R. J., Barron, J. C., Papendick, R. I., and Williams, G. J. (1981). Impact on agriculture of the Mount St. Helens eruptions. Science 211, 16–22.
| Impact on agriculture of the Mount St. Helens eruptions.Crossref | GoogleScholarGoogle Scholar | 17731222PubMed |
Cox, G. W., and Cox, D. G. (1977). Succession islands. Environmental Science 478, 3–9.
Craig, R. (1996). Seasonal population surveys and natural history of a micronesia bird community. The Wilson Bulletin 108, 246–267.
Cruz, J. B., and Cruz, F. (1990). Effect of El Niño–Southern Oscillation conditions on nesting growth rate in the dark-rumped petrel. The Condor 92, 160–165.
| Effect of El Niño–Southern Oscillation conditions on nesting growth rate in the dark-rumped petrel.Crossref | GoogleScholarGoogle Scholar |
Curry, R. L., and Stoleson, S. H. (1988). New bird records from the Galápagos associated with the El Niño–Southern Oscillation. The Condor 90, 505–507.
| New bird records from the Galápagos associated with the El Niño–Southern Oscillation.Crossref | GoogleScholarGoogle Scholar |
Dale, V. H., Swanson, F. J., and Crisafulli, E. M. (Eds) (2005a). ‘Ecological Responses to the 1980 Eruption of Mount St. Helens.’ (Springer: New York.)
Dale, V. H., Swanson, F. J., and Crisafulli, E. M. (2005b). 25 years of ecological change at Mount St. Helens. Science 308, 961–962.
| 25 years of ecological change at Mount St. Helens.Crossref | GoogleScholarGoogle Scholar | 15890864PubMed |
Davis, N. E., O’Dowd, D. J., Green, P. T., and Mac Nally, R. (2008). Effects of an alien ant invasion on abundance, behavior and reproductive success of endemic island birds. Conservation Biology 22, 1165–1176.
| Effects of an alien ant invasion on abundance, behavior and reproductive success of endemic island birds.Crossref | GoogleScholarGoogle Scholar | 18637918PubMed |
Day, T. A., and Detling, J. K. (1990). Grassland patch dynamics and herbivore grazing preference following urine deposition. Ecology 71, 180–188.
| Grassland patch dynamics and herbivore grazing preference following urine deposition.Crossref | GoogleScholarGoogle Scholar |
del Moral, R. (2010). Thirty years of permanent vegetation plots, Mount St. Helens, Washington, USA. Ecology 91, 2185.
| Thirty years of permanent vegetation plots, Mount St. Helens, Washington, USA.Crossref | GoogleScholarGoogle Scholar |
Denslow, J. S., Space, J. C., and Thomas, P. A. (2009). Invasive exotic plants in the tropical Pacific islands: patterns of diversity. Biotropica 41, 162–170.
| Invasive exotic plants in the tropical Pacific islands: patterns of diversity.Crossref | GoogleScholarGoogle Scholar |
Devney, C. A., Short, M., and Congdon, B. C. (2009). Sensitivity of tropical seabirds to El Niño precursors. Ecology 90, 1175–1183.
| Sensitivity of tropical seabirds to El Niño precursors.Crossref | GoogleScholarGoogle Scholar | 19537539PubMed |
Diamond, A. W. (1973). Notes on the breeding biology and behavior of the magnificent frigate bird. The Condor 75, 200–209.
| Notes on the breeding biology and behavior of the magnificent frigate bird.Crossref | GoogleScholarGoogle Scholar |
Diamond, J. M. (1974). Colonization of exploded islands by birds: the super-tramp strategy. Science 184, 803–806.
| Colonization of exploded islands by birds: the super-tramp strategy.Crossref | GoogleScholarGoogle Scholar | 17783475PubMed |
Dodson, J. J., and Fitzgerald, G. J. (1980). Observations on the breeding biology of the boobies (Sulidae) at Clipperton Island, eastern Pacific Naturaliste Canadien 107, 259–267.
Dorward, D. F. (1962). Comparative biology of the white booby and the brown booby, Sula sp. at Ascension. The Ibis 103, 174–220.
Douglas, H. D. (1998). Changes in the distribution and abundance of waved albatrosses at Isla Espańola, Galapagos Islands, Ecuador. The Condor 100, 737–740.
| Changes in the distribution and abundance of waved albatrosses at Isla Espańola, Galapagos Islands, Ecuador.Crossref | GoogleScholarGoogle Scholar |
Drake, D. R., and Mueller-Dombois, D. (1993). Population development of rain forest trees on a chronosequence of Hawaiian lava flows. Ecology 74, 1012–1020.
| Population development of rain forest trees on a chronosequence of Hawaiian lava flows.Crossref | GoogleScholarGoogle Scholar |
Duellman, W. E. (1954). The amphibians and reptiles of Jorullo Volcano, Michoacán, Mexico. Museum of Zoology University of Michigan Occasional Paper No. 560.
Duffy, D. C. (1984). Nest site selection by masked and blue-footed boobies on Isla Espanola, Galapagos. The Condor 86, 301–304.
| Nest site selection by masked and blue-footed boobies on Isla Espanola, Galapagos.Crossref | GoogleScholarGoogle Scholar |
Dunning, J. B. (1991). Habitat occupancy by Bachman’s sparrow in the Francis Marion National Forest before and after Hurricane Hugo. The Auk 108, 723–725.
| Habitat occupancy by Bachman’s sparrow in the Francis Marion National Forest before and after Hurricane Hugo.Crossref | GoogleScholarGoogle Scholar |
Eason, P., Rabea, B., and Attum, O. (2012). Island shape, size, and isolation affect nest-site selection by little terns. Journal of Field Ornithology 83, 372–380.
| Island shape, size, and isolation affect nest-site selection by little terns.Crossref | GoogleScholarGoogle Scholar |
Eden, L. (2004). Whole world on fire - organizations knowledge, and nuclear weapons devastation. (Cornell University Press: Ithaca, NY).
Edwards, J. S., and Sugg, P. (1993). Arthropod fallout as a resource in the recolonization of Mount St. Helens. Ecology 74, 954–958.
| Arthropod fallout as a resource in the recolonization of Mount St. Helens.Crossref | GoogleScholarGoogle Scholar |
Eggler, W. A. (1948). Plant communities in the vicinity of the volcano El Paricutin, Mexico, after two and a half years of eruption. Ecology 29, 415–436.
| Plant communities in the vicinity of the volcano El Paricutin, Mexico, after two and a half years of eruption.Crossref | GoogleScholarGoogle Scholar |
Ehrhardt, J. P. (1971). Census of the birds of Clipperton Island, 1968. The Condor 73, 476–480.
| Census of the birds of Clipperton Island, 1968.Crossref | GoogleScholarGoogle Scholar |
Faaborg, J., and Arendt, W. J. (1995). Survival rates of Puerto Rican birds: are islands really that different? Ak 112, 503–507.
Flaccus, E. (1959). Revegetation of landslides in the White Mountains of New Hampshire. Ecology 40, 692–703.
| Revegetation of landslides in the White Mountains of New Hampshire.Crossref | GoogleScholarGoogle Scholar |
Fonnesbeck, C. J., and Dodd, C. K. (2003). Estimation of the flattened musk turtle (Sternotherus depressus). Survival, recapture, and recovery rate during and after a disease outbreak. Journal of Hepatology 37, 602–607.
