Managing dingoes on Fraser Island: culling, conflict, and an alternative
Adam J. O’Neill A E , Kylie M. Cairns B , Gisela Kaplan C and Ernest Healy DA Dingo for Biodiversity Project, PO Box 156, Mount Perry, Qld 4671, Australia.
B Ramaciotti Centre for Genome Analysis, School of Biotechnology and Biomolecular Sciences, Faculty of Science, University of New South Wales, Sydney, NSW 2052, Australia.
C Centre for Neuroscience and Animal Behaviour, S&T, McClymont Building, Faculty of Arts and Science, University of New England, Armidale, NSW 2351, Australia.
D Centre for Population and Urban Research, Faculty of Arts, Monash University, Clayton, Vic. 3168, Australia.
E Corresponding author. Email: adam.oneill@bigpond.com
Pacific Conservation Biology 23(1) 4-14 https://doi.org/10.1071/PC16026
Submitted: 22 June 2016 Accepted: 25 September 2016 Published: 12 December 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Globally, the role of large predators is increasingly understood as essential for the restoration and maintenance of ecosystems. Consequently, predator conservation represents a paradigm shift in ecological thinking, yet the management of predators sets conflicting goals because of ongoing conflict with humans. This is exemplified on Fraser Island where dingoes come into conflict with tourists, and dingoes perceived to be dangerous are regularly culled. It is argued here that this new conservation paradigm premised on protecting predators in conjunction with conventional wildlife management can result in predator populations being held in a perpetual state of social disorder, exacerbating rather than alleviating conflict. We consider the intensity and frequency of lethal control and how this may impact upon predator social structures, healthy ecological function, stable breeding patterns and stable territoriality. The direct effects of management-induced psychological stress for the survivors of episodic culls are discussed, as well as the indirect flow-on effects of social dysfunction. A final consideration is the cyclical nature of lethal control, whereby conflict with humans results in culling which, in turn, gives rise to further social disruption and conflict. In part, our assessment is derived from official data collected in the course of the management of dingoes on Fraser Island. On this basis, and on the basis of the international literature available, we offer new insights, which may inform predator management more broadly.
Additional keywords: Canis dingo, dingo behaviour, human–wildlife conflict, lethal control, predator, social stability
Introduction
Ecosystems are significantly influenced by the presence of large predators. At the apex of ecological communities, they play pivotal roles in the maintenance of healthy, productive and diverse systems (Crooks and Soulé 1999; Johnson et al. 2007; Glen et al. 2007; Letnic et al. 2009; Ripple et al. 2013) with striking examples found globally (Estes et al. 2011). Yet predator persecution often continues under the rationales of ‘sustainable use’, ‘conflict mitigation’ and ‘population control’ (Treves and Karanth 2003; Packer et al. 2009; Hervieux et al. 2014; Allen et al. 2015). The dingo, Canis dingo, is a wild canid extant across much of the Australian continent and is no exception to this rule (Crowther et al. 2014).
Since European occupation, dingoes have been severely persecuted throughout most of their range (O’Neill 2002). Indeed, lethal control remains the dominant feature of their management and, in spite of intensive efforts to resolve conflict, livestock depredations have not declined (Smith 2015). A seemingly entrenched and failing management paradigm continues to impede progress to protect dingoes and a restoration of their important ecological role (Johnson and Wallach 2016). Hence, investigating human–dingo conflict is an obvious focus to improve the dingo’s conservation status. We propose that the biology, ecology and sociality of dingoes provide important cues for resolving conflict. These are particularly important considerations in World Heritage–listed or internationally recognised locations that are biodiversity rich.
Fraser Island, a World Heritage–listed site located off Australia’s eastern coast, constitutes 1840 km2 of subtropical wilderness, is an international tourist destination and home to a unique population of dingoes (Crowther et al. 2014). The current dingo population is estimated to be ~120 individuals (Appleby and Jones 2011). Dingoes on Fraser Island are particularly important as they form a genetically distinct population (Cairns 2015) that is relatively free from introgression with domestic dogs (Woodall et al. 1996; Stephens et al. 2015). As such they are currently managed as ecologically important and for their intrinsic value as an iconic Australian species (EPA 2013) (Fig. 1).

|
Despite their protected status, culling was established relatively early by the Queensland Parks and Wildlife Service (QPWS) following the transfer from forestry management in 1991 (Williams 2002). Reconciling tourism with dingo conservation came to a head in April 2001 when a nine-year-old boy was fatally injured by two young dingoes. The management response was a large cull of the dingo population. Despite objections (e.g. Peace 2001), management policy has persisted, with the view that the killing of potentially dangerous dingoes is necessary and justified as long as numbers are maintained and the population is not at risk of extinction (Allen et al. 2015).