Fontaine, C. (2013). Abundant equals nested. Nature 500, 411–412.
| Abundant equals nested.Crossref | GoogleScholarGoogle Scholar | 23969457PubMed |
Frangi, J. L., and Lugo, A. E. (1998). A flood plain palm forest in the Luguillo Mountains of Puerto Rico, five years after Hurricane Hugo. Biotropica 30, 339–348.
| A flood plain palm forest in the Luguillo Mountains of Puerto Rico, five years after Hurricane Hugo.Crossref | GoogleScholarGoogle Scholar |
Frankham, R. (1998). Inbreeding and extinction: island populations. Conservation Biology 12, 665–675.
| Inbreeding and extinction: island populations.Crossref | GoogleScholarGoogle Scholar |
Fraser, N. C., and Sues, H. (1994). ‘In the Shadow of the Dinosaurs.’ (Cambridge University Press: London.)
Freeman, J. P., Stolhgren, T. J., Hunter, M. E., Omi, P. N., Martinson, E. J., Chong, G. W., and Brown, C. S. (2007). Rapid assessment of post-fire plant invasions in coniferous forests of the western United States. Ecological Applications 17, 1656–1665.
| Rapid assessment of post-fire plant invasions in coniferous forests of the western United States.Crossref | GoogleScholarGoogle Scholar | 17913130PubMed |
Fridriksson, S. (1975). ‘Evolution of Life on a Volcanic Island.’ (Wiley: New York.)
Friedman, J. M., Osterkamp, W. R., and Lewis, W. M. (1996). Channel narrowing and vegetation development following a Great Plains flood. Ecology 77, 2167–2181.
| Channel narrowing and vegetation development following a Great Plains flood.Crossref | GoogleScholarGoogle Scholar |
Fryxell, J. M., Falls, J. B., Falls, E. A., and Brooks, R. J. (1998). Long-term dynamics of small-mammal populations in Ontario. Ecology 79, 213–225.
| Long-term dynamics of small-mammal populations in Ontario.Crossref | GoogleScholarGoogle Scholar |
Fuller, D. O., Jessup, T. C., and Salim, A. (2004). Loss of forest cover in Kalimantan, Indonesia, since the 1997–1998 El Niño. Conservation Biology 18, 249–254.
| Loss of forest cover in Kalimantan, Indonesia, since the 1997–1998 El Niño.Crossref | GoogleScholarGoogle Scholar |
Furness, R. W., and Barrett, R. T. (1991). Seabirds and fish declines. National Geographic Research Explorations 7, 82–95.
Gibbs, H. L., and Grant, P. R. (1987). Ecological consequences of an exceptionally strong El Niño event on Darwin’s finches. Ecology 68, 1735–1746.
| Ecological consequences of an exceptionally strong El Niño event on Darwin’s finches.Crossref | GoogleScholarGoogle Scholar |
Gibbs, H. L., Grant, P. R., and Weiland, J. (1984). Breeding of Darwin’s finches at an unusually early age in an El Niño year. The Auk 101, 872–874.
| Breeding of Darwin’s finches at an unusually early age in an El Niño year.Crossref | GoogleScholarGoogle Scholar |
Gibbs, H. L., Latta, S. C., and Gibbs, J. P. (1987). Effects of the 1982–83 El Niño event on blue-footed and masked booby populations on Isla Daphne Major, Galapagos. The Condor 89, 440–442.
| Effects of the 1982–83 El Niño event on blue-footed and masked booby populations on Isla Daphne Major, Galapagos.Crossref | GoogleScholarGoogle Scholar |
Gill, V. A., Hatch, S. A., and Lanctot, R. B. (2004). Colonization, population growth, and nesting success of black oystercatchers following a seismic uplift. The Condor 106, 791–800.
| Colonization, population growth, and nesting success of black oystercatchers following a seismic uplift.Crossref | GoogleScholarGoogle Scholar |
Gill, A. M., Stephens, S. L., and Cary, G. J. (2013). The worldwide “Wildfire” problem. Ecological Applications 23, 438–454.
| The worldwide “Wildfire” problem.Crossref | GoogleScholarGoogle Scholar | 23634593PubMed |
Grant, P. R., Grant, B. R., Keller, L. F., and Petren, K. (2000). Effects of El Niño events on Darwin’s finch productivity. Ecology 81, 2442–2457.
Graves, M. B., Rodewald, A. D., and Hull, S. D. (2010). Influence of woody vegetation on grassland birds within reclaimed surface mines. The Wilson Journal of Ornithology 122, 646–654.
| Influence of woody vegetation on grassland birds within reclaimed surface mines.Crossref | GoogleScholarGoogle Scholar |
Green, P. J. (1998). Litter-fall in the rain forest on Christmas Island, Indian Ocean: quantity, seasonality, and composition. Biotropica 30, 671–676.
| Litter-fall in the rain forest on Christmas Island, Indian Ocean: quantity, seasonality, and composition.Crossref | GoogleScholarGoogle Scholar |
Grodsky, S. M., Behr, M. J., Gendler, A., Drake, D., Dieterle, B. D., Rudd, R. J., and Walrath, N. L. (2011). Investigating the causes of death for wind turbine associated bat fatalities. Journal of Mammalogy 92, 917–925.
| Investigating the causes of death for wind turbine associated bat fatalities.Crossref | GoogleScholarGoogle Scholar |
Halpern, D., Hays, S. P., Leetmaa, A., Hansen, D. V., and Philander, S. G. H. (1983). Oceanographic observations of the 1982 warming of the tropical Eastern Pacific Science 211, 1173–1174.
Haney, J. C. (1985). Counting seabirds at sea from ships: comments on Inter study comparisons and methodological standardization. The Auk 102, 897–898.
Hanna, G. D. (1926). Expedition to the Revillagigedo Islands, Mexico in 1925. Proceedings of the California Academy of Science 15, 1–113.
Harris, G. D. (1974). ‘A Field Guide to the Birds of the Galápagos.’ (Talpinger: New York.)
Hawley, T. J. (2006). Alterations in frog assemblages after Hurricane Iris in Belize. Herpetological Review 37, 407–411.
Hayward, J. L., Miller, D. E., and Hill, C. R. (1982). Mount St. Helens ash: its impact on breeding ring-billed and California gulls. The Auk 99, 623–631.