The current management perspective
Most of Fraser Island came under the management of QPWS in 1991. Active dingo management by QPWS began in 1992, with the first major cull of 16 dingoes occurring in 1994 (Williams 2002) and a further 35 dingoes were reportedly culled for scientific purposes in 1996 (Fleming et al. 2001; Williams 2002). Sporadic killing of dingoes deemed dangerous has continued since 1992 (Williams 2002; Allen et al. 2012, 2015; Right To Information (RTI) data from QPWS). In 1998, due to increasing reports of attacks on humans, a scientific evaluation and report was commissioned to guide management policy. The primary focus of this document was centred on human food subsidies and habituation, and recommended that ‘problem dingoes should be shot as a general rule’ (Corbett 1998). After the death of the boy in 2001, the QPWS argued again that dingo habituation and loss of natural fear towards humans was the cause of the boy’s death (Healy 2007). Specifically, the feeding of dingoes by humans was believed to be a primary cause of dingo habituation and aggression. As part of an audit of the Fraser Island Dingo Management Strategy in 2009, Corbett reiterated his view that the feeding of dingoes by humans was the primary catalyst driving habituation and dingo-related incidents (Corbett 2009).
Although the assumption of habituation being equal to, or leading to, aggression remains controversial, this management perspective can also be found in a recent study by Allen et al. (2015), which sought to assess the sustainability of the island’s dingoes under current levels of lethal control. This study concluded that the current level of culling is sustainable and within the parameters of an acceptable numerical response. Hence, the QPWS management persists with a focus on population numbers as a primary measure of sustainability, and the belief that habituation leads to aggression and the need for culling to mitigate human risk (EPA 2013).
Allen et al. (2015) aimed ‘to establish whether these destructions might adversely affect dingo population growth or breeding success into the future’. Primarily focussed on the numerical outcomes of lethal control, the authors’ modelling foreclosed any serious consideration of pack destabilisation, dispersal and conflict as a broader consequence of culling. The authors argued that major culls were atypical and that other mercy killings (euthanasia) or deaths due to sickness, injury, vehicle strikes and injuries resulting from other dingoes, occurred independently of the active management of human–dingo conflict. Data relating to such deaths were therefore considered beyond the scope of their study.
A challenge to the current management perspective; potential implications of lethal control
The current QPWS framework for understanding the cause of conflict can be succinctly described as (feeding → habituation → interaction → aggression) in response, their management model can be described as (kill problem individuals → human safety). This model has been supported in one study (Allen et al. 2015) as ‘sustainable’. These views strongly drive and inform the management of dingoes on Fraser Island, and are problematic for several reasons.
First, the view that habituated dingoes are more likely to harm humans is contested. Second, little consideration has been given to the potential effects of killing individual dingoes on the population’s social structure. Third, the ecological consequences of social disruption can potentially promote conflict. Fourth, that cull-induced stress is a potential cause of aggression. We also contend that management-induced aggression might entrain a cycle of culls and conflict and lead to mortality rates that threaten the population’s sustainability.
We propose instead that: (cull → social disruption → elevated breeding rates and dispersal → conspecific conflict and ecological decline → stress and aggression → human conflict → further culls). Here we provide an overview of research on the biology and ecology of dingoes and other large predators that suggests that ending lethal control will help promote the social stability and well being of dingoes, reduce conflict with humans, and ensure the sustainability of this unique population.
Demographic outcomes of lethal control
We argue that the methodology of Allen et al. (2015) reflects a significant flaw in the current management perspective. Both cull and non-cull mortalities are relevant in assessing the sustainability of the dingo population, particularly in context of large culling events. Further, the methodology of Allen et al. (2015) does not consider the possibility that some non-cull deaths may be an indirect result of management culling activity.
Research on grey wolves, Canis lupus, has shown lethal control to have additive, even super-additive effects on total mortality, with social disruption compounding the direct mortality of culls. Reduced survival rates in pups, increased non-cull mortality and sustained population declines were recorded in 10 separate studies of grey wolves (Creel and Rotella 2010). When socially compromised, natural reproductive suppression (infanticide and territorial behaviour) also becomes compromised in grey wolves (Wallach et al. 2015). However, an increase in the number of pups produced does not necessarily reflect increased recruitment into a population, because social disruption promotes emigration and transient unaffiliated wolves are less likely to survive (Creel and Rotella 2010). Hence, social stability is a crucial variable when modelling wolf population sustainability. Given the similarities between wolf and dingo biology, this consequence of culling should have been considered. Factoring in the potentially additive effect of cull and non-cull deaths is all the more important given that there are no reliable data to confirm dingo demographic trends on Fraser Island.
The ‘habituation equals aggression’ assumption
Several studies and reviews relating to coyote, Canis latrans, habituation to, and aggression towards, humans were used to inform the Fraser Island dingo management policy, particularly after 2001. However, evidence for a causal link between coyote habituation and aggression is weak (Timm 2006; Schmidt and Timm 2007). As of 2009, 70% of recorded coyote attacks on humans were found not to be associated with habituation (White and Gehrt 2009).
The pivotal document cited in QPWS literature to support the ‘habituation equals aggression’ assumption was an unpublished private consultancy report (Corbett 1998). The primary source of evidence in Corbett (1998) was a brief paper written by Dr Ludwig Carbyn of the Canadian Wildlife Service (Carbyn 1989). However, Carbyn did not consider coyote habituation alone as a sufficient explanation for aggression towards humans. He points to other possible factors, such as food stress, breeding cycles and idiosyncratic coyote behaviour (Carbyn 1989).
Typically, social disruption as a driver of stress-induced aggression is not considered by US authorities. Rather, state departments have adopted a protocol of lethal control once certain coyote behaviours are observed, primarily in an attempt to curtail potential aggression towards humans (Schmidt and Timm 2007; White and Gehrt 2009). That lethal control is undertaken before observed aggression begs the question of whether lethal control may be the trigger that actually stimulates coyote aggression.
Stress-induced aggression
Conceptualising animals as purely mechanistic has long since been supplanted by a more sophisticated understanding that animals feel, make choices and form deep emotional bonds (Johnson 1991; Haber 1996; Rogers and Kaplan 2003; Bekoff 2007; Ferdowsian et al. 2011). Animals have been shown to experience trauma and this can be triggered by loss of family or companions, by abuse and resulting stress (Ferdowsian et al. 2011). One of the mechanisms of coping with trauma is aggression, born sometimes of fearfulness or confusion and denoting, in its true meaning, a dysfunctional behaviour that carries little to no benefit for the individual displaying it (Rogers and Kaplan 2003). For example, diagnosis of post-traumatic stress disorder in socially compromised elephants has been associated with symptoms of aggression (Bradshaw et al. 2005). Veterinary analysis has observed similar aggressive tendencies in traumatised military working dogs (Brait 2015). Further, domestic dogs that are subjected to trauma and abuse are reported to display significantly higher rates of aggression (McMillan et al. 2015).
Elevated stress levels have been recorded in predators following social collapse (Bryan et al. 2015) and stress-induced aggression has been recorded in domestic dogs. Although empirical evidence is scant, to fully appreciate the significance of psychological disorders in predators that may develop in the wake of social collapse, findings relating to other socially complex species are informative, particularly for understanding effective remedial actions. Slowtow and Van Dyk (2001) and Bradshaw et al. (2005) demonstrate the importance of restoring stable social structures to mitigate hyperaggression in elephants, Loxodonta africana, a socially complex species. Given that behaviour is largely determined by ‘psychological state’ in socially complex species, and psychological state is influenced by the stability of social structures (Bradshaw et al. 2005), it is imperative that we consider predator psychology associated with conflict.
The ecological consequences of lethal control can potentially drive conflict
The logic behind human food supplements leading to aggression comprises four major components: attraction (human food), habituation, interaction and aggression. The first three are obviously connected, but how and whether these lead to aggression is unclear (Smith 2015). Above, we provide some insights to explain why culling and social disruption may drive aggressive behaviour; below we propose some mechanisms that may promote human–predator ‘interaction’.
It has been suggested that human food supplements support predators in ‘super-abundance’ that can impact prey bases and drive interaction with humans (Newsome et al. 2015). Similarly, the conventional management approach suggests that supplemental food from humans is the limiting factor for dingo densities, and surplus is dealt with by lethal control. By contrast, we propose that the limiting factor is self-regulation, a socially mediated process, which breaks down in the advent of lethal control. Before further describing this social and ecological dynamic, a description of the relevant aspects of dingo ecology and social behaviour is required.
Dingoes and wolves share a common ancestry and both display similar social behaviours. Characterised as ‘eusocial’, they are part of a small group of predators that are highly sentient and represent the most advanced level of vertebrate social development (Haber 1996). Offspring are nurtured by in-pack non-breeders and are dependent on parental support for extended periods, and, under stable conditions, packs maintain distinct family lineages for decades (Haber 1996). In stable populations, wild canids such as dingoes, wolves and coyotes maintain distinct territories with little or no overlap with neighbouring packs (Thomson 1992; Knowlton et al. 1999), and self-regulate via hierarchical dominance and territorial defence. Self-regulation is achieved through several mechanisms and all are socially mediated. These mechanisms include reproductive strategies that invest more energy into fewer offspring, limit the number of offspring below the species’ potential, and maintain territories to limit population densities; finally, family groups engage cooperatively to defend large territories and resources (Wallach et al. 2015). Due to their position in the food web and historic Indigenous totemic status (Rose 2000; Parkhurst 2010), dingoes experienced relatively stable social structures until the arrival of Europeans. Arguably, socially complex top predators may generally not be expected to, and thus not be adapted to, cope with episodes of high kin-based mortality (Promislow and Harvey 1990).
As a result of lethal control, wild canids typically have younger age structures, larger litter size, smaller pack size, higher emigration and higher non-cull mortality (Brainerd et al. 2008; Wallach et al. 2015). Following lethal control, profound changes to social dynamics have also been observed in other top predator groups (wolves: Haber 1996; Brainerd et al. 2008; bears: Wielgus et al. 2001; large cats: Cooley et al. 2009) and these changes influence predator–prey dynamics (Wallach et al. 2015). Social integrity, therefore, is a key consideration when evaluating the ecological influence of top predators, such as dingoes (Wallach et al. 2015). The importance of top predators maintaining the structure and function in diverse ecosystems is unquestioned (Estes et al. 2011), but their presence alone may be insufficient to facilitate their functional ecological roles (Ordiz et al. 2013). Research suggests that social stability and ecological function are intrinsically linked and when predator social systems are compromised ecosystems can potentially collapse (Wallach et al. 2010; Ordiz et al. 2013). We are only beginning to understand the profound effects that top predators exert on ecosystems, and more recently the significance of social integrity.