Hjerpe, J., Hedenás, H., and Elmquist, T. (2001). Tropical rain forest recovery from cyclone damage and fire in Samoa. Biotropica 33, 249–259.
| Tropical rain forest recovery from cyclone damage and fire in Samoa.Crossref | GoogleScholarGoogle Scholar |
Hodder, J., and Graybill, M. R. (1985). Reproduction and survival of seabirds in Oregon during the 1982–1983 El Niño. The Condor 87, 535–541.
| Reproduction and survival of seabirds in Oregon during the 1982–1983 El Niño.Crossref | GoogleScholarGoogle Scholar |
Holl, K. D. (1999). Factors limiting tropical rainforest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31, 229–242.
| Factors limiting tropical rainforest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil.Crossref | GoogleScholarGoogle Scholar |
Holmes, R. T., and Sherry, T. W. (2001). Thirty-year bird population trends in an unfragmented temperate deciduous forest: importance of habitat change. The Auk 118, 589–609.
| Thirty-year bird population trends in an unfragmented temperate deciduous forest: importance of habitat change.Crossref | GoogleScholarGoogle Scholar |
Hopkins, W. A., Mendonca, M. T., and Congdon, J. D. (1997). Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. General and Comparative Endocrinology 108, 237–246.
| Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste.Crossref | GoogleScholarGoogle Scholar | 9356219PubMed |
Horton, J. S., and Kraebel, C. J. (1955). Development of vegetation after fire in the Chamise Chaparral of southern California. Ecology 36, 244–262.
| Development of vegetation after fire in the Chamise Chaparral of southern California.Crossref | GoogleScholarGoogle Scholar |
Howell, S. N. G., and Webb, S. (1990). The seabirds of Las Islas Revillagigedo, Mexico. The Wilson Bulletin 102, 140–146.
Howell, S. N. G., and Webb, S. (1992a). Changing status of the Laysan albatross in Mexico. American Birds 46, 220–223.
Howell, S. N. G., and Webb, S. (1992b). Observations of North American migrant birds in the Revillagigedo Islands. Euphonia 1, 27–33.
Hunt, G. L., Eppley, Z. A., and Schneider, D. C. (1986). Reproductive performance of seabirds: the importance of population and colony size. The Auk 103, 306–317.
Hunter, M. D., and Forkner, R. E. (1999). Hurricane damage influences foliar polyphenolics and subsequent herbivory on surviving bees. Ecology 80, 2676–2682.
| Hurricane damage influences foliar polyphenolics and subsequent herbivory on surviving bees.Crossref | GoogleScholarGoogle Scholar |
Iverson, S. A., and Esler, D. (2010). Harlequin duck population injury and recovery dynamics following the 1989 Exxon Valdez oil spill. Ecological Applications 20, 1993–2006.
| Harlequin duck population injury and recovery dynamics following the 1989 Exxon Valdez oil spill.Crossref | GoogleScholarGoogle Scholar | 21049885PubMed |
Jaksic, F. B., and Lazo, I. (1999). Response of a bird assemblage in semiarid Chile to the 1997–1998 El Niño. The Wilson Bulletin III, 527–535.
Jehl, J. R., and Parkes, K. C. (1982a). The status of the avifauna of the Revillagigedo Islands, Mexico. The Wilson Bulletin 94, 1–19.
Jehl, J. R., and Parkes, K. C. (1982b). The biology and taxonomy of Townsend’s shearwater. Le Gerfaut 72, 121–135.
Jehl, J. R., and Parkes, K. C. (1983). “Replacements” of land bird species on Socorro Island, Mexico. The Auk 100, 551–559.
Jehl, J. R., Boyd, W. S., Paul, D. S., and Anderson, D. W. (2002). Massive collapse and rapid rebound: population dynamics of eared grebes (Podiceps nigricollis) during an ENSO event. The Auk 119, 1162–1166.
| Massive collapse and rapid rebound: population dynamics of eared grebes (Podiceps nigricollis) during an ENSO event.Crossref | GoogleScholarGoogle Scholar |
Jenkins, K. M., and Boulton, A. J. (2003). Connectivity in a dry-land river: short-term aquatic microinvertebrate recruitment following floodplain inundation. Ecology 84, 2708–2723.
| Connectivity in a dry-land river: short-term aquatic microinvertebrate recruitment following floodplain inundation.Crossref | GoogleScholarGoogle Scholar |
Johnston, I. M. (1931). The flora of the Revillagigedo Islands. Proceedings of the California Academy of Science Series 4, 9–104.
Jones, H. P. (2010a). Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago. Ecological Applications 20, 1204–1216.
| Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago.Crossref | GoogleScholarGoogle Scholar | 20666244PubMed |
Jones, H. P. (2010b). Seabird islands take mere decades to recover following rat eradication. Ecological Applications 20, 2075–2080.
| Seabird islands take mere decades to recover following rat eradication.Crossref | GoogleScholarGoogle Scholar | 21265441PubMed |
Jones, H. P., and Kress, S. W. (2012). A review of the world’s active seabird restoration projects. Journal of Wildlife Management 76, 2–9.
| A review of the world’s active seabird restoration projects.Crossref | GoogleScholarGoogle Scholar |
Jorgenson, J. C., Ver Hoef, J. M., and Jorgenson, M. T. (2010). Long term recovery patterns of arctic tundra after winter seismic exploration. Ecological Applications 20, 205–221.
| Long term recovery patterns of arctic tundra after winter seismic exploration.Crossref | GoogleScholarGoogle Scholar | 20349841PubMed |
Kaeding, H. B. (1905). Birds from the west coast of Lower California and adjacent islands. The Condor 7, 105–111, 134–138.
| Birds from the west coast of Lower California and adjacent islands.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2011). Climate change in Oceania – a synthesis of biodiversity impacts and adaptations. Pacific Conservation Biology 17, 270–284.
Knapp, C. R., and Valeri, S. (2008). Iguana delicatissima (lesser Antillean iguana), mortality. Herpetological Review 39, 227–228.
Knopf, F. L., and Sedgwick, J. A. (1987). Latent population responses of summer birds to a catastrophic, climatological event. The Condor 89, 869–873.
| Latent population responses of summer birds to a catastrophic, climatological event.Crossref | GoogleScholarGoogle Scholar |
Knops, J. M. H., and Tilman, D. (2000). Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology 81, 88–98.
| Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment.Crossref | GoogleScholarGoogle Scholar |
Knutson, M. G., and Klaas, E. E. (1997). Declines in abundance and species richness of birds following a major flood on the upper Mississippi River. The Auk 114, 367–380.
| Declines in abundance and species richness of birds following a major flood on the upper Mississippi River.Crossref | GoogleScholarGoogle Scholar |
Koenig, W. D., Walters, E. L., and Liebhold, A. M. (2011). Effects of gypsy moth outbreaks on North American woodpeckers. The Condor 113, 352–361.
| Effects of gypsy moth outbreaks on North American woodpeckers.Crossref | GoogleScholarGoogle Scholar |
Kramer, M. G., Hansen, A. J., Taper, M. L., and Kissinger, E. J. (2001). Abiotic controls on long-term wind-throw disturbance and temperate rain forest dynamics in southeastern Alaska. Ecology 82, 2749–2768.
| Abiotic controls on long-term wind-throw disturbance and temperate rain forest dynamics in southeastern Alaska.Crossref | GoogleScholarGoogle Scholar |
Lai, Y. C., Shieh, B. S., and Kam, Y. C. (2007). Population patterns of a riparian frog (Rana swinhoana) before and after an earthquake in subtropical Taiwan. Biotropica 39, 731–736.
| Population patterns of a riparian frog (Rana swinhoana) before and after an earthquake in subtropical Taiwan.Crossref | GoogleScholarGoogle Scholar |
Latta, S. C., Wunderle, J. M., Terranova, E., and Pagan, M. (1995). An experimental study of nest predation in a subtropical wet forest following hurricane disturbance. The Wilson Bulletin 107, 590–602.