For example, grey wolf populations were traditionally considered to be limited by prey biomass, but when revisiting old datasets and considering social stability, wolf populations were found to be self-regulating (Cariappa et al. 2011). It has also been assumed that culling or ‘hunter take’ has little effect on population growth in grey wolves, that the effects of culling are compensatory, and that harvests of 28–50% are sustainable. However, when revaluating datasets by testing the relationship between culled and non-culled mortality, researchers found social factors contributing to super additive effects, suggesting that conventional management is insufficient to sustain wolf populations in the long term, even with low-intensity culls (Creel and Rotella 2010). It has also been assumed that lethal control on park boundaries has little effect on wolf populations within protected areas. However, Rutledge et al. (2010) found the effects of control (social disruption) to carry over very large spatial scales: 7000 km2 of protected area proved insufficient to sustain the social and genetic integrity in grey wolves.
An overwhelming weight of evidence supports dingoes as having important ecological roles (Glen et al. 2007), and also places them in the unique group of ‘top-predators’ that ‘self regulate’ (Wallach et al. 2015). Compromising the ecological functions of top predators may also be associated with conflict because resource depletion through the loss of top-down effects could potentially drive predators into domestic environments. A pair of socially unaffiliated wolves will kill the same number of moose as a socially stable large pack, causing an increase in predation rates. These effects are exacerbated by the loss of territorial boundaries: several socially dislocated pairs of wolves can occupy the same area as a single stable pack (Haber 1996). Surplus killing (Hayes et al. 1991) and mesopredator release (Johnson et al. 2007) are other potential drivers of resource depletion. Such ecological declines are widely documented (Johnson et al. 2007; Estes et al. 2011; Ripple et al. 2014) and not only culminate as a result of predator extirpation (prey release), but may be expedited through the effects of social disruption (overexploitation of prey) (Hayes et al. 1991; Haber 1996; Wallach et al. 2010; Ordiz et al. 2013)
In summary, top predators operate within intricate and complex social systems (Haber 1996) that are critical for population self-regulation (Wallach et al. 2015), territorial behaviour (Cooley et al. 2009) and for maintaining the integrity of ecosystems (Wallach et al. 2010). Essentially then, top predators provide a service that balances the ledgers in ecological economies, and, to use a familiar human comparison, when governing/regulatory systems are compromised and economies collapse, depression and crime can predominate. To reiterate, social disruption and the loss of top-down regulation can impoverish local ecologies (Estes et al. 2011), stimulate the dispersal of predators (Haber 1996; Knowlton et al. 1999; Wielgus et al. 2001), increase conspecific conflict (Wielgus et al. 2001; Cooley et al. 2009), and induce trauma and raise stress levels in predators (Van Meter et al. 2009; Bryan et al. 2015).
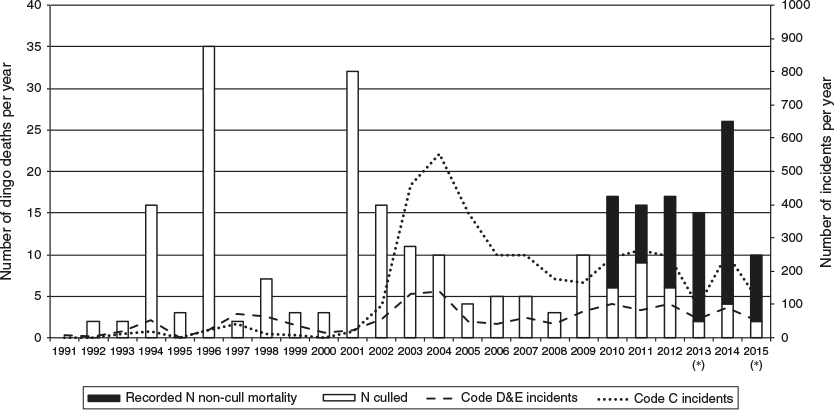
Increases in conflict following episodic predator culls/harvesting are not always observed, but have been recorded in dingoes (Wallach et al. 2009; Allen 2014, 2015), wolves (Fernández-Gil et al. 2016), cougars, Puma concolor (Gross 2008; Peebles et al. 2013), coyotes (Conner et al. 1998), jackals, Canis mesomelas (McManus et al. 2015) and black bears, Ursus americanus (Treves et al. 2010). As discussed below, following a major dingo cull on Fraser Island in 2001, human–dingo conflict rose markedly, with the number of incidents eventually reaching an asymptote in 2004 (Fig. 2).
On the basis of available data from Fraser Island and literature concerning dingoes and other socially complex top predators, we believe that the destruction of alleged ‘problem’ dingoes on the island may also have had a substantial destabilising effect on dingo social structures, potentially facilitating negative consequences for dingo physio-biological, behavioural and ecological functions and may be exacerbating, rather than alleviating, conflict.
Social disruption and cull characteristics on Fraser Island
The effects of lethal control on population densities vary considerably depending on the frequency and intensity of control events. High-intensity and frequent control can drastically reduce populations (Thomson 1986), whereas sporadic control can trigger higher rates of reproduction (Wallach et al. 2009, 2015). On Fraser Island, culls of dingoes are undertaken when individuals are assessed by QPWS as dangerous and/or posing a high risk to humans. As such, Fraser Island is representative of sporadic, low-intensity control. Perhaps counter intuitively, this may drive reproduction increase in areas where conflict and destruction are coincident and recurring.
Official culling has not occurred uniformly across Fraser Island, but has been concentrated in relatively few areas with a strong human presence (Allen et al. 2012). What may ostensibly appear to be relatively low official cull counts, together with non-cull deaths, may have a disproportionate impact on the dingo packs in and near particular locations. In such situations, localised control may facilitate higher dispersal and an increase in conspecific conflict with surrounding packs, inducing stress and trauma across pack territories. This possibility is supported by Rutledge et al. (2010), who demonstrated the propensity of ‘social collapse’ symptoms in wolves to transmit over large spatial scales.
Consideration of the ‘Eurong’ dingo pack on Fraser Island is instructive. It is situated in a location that has been subject to the highest levels of destruction since 2001. In fact, all of the destructions for ‘high risk behaviour’ reported for 2014–16 were at the Eurong location (RTI data from QPWS). Between 1992 and 2012, 24 of 132 dingoes culled were killed at Eurong. Incidence levels are also reported to be high, with 41% of all incidents occurring at this location (Allen et al. 2012). The Eurong location is also subject to the highest density of tourists on the island. This pack has produced multiple litters in some years of up to eight pups (Allen et al. 2015). Litter size may vary depending on seasonal resources, but multiple litters per pack are uncommon and are likely an indicator of social instability for dingoes, coyotes and wolves (Corbett 1988; Haber 1996; Knowlton et al. 1999; Corbett 2001; Wallach et al. 2015). Instances of multiple litters per pack are largely reported in dingo packs under lethal control management (Jones and Stevens 1988; Catling et al. 1992; Purcell 2010).
By contrast, in their assessment of the Eurong pack, Allen et al. (2015) argue that this particular situation is proof that the culling of a few individuals of a pack each year does not inhibit the reproduction, stability and persistence of dingo packs. It is important to recognise, however, that an increase in offspring should not be interpreted as population ‘stability’. An alternative explanation is that the recurring destruction of pack individuals may increase reproduction and thus lead to higher dispersal rates into neighbouring pack territories, resulting in intensified territorial conflict. While the authors argue that ongoing lethal control has no detrimental impact on the population size, and view the increase of dispersing animals as ‘doomed surplus anyway’, the likelihood of increased interpack conflict is not considered. Situations like this, of which there are several on the island, for example at Hook Point and Waddy Point (Allen et al. 2015), may lead to increases in conspecific conflict and mortality.
Further evidence of social disruption is provided by observations made in the years immediately following the fatality in 2001. These observations have suggested that dingoes on Fraser Island were a lot less territorial than at mainland locations, had weaker pack structures and island-wide dingo movements were common (Baxter and Davies 2013). Some hypothesise this to be the result of food subsidisation by humans (Corbett 1998). However, weakened pack structure and heightened, territorially unattached movement across the island may just as easily be explained by pack fracturing and dingo disorientation following the large 2001 culling event. Similarly, wildlife ecologist Rob Appleby observed that many dingoes on the island do not appear to have defined territories, being regularly found alone or with other packs at numerous and random locations (Smith 2015).
Supplemental food, intentional feeding and dingo habituation to humans
Intentional feeding features prominently in the literature in relation to habituation and wild animal aggression (Schmidt and Timm 2007), but confirming a conclusive link remains contentious. This is not surprising given the complex nature of predators, their social systems and interactions within food web structures. In their review of the subject, Newsome et al. (2015) found that supplemental food can alter the ecology and behaviour of predators, but caution that there are still many knowledge gaps. Studying the effects of food supplementation is also problematic because human food supplements for predators are often associated with conflict and lethal control, as with farm stock predation. For example, the most striking case study in the review by Newsome et al. (2015) review found an 8-fold increase in coyotes that were subsidised by human food, but they were also subjected to low-intensity lethal control (Fedriani et al. 2001).