Laurance, S. G. W. (2004). Responses of understory rain forest birds to road edges in central Amazonia. Ecological Applications 14, 1344–1357.
| Responses of understory rain forest birds to road edges in central Amazonia.Crossref | GoogleScholarGoogle Scholar |
Lawrence, R. L., and Ripple, W. J. (2000). Fifteen years of revegetation of Mount St. Helens: a landscape scale analysis. Ecology 81, 2742–2752.
| Fifteen years of revegetation of Mount St. Helens: a landscape scale analysis.Crossref | GoogleScholarGoogle Scholar |
Le Corre, M. (1997). Diving depths of two tropical pelecaniformes: the red-tailed tropicbird and the red-footed booby. The Condor 99, 1004–1007.
| Diving depths of two tropical pelecaniformes: the red-tailed tropicbird and the red-footed booby.Crossref | GoogleScholarGoogle Scholar |
Levin, G. A., and Moran, R. (1989). The vascular flora of Isla Socorro, Mexico. Memoirs of the San Diego Society of Natural History 16, 1–71.
Lewis, D. (1973). ‘We the Navigators.’ (University of Hawaii Press: Honolulu, HI)
Lindig-Cisneros, R., Galindo-Vallejo, S., and Lara-Cabrera, S. (2006). Vegetation of tephra deposits 50 years after the end of the eruption of the Paricutin volcano, Mexico. The Southwestern Naturalist 51, 455–461.
| Vegetation of tephra deposits 50 years after the end of the eruption of the Paricutin volcano, Mexico.Crossref | GoogleScholarGoogle Scholar |
Logan, J. A., Mafarlane, W. W., and Willcox, L. (2010). Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the greater Yellowstone ecosystem. Ecological Applications 20, 895–902.
| Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the greater Yellowstone ecosystem.Crossref | GoogleScholarGoogle Scholar | 20597278PubMed |
Mabry, C. M., Hamburg, S. P., Lin, T. C., Horng, F. W., King, H. B., and Hsia, Y. J. (1998). Typhoon disturbance and stand-level damage patterns at a subtropical forest in Taiwan. Biotropica 30, 238–250.
| Typhoon disturbance and stand-level damage patterns at a subtropical forest in Taiwan.Crossref | GoogleScholarGoogle Scholar |
Manner, H. I., Thaman, R. R., and Hassall, D. C. (1984). Phosphate mining induced vegetation changes on Nauru Island. Ecology 65, 1454–1465.
| Phosphate mining induced vegetation changes on Nauru Island.Crossref | GoogleScholarGoogle Scholar |
Manolis, J. C., Andersen, D. E., and Cuthbert, F. J. (2002). Edge effect on nesting success of ground nesting birds near regenerating clear-cuts in a forest-dominated landscape. The Auk 119, 955–970.
| Edge effect on nesting success of ground nesting birds near regenerating clear-cuts in a forest-dominated landscape.Crossref | GoogleScholarGoogle Scholar |
Marshall, J. T. (1988). Birds lost from a giant sequoia forest during fifty years. The Condor 90, 359–372.
| Birds lost from a giant sequoia forest during fifty years.Crossref | GoogleScholarGoogle Scholar |
Martin, P. H., Sherman, R. E., and Fahey, T. J. (2004). Forty years of tropical forest recovery from agriculture: structure and floristics of secondary and old-growth riparian forests in the Dominican Republic. Biotropica 36, 297–317.
McCarley, H. (1959). The effect of flooding on a marked population of Peromyscus. Journal of Mammalogy 40, 57–62.
| The effect of flooding on a marked population of Peromyscus.Crossref | GoogleScholarGoogle Scholar |
McGlinn, D. J., Churchwell, R. T., and Palmer, M. (2010). Effects of a tornado on birds in cross timbers community. The Southwestern Naturalist 55, 460–466.
| Effects of a tornado on birds in cross timbers community.Crossref | GoogleScholarGoogle Scholar |
McIntire, E. J. B. (2004). Understanding natural disturbance boundary formation using spatial data and path analysis. Ecology 85, 1933–1943.
| Understanding natural disturbance boundary formation using spatial data and path analysis.Crossref | GoogleScholarGoogle Scholar |
McKnight, D. T. (2012). Terrapene carolina carolina (eastern box turtle) movement after a hurricane. Herpetological Review 43, 327.
McNab, B. K. (1994). Resource use and the survival of land and fresh water vertebrates on oceanic islands. American Naturalist 144, 643–660.
| Resource use and the survival of land and fresh water vertebrates on oceanic islands.Crossref | GoogleScholarGoogle Scholar |
Meyer, W. M., and Cowie, R. H. (2010). Invasive temperate species are a threat to tropical island biodiversity. Biotropica 42, 732–738.
| Invasive temperate species are a threat to tropical island biodiversity.Crossref | GoogleScholarGoogle Scholar |
Mills, K. L. (1998). Multispecies seabird feeding flocks in the Gálápagos Islands. The Condor 100, 277–285.
| Multispecies seabird feeding flocks in the Gálápagos Islands.Crossref | GoogleScholarGoogle Scholar |
Munoz, M. M., and Hewlett, J. (2011). Ecological consequences of continual volcanic activity on the lizard Anolis lividus, from Montserrat. Herpetological Review 42, 160–165.
Nelson, J. G. (1968). ‘Galápagos, Islands of Birds.’ (Longmans.)
Nelson, J. B. (1975). The breeding biology of frigate birds, a comparative review. Living Bird 14, 113–156.
Niemi, G. J., and Hanowski, J. M. (1984). Effects of a transmission line on bird populations in the Red Lake Peatland, northern Minnesota. The Auk 101, 487–498.
Norvell, R. E., Howe, F. P., and Parrish, J. R. (2003). A seven-year comparison of relative-abundance and distance-sampling methods. The Auk 120, 1013–1028.
| A seven-year comparison of relative-abundance and distance-sampling methods.Crossref | GoogleScholarGoogle Scholar |
Novak, M., Wooten, J. T., Doak, D. F., Emmerson, M., Estes, J. A., and Tinker, M. T. (2011). Predicting community responses to perturbations in the face of imperfect knowledge and network complexity. Ecology 92, 836–846.
| Predicting community responses to perturbations in the face of imperfect knowledge and network complexity.Crossref | GoogleScholarGoogle Scholar | 21661547PubMed |
O’Shea, M., and Cook, S. (2006). Gehyra mutilata (mutilated gecko), Indonesia Krakatau Island. Herpetological Review 37, 360–361.
Officer, C., and Page, J. (1996). ‘The Great Dinosaur Extinction Controversy.’ (Addison-Wesley Publishing Co.: Reading, MA.)
Ostertag, R., Silver, W. L., and Luge, A. E. (2005). Factors affecting mortality and resistance to damage following hurricanes in a rehabilitated subtropical moist forest. Biotropica 37, 16–24.
| Factors affecting mortality and resistance to damage following hurricanes in a rehabilitated subtropical moist forest.Crossref | GoogleScholarGoogle Scholar |
Palacios-Vargas, J. G., Llampallas, J., and Hogue, C. L. (1982). Preliminary list of the insects and related terrestrial arthropods of Socorro Island, Islas Revillagigedo, Mexico. Bulletin of the Southern California Academy of Sciences 81, 138–147.
Parker, K. R., and Wiens, J. A. (2005). Assessing recovery following environmental accidents: environmental variation, ecological assumptions, and strategies. Ecological Applications 15, 2037–2051.
| Assessing recovery following environmental accidents: environmental variation, ecological assumptions, and strategies.Crossref | GoogleScholarGoogle Scholar |
Pavelka, M. S. M., and Behie, A. M. (2005). The effect of Hurricane Iris on the food supply of black houders (Alouatta pigra) in southern Belize. Biotropica 37, 102–108.
| The effect of Hurricane Iris on the food supply of black houders (Alouatta pigra) in southern Belize.Crossref | GoogleScholarGoogle Scholar |
Pedersen, S. C., Popowics, T. E., Kwiecinski, G. G., and Knudsen, D. E. B. (2012). Sublethal pathology in bats associated with stress and volcanic activity on Montserrat, West Indies. Journal of Mammalogy 93, 1380–1392.
| Sublethal pathology in bats associated with stress and volcanic activity on Montserrat, West Indies.Crossref | GoogleScholarGoogle Scholar |
Penteriani, V., Mathiaut, M., and Boisson, G. (2002). Immediate species responses to catastrophic natural disturbances: wind-throw effects on density, productivity, nesting stand choice, and fidelity in northern goshawks (Accipiter gentilis). The Auk 119, 1132–1137.
| Immediate species responses to catastrophic natural disturbances: wind-throw effects on density, productivity, nesting stand choice, and fidelity in northern goshawks (Accipiter gentilis).Crossref | GoogleScholarGoogle Scholar |
Pike, D. A., Webb, J. K., and Shine, R. (2011). Removing forest canopy cover restores a reptile assemblage. Ecological Applications 21, 274–280.
| Removing forest canopy cover restores a reptile assemblage.Crossref | GoogleScholarGoogle Scholar | 21516904PubMed |
Piou, C., Feller, I. C., Berger, U., and Chi, F. (2006). Zonation patterns of Belizean offshore mangrove forests 41 years after a catastrophic hurricane. Biotropica 38, 365–374.
| Zonation patterns of Belizean offshore mangrove forests 41 years after a catastrophic hurricane.Crossref | GoogleScholarGoogle Scholar |
Pitman, R. L., and Ballance, L. (1990). Daytime feeding by Leach’s storm petrel on a mid-water fish in the eastern tropical Pacific. The Condor 92, 524–527.
| Daytime feeding by Leach’s storm petrel on a mid-water fish in the eastern tropical Pacific.Crossref | GoogleScholarGoogle Scholar |
Pitman, R. L., and Ballance, L. T. (2002). The changing status of marine birds breeding at San Benedicto Island, Mexico. The Wilson Bulletin 114, 11–19.
| The changing status of marine birds breeding at San Benedicto Island, Mexico.Crossref | GoogleScholarGoogle Scholar |
Pitman, R. L., and Jehl, J. R. (1998). Geographic variation and reassessment of species limits in the “masked” boobies of the eastern Pacific Ocean. The Wilson Bulletin 110, 155–170.
Planes, W., Galzin, R., Bablet, J. P., and Sale, P. F. (2005). Stability of coral reef fish assemblages impacted by nuclear tests. Ecology 86, 2578–2585.
| Stability of coral reef fish assemblages impacted by nuclear tests.Crossref | GoogleScholarGoogle Scholar |
Platt, R. B. (1961). Ecological effects of Ionizing Radiation on organisms, communities, and ecosystems. In ‘First National Symposium of Radioecology‘. pp. 1–29.
Platt, W. J., and Connell, J. H. (2003). Natural disturbances and directional replacement of species. Ecological Monographs 73, 507–522.
| Natural disturbances and directional replacement of species.Crossref | GoogleScholarGoogle Scholar |
Platt, R. B., and Mohrbracher, J. A. (1959). Studies in radiation ecology: I. The program of study. Bulletin of the Georgia Academy of Science 17, 1–30.
Plotkin, A. B., Foster, D., Carlson, J., and Magill, A. (2013). Survivors, not invaders, control forest development following simulated hurricane. Ecology 94, 414–423.
| Survivors, not invaders, control forest development following simulated hurricane.Crossref | GoogleScholarGoogle Scholar | 23691660PubMed |
Podolsky, R. H. (1990). Effectiveness of social stimuli in attracting Layson albatross to new potential nesting sites. The Auk 107, 119–124.
Polis, G. A., Hurd, S. D., Jackson, C. T., and Pińero, F. S. (1997). El Niño effects on the dynamics and control of an island ecosystem in the Gulf of California. Ecology 78, 1884–1897.
Pollack, J. B. (1981). Measurements of the volcanic plumes of Mount St. Helens in the stratosphere and troposphere: introduction. Science 211, 815–816.
| Measurements of the volcanic plumes of Mount St. Helens in the stratosphere and troposphere: introduction.Crossref | GoogleScholarGoogle Scholar | 17740387PubMed |
Prather, J. W., and Smith, K. G. (2003). Effects of tornado damage on forest bird populations in the Arkansas Ozarks. The Southwestern Naturalist 48, 292–297.
| Effects of tornado damage on forest bird populations in the Arkansas Ozarks.Crossref | GoogleScholarGoogle Scholar |
Quanstrom, W. R. (1966). Flood tolerance in Richardson’s ground squirrel. Journal of Mammalogy 47, 323.
| Flood tolerance in Richardson’s ground squirrel.Crossref | GoogleScholarGoogle Scholar |
Rabor, D. S. (1959). The impact of deforestation on birds of Cebu, Philippines, with new records for the island. The Auk 76, 37–43.
| The impact of deforestation on birds of Cebu, Philippines, with new records for the island.Crossref | GoogleScholarGoogle Scholar |
Ramírez-Barajas, P. J., Islebe, G. A., and Calmé, S. (2012). Impact of Hurricane Dean (2007) on game species of the Selva Maya, Mexico. Biotropica 44, 402–411.
| Impact of Hurricane Dean (2007) on game species of the Selva Maya, Mexico.Crossref | GoogleScholarGoogle Scholar |
Rebertus, A. J., Kitzberger, T., Veblen, T. T., and Roovers, L. M. (1997). Blowdown history and landscape patterns in the Andes of Tierra del Fuego, Argentina. Ecology 78, 678–692.
| Blowdown history and landscape patterns in the Andes of Tierra del Fuego, Argentina.Crossref | GoogleScholarGoogle Scholar |
Reichert, B. E., Cattau, C. E., Fletcher, R. J., Kendall, W. L., and Kitchens, W. M. (2012). Extreme weather and experience influence reproduction in an endangered bird. Ecology 93, 2580–2589.
| Extreme weather and experience influence reproduction in an endangered bird.Crossref | GoogleScholarGoogle Scholar | 23431589PubMed |
Restrepo, C., and Vitousek, P. (2001). Landslides, alien species, and the diversity of a Hawaiian montane mesic ecosystem. Biotropica 33, 409–420.