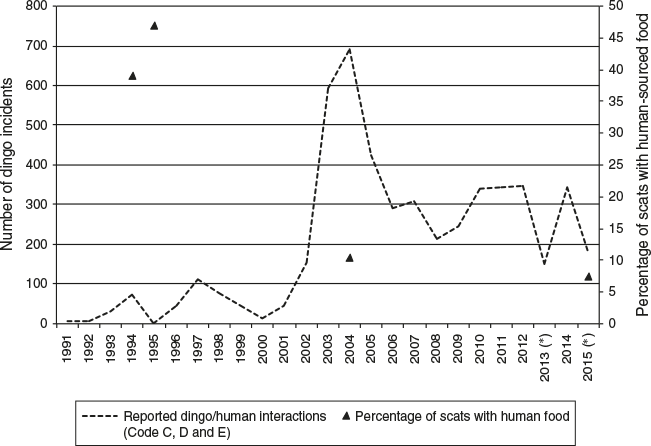
As noted, Fraser Island dingo management is structured on the premise of supplemental food and intentional feeding. This policy is supported by findings from several human food subsidisation studies. However, the policy is not really supported by the data. Over the last 22 years on Fraser Island, human food supplementation has been significantly reduced (EPA 2001), yet, simultaneously, dingo aggression is reported to have increased (Fig. 3). Analysis of ~1300 dingo scats in 1994 found 47% to contain human food (EPA 2001). A recent comparable survey found only 7.5% to contain human food (Behrendorff et al. 2016). Also, contrary to this argument, long-term resident and shopkeeper, Joe Mills, at Happy Valley from the 1960s to the 1980s, readily admits to feeding wild dingoes; he describes them as timid, well behaved and regular scavengers at the numerous dumps that existed across Fraser Island. After feeding them, ‘they would hang around for 10 minutes and then go back to the scrub, there were no attacks on any person to my knowledge while we lived on the island, nor did I hear any stories from anyone relating bad experiences with dingoes’ (reported in Healy 2007). This account is consistent with the general consensus among residents at this time: human–dingo interactions were relatively rare (Williams 2002). Through the 1990s, dingo–human interactions increased and three likely variables came into play: increased tourism, removal of human food sources and the introduction of lethal control.
|
As noted, Corbett’s 1998 report was commissioned to develop a strategy to better understand and curb the increasing number of dingo attacks on humans, particularly at the commonly used areas of the island (Corbett 1998). The increase in hostile encounters at this time is consistent with social disruption and conflict in the wake of a large cull in 1996, when 35 dingoes were killed (Fleming et al. 2001; Williams 2002).
Evidence from related and behaviourally similar species is instructive in this respect. For example, Rutledge et al. (2010) found that interactions between grey wolves and coyotes increased when social integrity was compromised. However, wolf–coyote interactions decreased when control measures were relaxed and stable pack structures reformed. In essence, as hunting pressure increased, the wolf population was socially compromised, leaving unaffiliated survivors that sought intraspecific contact with coyotes, with which they bred to produce hybrid offspring.
Parallels may be postulated between dingo populations subject to lethal control and socially disrupted emigrating dingoes on Fraser Island. The latter may be similarly isolated through pack dissolution and from competition with stable packs. Unlike wolves, however, through a long association with humans and, having undergone a rudimentary level of domestication in prehistory, dingoes may have a propensity to affiliate with humans (Corbett 2001; vonHoldt et al. 2010; Smith 2015). This propensity for human contact may increase during periods of social disturbance, triggered through mechanisms similar to those described by Rutledge et al. (2010). This tendency for dingoes to interact with humans may lead to increased conflict, as tourists are likely to interpret dingo behaviour as aggressive even when it is play.
Examination of the available data
The largest portion of data retrieved from dingo management relates to human–dingo incident reports. Incidents are categorised as Code C (largely benign interactions) and Code D (growling, snarling, stalking) and Code E (bailing-up, lunging, nipping, biting and human casualty) (Allen et al. 2015). Prior to 2001, incidents were poorly reported and recorded (Corbett 1998; Allen et al. 2015). However, those that were recorded at least represented the minimum number of incidents and thus may provide a fairly accurate representation of trends. The lack of Code C through this period suggests that QPWS were not actively seeking, but rather receiving, information about serious dingo attacks.
The possibility that lethal control is driving dingo–human encounters and conflict on Fraser Island is suggested in the data shown in Fig. 2. Incidents increase following high dingo mortality events. This is particularly evident in the period 2001–05, when the number of incidents spiked after the heavy cull of 2001 and again in 2002–04. The escalation of dingo–human incidents from 2001 onwards, which peaked around 2004–05, may have been the result of unnaturally high rates of dispersing individuals, resulting in unaffiliated, desperate dingoes seeking companionship and/or resources during an episode of severe social dislocation.