| Landslides, alien species, and the diversity of a Hawaiian montane mesic ecosystem.Crossref | GoogleScholarGoogle Scholar |
Richards, A. F. (1959). Geology of the Islas Revillagigedo, Mexico: I. Birth and development of Volcan Bárcena, Isla San Benedicto. Bulletin Volcanologique 22, 73–123.
| Geology of the Islas Revillagigedo, Mexico: I. Birth and development of Volcan Bárcena, Isla San Benedicto.Crossref | GoogleScholarGoogle Scholar |
Richards, A. F. (1965). Geology of the Islas Revillagigedo, 3. Effects of erosion on Isla San Benedicto 1952–61, following the birth of Volcan Bárcena. Bulletin Volcanologique 28, 381–403.
| Geology of the Islas Revillagigedo, 3. Effects of erosion on Isla San Benedicto 1952–61, following the birth of Volcan Bárcena.Crossref | GoogleScholarGoogle Scholar |
Richards, A. F. (1966). Geology of the Islas Revillagigedo, Mexico, 2. Geology and petrography of Isla San Benedicto. Proceedings of the California Academy of Sciences 33, 361–414.
Richards, A. F., and Brattstrom, B. H. (1959). Bibliography, cartography, discovery, and exploration of the Islas Revillagigedo. Proceedings of the California Academy of Sciences 29, 315–360.
Rickard, W. H., and Beatley, J. C. (1965). Canopy-coverage of the desert shrub vegetation mosaic of the Nevada test site. Ecology 46, 524–529.
| Canopy-coverage of the desert shrub vegetation mosaic of the Nevada test site.Crossref | GoogleScholarGoogle Scholar |
Rios-López, N., and Aide, T. M. (2007). Herpetofaunal dynamics during secondary succession. Herpetologica 63, 35–50.
| Herpetofaunal dynamics during secondary succession.Crossref | GoogleScholarGoogle Scholar |
Rivera-Milán, F. F. (1995). Detectability and population density of scaly-naped pigeons before and after Hurricane Hugo in Puerto Rico and Vieque Island. The Wilson Bulletin 107, 727–733.
Robinson, S. K. (1998). Another threat posed by forest fragmentation: reduced food supply. The Auk 115, 1–3.
| Another threat posed by forest fragmentation: reduced food supply.Crossref | GoogleScholarGoogle Scholar |
Robock, A., and Matson, M. (1983). Circumglobal transport of El Chichón volcanic dust. Science 221, 195–197.
| Circumglobal transport of El Chichón volcanic dust.Crossref | GoogleScholarGoogle Scholar | 17769219PubMed |
Roth, L. C. (1992). Hurricanes and mangrove regeneration: effects of Hurricane Joan, October 1988, on the vegetation of Isla del Venado, Bluefields, Nicaragua. Biotropica 24, 375–385.
| Hurricanes and mangrove regeneration: effects of Hurricane Joan, October 1988, on the vegetation of Isla del Venado, Bluefields, Nicaragua.Crossref | GoogleScholarGoogle Scholar |
Sakai, H. F. (1988). Avian response to mechanical clearing of a native rainforest in Hawaii. The Condor 90, 339–348.
| Avian response to mechanical clearing of a native rainforest in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Santaella, L., and Sada, A. M. (1991). The avifauna of the Revillagigedo Islands, Mexico: additional data and observations. The Wilson Bulletin 103, 668–675.
Santos, L. N., Garcia-Berthou, G., Agostinho, A. A., and Latini, J. G. (2011). Fish colonization of artificial reefs in a large Neotropical reservoir: material type and successional changes. Ecological Applications 21, 251–262.
| Fish colonization of artificial reefs in a large Neotropical reservoir: material type and successional changes.Crossref | GoogleScholarGoogle Scholar | 21516902PubMed |
Schreiber, E. A., and Burger, J. (Eds) (2003). ‘Biology of Marine Birds.’ (CRC Press: Boca Raton, FL.)
Schreiber, R. W., and Chovan, J. L. (1986). Roosting by pelagic seabirds: energetic populational, and social considerations. The Condor 88, 487–492.
| Roosting by pelagic seabirds: energetic populational, and social considerations.Crossref | GoogleScholarGoogle Scholar |
Schreiber, R. W., and Schreiber, E. A. (1984). Central Pacific seabirds and the El Niño Southern Oscillation: 1982 to 1983 perspectives. Science 225, 713–716.
| Central Pacific seabirds and the El Niño Southern Oscillation: 1982 to 1983 perspectives.Crossref | GoogleScholarGoogle Scholar | 17810291PubMed |
Schreiber, E. A., and Schreiber, R. W. (1989). Insights into seabird ecology from a global “Natural experiment”. National Geographic Research 5, 64–81.
Schreiber, E. A., Doherty, P. F., and Schenk, G. A. (2001). Effects of a chemical weapons incineration plant on red-tailed tropicbirds. Journal of Wildlife Management 65, 685–695.
| Effects of a chemical weapons incineration plant on red-tailed tropicbirds.Crossref | GoogleScholarGoogle Scholar |
Schulte, L. A., and Mladenoff, D. J. (2005). Severe wind and fire regimes in northern forests: historical variability at the regional scale. Ecology 86, 431–445.
| Severe wind and fire regimes in northern forests: historical variability at the regional scale.Crossref | GoogleScholarGoogle Scholar |
Seigel, R. A., Shel, C. A., and Doody, J. S. (1998). Changes in a population of an endangered rattlesnake Sistrurus catenatus following a severe flood. Biological Conservation 83, 127–131.
| Changes in a population of an endangered rattlesnake Sistrurus catenatus following a severe flood.Crossref | GoogleScholarGoogle Scholar |
Seki, M. P., and Harrison, C. S. (1989). Feeding ecology of two subtropical seabird species at French Frigate Shoals, Hawaii. Bulletin of Marine Science 45, 52–67.
Sherman, R. E., Fahey, T. J., and Martinez, P. (2001). Hurricane impacts on a mangrove forest in the Dominican Republic: damage patterns and early recovery. Biotropica 33, 393–408.
| Hurricane impacts on a mangrove forest in the Dominican Republic: damage patterns and early recovery.Crossref | GoogleScholarGoogle Scholar |
Shields, L. M., and Wells, P. V. (1962). Effects of nuclear testing on desert vegetation. Science 135, 38–40.
| Effects of nuclear testing on desert vegetation.Crossref | GoogleScholarGoogle Scholar | 17820186PubMed |
Shiels, A. B. (2006). Leaf litter decomposition and substrate chemistry of early successional species on landslides in Puerto Rico. Biotropica 38, 348–353.
| Leaf litter decomposition and substrate chemistry of early successional species on landslides in Puerto Rico.Crossref | GoogleScholarGoogle Scholar |
Shultz, A. J., Tingley, M. W., and Bowie, R. C. K. (2012). A century of avian community turnover in an urban green space in northern California. The Condor 114, 258–267.
| A century of avian community turnover in an urban green space in northern California.Crossref | GoogleScholarGoogle Scholar |
Simkin, T., and Fiske, R. S. (1983). ‘Krakatau 1883 – the Volcanic Eruption and its Effects.’ (Smithsonian Institution Press: Baltimore, MD.)