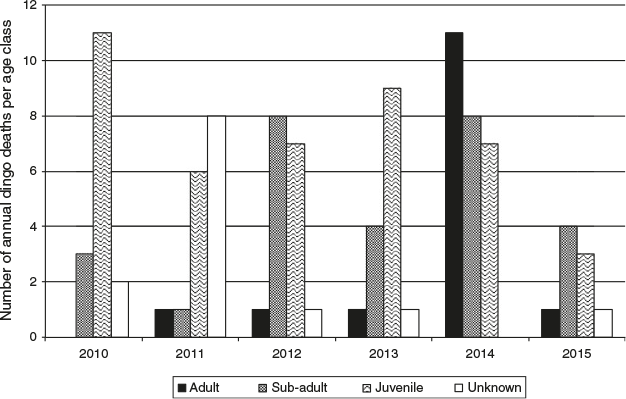
In this regard, the timing of the 2001 cull and the age of the dingoes killed appears important. Although official records of the age of the dingoes killed in the large cull of 2001 are fragmentary, the available information suggests that the cull may have been biased towards adults. This cull was also concentrated in a very narrow timeframe during April–May, in the middle of the breeding season. These factors combined would likely have maximised social and territorial disruption. In summary: when conspecific conflicts increase and unaffiliated survivors seeking entry to stable packs are turned away, lacking the necessary skills to secure resources in the wild, they may then seek out human companionship and resources.
Dingo mortality and incident data for the period 2009–14 lend further support to the hypothesis that ongoing low-level lethal control is facilitating dingo–human conflict through social disruption and increases in reproduction and dispersal (Figs 2, 4). For the previous several years, 2005–08, annual official cull counts declined to relatively low levels. After 2009, the annual cull counts increased moderately.
|
However, of particular concern are data relating to non-cull dingo mortality, for which we have data beginning in 2010 (RTI data from QPWS). When official cull counts are added to non-cull deaths, the overall known mortality is substantial, at ~15 per year. In 2014, known mortality rose to ~25 individuals. Prior to 2010, non-cull mortality records are lacking due to poor record-keeping. Nevertheless, the possibility remains that an increase in non-cull deaths accompanied official culling in the decade and a half after the 2001 cull, creating an ongoing pattern of pack instability and territorially unattached dingo behaviour that has not stabilised. The apparent increase in the number of incidents between 2010 and 2012 does appear to coincide with an increase in overall mortality counts, as one might expect in a context of pack destabilisation through lethal culling (Figs 2, 4).
Large culling events can be particularly disruptive because adult dingoes make up a larger proportion of mortality when compared with incremental culling. A hypothesis to explain the escalating rate of incidents following the major cull in 2001 is that many dingoes were deprived of pack solidarity, leadership of experienced adults and natural social constraints. Generally, hierarchal dominance ensures that a pack will successfully raise only one litter per year. In the absence of such constraints, adolescent females may conceive and raise large litters, with hierarchal infanticide unlikely. Without pack support, however, young dingo mothers would be less capable of providing for offspring, so may disperse early the following year. Non-socialised, unaffiliated and transient offspring are more likely to breed at first oestrous, adding to a spike in population and to further potential for both dingo and human conflict. Thus, incidents may peak in the second or third year after a cull. It is likely that a larger proportion of this ‘surplus’ would perish or be killed by remaining stable dingoes or appear in the statistics in the next round of culls. Within this cycle, culling temporarily reduces incidents, but also provides the basis for a new round of non-socialised breeding and conflict.
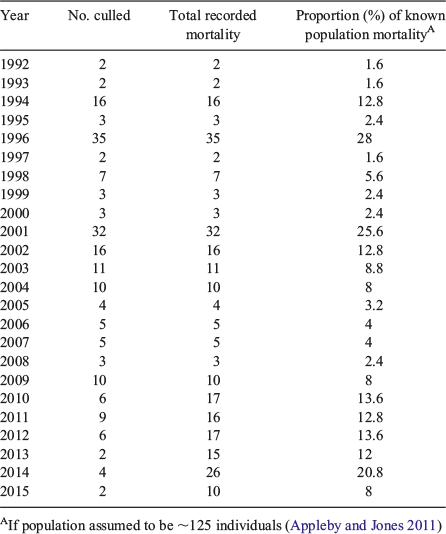
Dingo behaviour on Fraser Island does appear to have become problematic, with up to 141 serious incidents recorded annually (Allen et al. 2015). However, as we have argued, this is not typical dingo behaviour, but, once manifest, can change people’s perceptions of dingoes and encourage intolerance. Reports of tourists deliberately killing dingoes appear to be increasing, with several deliberately run over by cars in 2014–15 and the discovery of six dingoes killed by 1080 poison in 2016 (RTI data from QPWS; Bartholomew and Gaffney 2016). These additional deaths may have consequences for population sustainability. Considering the proportion of known dingo mortality as a proportion of population size, there are years where population growth could be seriously affected, with a greater than 13% yearly known mortality rate (Table 1) (Hone et al. 2010).
|
Genetic consequences
Anthropogenic pressures on predator populations that are selective in removing animals displaying unique behavioural and physical characteristics override ‘natural selection’ and facilitate rapid and dramatic changes in phenotype (Darimont et al. 2009). In eastern wolves the implementation of harvest bans beyond the boundaries of protected areas greatly improved the genetic integrity of kin-based groups within park boundaries (Rutledge et al. 2010). Thus, lethal control has far-reaching spatial and severe genetic consequences when social structures are disturbed.