Simkin, T., and Fiske, R. S. (2003). Clouded picture of a big bang. Science 301, 50–51.
| Clouded picture of a big bang.Crossref | GoogleScholarGoogle Scholar |
Sinton, D. S., Jones, J. A., Ohmann, J. L., and Swanson, F. J. (2000). Windthrow disturbance, forest composition, and structure in the Bull Run Basin, Oregon. Ecology 81, 2539–2556.
| Windthrow disturbance, forest composition, and structure in the Bull Run Basin, Oregon.Crossref | GoogleScholarGoogle Scholar |
Sirotnak, J. M., and Huntly, N. J. (2000). Direct and indirect effects of herbivores on nitrogen dynamics: voles in riparian areas. Ecology 81, 78–87.
| Direct and indirect effects of herbivores on nitrogen dynamics: voles in riparian areas.Crossref | GoogleScholarGoogle Scholar |
Skousen, J. G., Call, C. A., and Knight, R. W. (1990). Natural revegetation of an unreclaimed lignite surface mine in east-central Texas. The Southwestern Naturalist 35, 434–440.
| Natural revegetation of an unreclaimed lignite surface mine in east-central Texas.Crossref | GoogleScholarGoogle Scholar |
Southern, L. K., and Southern, W. E. (1982). Effect of habitat decimation on ring-billed gull colony and nest-site tenacity. The Auk 99, 328–332.
Spear, L. B., Ainley, D. G., Nur, N., and Howell, S. N. G. (1995). Population size and factors affecting at-sea distributions of four endangered procellariids in the tropical Pacific. The Condor 97, 613–638.
| Population size and factors affecting at-sea distributions of four endangered procellariids in the tropical Pacific.Crossref | GoogleScholarGoogle Scholar |
Spear, S. F., Crisafulli, C. M., and Storfer, A. (2012). Genetic structure among coastal tailed frog populations at Mount St. Helens is moderated by post-disturbance management. Ecological Applications 22, 856–869.
| Genetic structure among coastal tailed frog populations at Mount St. Helens is moderated by post-disturbance management.Crossref | GoogleScholarGoogle Scholar | 22645816PubMed |
Spendelow, P. (1985). Starvation of a flock of chimney swifts on a very small Caribbean island. The Auk 102, 387–388.
| Starvation of a flock of chimney swifts on a very small Caribbean island.Crossref | GoogleScholarGoogle Scholar |
Spiller, D. A., and Schoener, T. W. (2007). Alteration of island food-web dynamics following major disturbance by hurricanes. Ecology 88, 37–41.
| Alteration of island food-web dynamics following major disturbance by hurricanes.Crossref | GoogleScholarGoogle Scholar | 17489451PubMed |
Stauffer, G. E., Diefenbach, D. R., Marshall, M. R., and Brauning, D. W. (2011). Nest success of grassland sparrows on reclaimed surface mines. Journal of Wildlife Management 75, 548–557.
| Nest success of grassland sparrows on reclaimed surface mines.Crossref | GoogleScholarGoogle Scholar |
Stolzenburg, W. (2011). ‘Rat Island.’ (Bloomsbury: London.)
Sullivan, N. H., Bowden, W. B., and McDowell, W. H. (1999). Short-term disappearance of foliar litter in three species before and after a hurricane. Biotropica 31, 382–393.
| Short-term disappearance of foliar litter in three species before and after a hurricane.Crossref | GoogleScholarGoogle Scholar |
Sweeney, J. R. (1956). Responses of vegetation to fire. University of California Publications in Botany 28, 143–250.
Thompson, W. L. (2002). Towards reliable bird surveys: accounting for individuals present but not detected. The Auk 119, 18–25.
| Towards reliable bird surveys: accounting for individuals present but not detected.Crossref | GoogleScholarGoogle Scholar |
Thompson, G. G., and Thompson, S. A. (2007). Early and late colonizers in mine site rehabilitated waste dump in the goldfields of Western Australia. Pacific Conservation Biology 13, 235–243.
Thorarinsson, S. (1964). ‘Surtsey, the New Island in the North Atlantic.’ (Reykjovck, Iceland.)
Thornton, I. W. B. (2000). The ecology of volcanoes: recovery and reassembly of living communities. In ‘Encyclopedia of Volcanoes’. (Ed. H Sigurdsson) pp. 1057–1081. (Academic Press: San Diego.)
Thornton, I. W. B., Zann, R. A., Rawlinson, P. A., Tidemann, C. R., Adikerana, A. S., and Widjoya, A. H. T. (1988). Colonization of the Krakatau Islands by vertebrates: equilibrium, succession and possible delayed extinction. Proceedings of the National Academy of Sciences of the United States of America 85, 515–518.
| Colonization of the Krakatau Islands by vertebrates: equilibrium, succession and possible delayed extinction.Crossref | GoogleScholarGoogle Scholar |
Thornton, I. W. B., New, T. R., Zann, R. A., and Rawlinson, P. A. (1990). Colonization of the Krakatau Islands by animals: a perspective from the 1980s. Philosophical Transactions of the Royal Society London, B 328, 131–165.
| Colonization of the Krakatau Islands by animals: a perspective from the 1980s.Crossref | GoogleScholarGoogle Scholar |
Tisdale, E. H., Fasberg, M. A., and Poulton, C. E. (1966). Vegetation and soil development on a recently glaciated area near Mount Robson, British Columbia. Ecology 47, 517–523.
| Vegetation and soil development on a recently glaciated area near Mount Robson, British Columbia.Crossref | GoogleScholarGoogle Scholar |
Titus, J. H., and Landau, F. (2003). Ski slope vegetation of Lee Canyon, Nevada, USA. The Southwestern Naturalist 48, 491–504.
| Ski slope vegetation of Lee Canyon, Nevada, USA.Crossref | GoogleScholarGoogle Scholar |
Tobin, M. F., Lopez, O. R., and Kuiar, T. A. (1999). Responses of tropical understory plants to a severe drought: tolerance and avoidance of water stress. Biotropica 31, 570–578.
| Responses of tropical understory plants to a severe drought: tolerance and avoidance of water stress.Crossref | GoogleScholarGoogle Scholar |
Townsend, C. H. (1890). Birds from the coasts of western North America and adjacent islands, collected in 1888–89, with descriptions of new species. Proceedings of the United States National Museum 13, 131–142.
| Birds from the coasts of western North America and adjacent islands, collected in 1888–89, with descriptions of new species.Crossref | GoogleScholarGoogle Scholar |
Tucker, J. K., Paukstis, G. L., and Theiling, C. H. (2001). Anuran response to catastrophic flooding. Herpetological Natural History 81, 83–86.
Turner, M. G. (2010). Disturbance and landscape dynamics in a changing world. Ecology 91, 2833–2849.
| Disturbance and landscape dynamics in a changing world.Crossref | GoogleScholarGoogle Scholar | 21058545PubMed |
Turner, F. B., and Gist, C. S. (1965). Influences of a thermonuclear cratering test on close-in populations of lizards. Ecology 46, 845–852.
| Influences of a thermonuclear cratering test on close-in populations of lizards.Crossref | GoogleScholarGoogle Scholar |
Vandecar, K. L. (2011). High mortality for rare species following hurricane disturbance in the southern Yucatán. Biotropica 43, 676–684.
| High mortality for rare species following hurricane disturbance in the southern Yucatán.Crossref | GoogleScholarGoogle Scholar |
Vandermeer, J., Brenner, A., and de la Cerda, I. G. (1998). Growth rates of tree height six years after hurricane damage at four localities in eastern Nicaragua. Biotropica 30, 502–509.
| Growth rates of tree height six years after hurricane damage at four localities in eastern Nicaragua.Crossref | GoogleScholarGoogle Scholar |
Velarde, E., Excurra, E., Cisneros-Mata, M. A., and Lavin, M. T. (2004). Seabird ecology, El Niño anomalies, and prediction of sardine fisheries in the Gulf of California. Ecological Applications 14, 607–615.
| Seabird ecology, El Niño anomalies, and prediction of sardine fisheries in the Gulf of California.Crossref | GoogleScholarGoogle Scholar |
Velázquez, E., and Gomez-Sal, A. (2007). Environmental control of early succession on a large landslide in a tropical dry ecosystem (Casita Volcano, Nicaragua). Biotropica 39, 601–609.
| Environmental control of early succession on a large landslide in a tropical dry ecosystem (Casita Volcano, Nicaragua).Crossref | GoogleScholarGoogle Scholar |
Walker, L. R., and Chapin, F. S. (1986). Physiological controls over seedling growth in primary success on an Alaskan floodplain. Ecology 67, 1508–1523.
| Physiological controls over seedling growth in primary success on an Alaskan floodplain.Crossref | GoogleScholarGoogle Scholar |
Walker, L. R., and del Moral., R. (2003). ‘Primary Succession and Ecosystem Rehabilitation.’ (Cambridge University Press.)
Walker, L. R., Lodge, J., Guzmán-Grajales, S. M., and Fletcher, N. (2003). Species-specific seedling responses to hurricane disturbance in a Puerto Rican rain forest. Biotropica 35, 472–485.
| Species-specific seedling responses to hurricane disturbance in a Puerto Rican rain forest.Crossref | GoogleScholarGoogle Scholar |
Wardle, D. A., Wiser, S. K., Allen, R. B., Doherty, J. E., Bonner, K. I., and Williamson, W. M. (2008). Aboveground and belowground effects of single-tree removals in New Zealand rain forest. Ecology 89, 1232–1245.
| Aboveground and belowground effects of single-tree removals in New Zealand rain forest.Crossref | GoogleScholarGoogle Scholar | 18543618PubMed |
Watts, B. D., and Byrd, M. A. (2007). Impact of Hurricane Isabel on bald eagle nests and reproductive performance in the Lower Chesapeake Bay. The Condor 109, 206–209.
| Impact of Hurricane Isabel on bald eagle nests and reproductive performance in the Lower Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., and Scanga, S. E. (2001). Windstorm disturbance without patch dynamics: twelve years of change in a Minnesota forest. Ecology 82, 893–897.
| Windstorm disturbance without patch dynamics: twelve years of change in a Minnesota forest.Crossref | GoogleScholarGoogle Scholar |
Whittaker, R. J., Schmitt, S. F., Jones, S. H., Partomihardjo, T., and Bush, M. B. (1998). Stand biomass and tree mortality from permanent forest plots on Krakatau, Indonesia, 1989–1995. Biotropica 30, 519–529.
| Stand biomass and tree mortality from permanent forest plots on Krakatau, Indonesia, 1989–1995.Crossref | GoogleScholarGoogle Scholar |
Wikramanayake, E., Fernando, P. V., and Leimgruber, P. (2006). Behavioral response of satellite-collared elephants to the tsunami in southern Sri lanka. Biotropica 38, 775–777.
| Behavioral response of satellite-collared elephants to the tsunami in southern Sri lanka.Crossref | GoogleScholarGoogle Scholar |
Wilkie, D. S., and Finn, J. T. (1990). Slash–burn cultivation and mammal abundance in the Ituri Forest, Zaïre. Biotropica 22, 90–99.
| Slash–burn cultivation and mammal abundance in the Ituri Forest, Zaïre.Crossref | GoogleScholarGoogle Scholar |
Wilson, U. W. (2005). The effect of the 1997–1998 El Niño on rhinoceros auklets on Protection Island, Washington. The Condor 107, 462–468.
| The effect of the 1997–1998 El Niño on rhinoceros auklets on Protection Island, Washington.Crossref | GoogleScholarGoogle Scholar |
Wilson, J. A., Lochmiller, R. L., and Jang, D. M. (2004). Dynamics of rodent assemblages inhabiting abandoned petroleum land-farms in Oklahoma. Ecological Applications 14, 1016–1027.
| Dynamics of rodent assemblages inhabiting abandoned petroleum land-farms in Oklahoma.Crossref | GoogleScholarGoogle Scholar |
Winchester, S. (2003). ‘Krakatoa.’ (Harper Perennial: New York.)
Wood, D. M., and Del Moral, R. (1987). Mechanisms of early primary succession in subalpine habitats on Mount St. Helens. Ecology 68, 780–790.
| Mechanisms of early primary succession in subalpine habitats on Mount St. Helens.Crossref | GoogleScholarGoogle Scholar |
Wray, T., Strait, K. A., and Whitmore, R. C. (1982). Reproductive success of grassland sparrows on a reclaimed surface mine in West Virginia. The Auk 99, 157–164.
| Reproductive success of grassland sparrows on a reclaimed surface mine in West Virginia.Crossref | GoogleScholarGoogle Scholar |
Wunderle, J. M. (1995). Responses of bird populations in a Puerto Rican forest to Hurricane Hugo: the first 18 months. The Condor 97, 879–896.
| Responses of bird populations in a Puerto Rican forest to Hurricane Hugo: the first 18 months.Crossref | GoogleScholarGoogle Scholar |
Wunderle, J. M., Henriques, L. M. P., and Willig, M. R. (2006). Short-term response of birds to forest gaps and understory: an assessment of reduced impact logging in a lowland Amazon forest. Biotropica 38, 235–255.
| Short-term response of birds to forest gaps and understory: an assessment of reduced impact logging in a lowland Amazon forest.Crossref | GoogleScholarGoogle Scholar |
Yamaguchi, D. K. (1993). Forest history, Mount St. Helens. National Geographic Research Explorations 9, 294–325.
Yaukey, P. H. (2008). Effects of Hurricane Katrina on the urban resident land-birds of New Orleans, Louisiana. The Condor 110, 158–161.
| Effects of Hurricane Katrina on the urban resident land-birds of New Orleans, Louisiana.Crossref | GoogleScholarGoogle Scholar |
Zobel, D., et al. (1992). Survival of plants buried for eight growing seasons by volcanic tephra. Ecology 73, 698–700.
| Survival of plants buried for eight growing seasons by volcanic tephra.Crossref | GoogleScholarGoogle Scholar |