Allen et al. (2015) suggest that, because culling is significantly biased towards adolescent males, it is less likely to affect overall population growth, but do caution that there may be genetic implications. This caution is warranted. Research on the genetic diversity of dingoes on Fraser Island observed a high level of inbreeding compared with mainland populations (Cairns 2015). Further, it should be noted that a population is not considered to be sustainable when the effective population size drops below 100–200 (Ballou et al. 1998; Keller and Waller 2002). Combined, this suggests that the dingo population on Fraser Island may be at risk of inbreeding depression and collapse. Removing potentially reproductive animals, male or female, may exacerbate genetic instability of the population. Episodic severe culls, combined with ongoing incremental culling and high pup mortality rates, may have created additional genetic bottlenecks in an already genetically isolated island population.
Yet other genetic processes may be at work, which may make the long-term implications of lethal control for dingo survival on Fraser Island uncertain. Recent genetic research has identified that conditions such as anxiety, stress and depression can cause modifications (genetic or epigenetic) to the DNA of affected individuals (Franklin et al. 2010; Cai et al. 2015; Swartz et al. 2016; Tyrka et al. 2016). Additionally, studies have identified genetic or epigenetic mutations in both humans and animals in response to both social and physical environmental conditions (Popova 2006; Brendgen et al. 2008; Jablonka and Raz 2009; Pavlov et al. 2012; Dasgupta 2015). Stressful events can cause epigenetic mutations that may alter an individual’s response to future stressors (Franklin et al. 2010). These epigenetic modifications can be heritable (Jablonka and Raz 2009; Franklin et al. 2010). Potentially, genetic selection and epigenetic mutation as a consequence of lethal control may be facilitating negative changes in dingo behaviour on Fraser Island.
Conclusion
We propose that a focus on dingo population numbers in the context of lethal control is not a sufficient basis for responsible management, be this in terms of animal welfare or in terms of cultural, genetic and ecological conservation values, nor is it a way of mitigating the potential threat to humans. The available data relating to dingo mortality and human–dingo ‘incidents’ on Fraser Island are consistent with the hypothesis that the current management approach is contributing to social instability and diminished territorial integrity in the dingo population, resulting in both increased human–dingo and conspecific conflict, heightened stress, elevated breeding rates and fatal dispersal of poorly socialised juveniles into neighbouring pack territories.
As it stands, the long-established management perspective on Fraser Island might entail a cycle of cull and conflict, and consequently fail to account for the possibility that current policy may be contributing to, rather than alleviating conflict. Paradoxically, while being obliged to restrict lethal control of the dingo population to a minimum, the Fraser Island dingo management regime may be applying lethal control at a level that maximises social disruption and conflict. It is also of concern that little consideration is given to the well researched link between social stability and ecological function. Trophic cascades theory, widely accepted, predicts a degrading ecology on Fraser Island – a depleted prey base may be promoting conspecific conflict and stress.
The initial incorporation of the ‘habituation equals aggression’ thesis into the dingo management strategy must remain controversial because of its weak empirical justification and management practices should therefore be reviewed. Single-cause theories rarely hold water in a complex context. The context and consequences of specific points of interception should be revisited by joint efforts of experts in the fields of genetics, animal behaviour, biology and ecology with a view to developing an alternative management perspective and strategy focussed on entire ecosystem outcomes, which are not reliant upon lethal control. Regular scat surveys to monitor stress levels and genetic variability throughout the population may help in the development of viable management policy alternatives by informing scientists and the QPWS regarding the biological, genetic and psychological health of the dingo population.
Acknowledgements
The authors thank reviewers for helpful comments, with special thanks to Al Glen and Arian Wallach for guidance and advice in preparing this manuscript. We appreciate Karin Kilpatrick from Save Fraser Island Dingoes Inc. for sharing Right to Information (RTI) data from QPWS concerning dingo mortalities and incidents. We would also pay tribute to the late Gordon Haber for his inspirational work with wolves.
References
Allen, B. L., Boswell, J., and Higginbottom, K. (2012). Fraser Island dingo management strategy review: report to Department of Environment and Heritage Protection. Ecosure Pty Ltd, West Burleigh. , .Allen, B. L., Higginbottom, K., Bracks, J. H., Davies, N., and Baxter, G. S. (2015). Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingoes. Australasian Journal of Environmental Management 22, 197–215.
| Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingoes.Crossref | GoogleScholarGoogle Scholar |
Allen, L. R. (2014). Wild dog control impacts on calf wastage in extensive beef cattle enterprises. Animal Production Science 54, 214–220.
| Wild dog control impacts on calf wastage in extensive beef cattle enterprises.Crossref | GoogleScholarGoogle Scholar |
Allen, L. R. (2015). Demographic and functional responses of wild dogs to poison baiting. Ecological Management & Restoration 16, 58–66.
| Demographic and functional responses of wild dogs to poison baiting.Crossref | GoogleScholarGoogle Scholar |
Angel, D. C. (2006). Dingo diet and prey availability on Fraser Island. Masters thesis, University of the Sunshine Coast, Sippy Downs.
Allen, B. L., Higginbottom, K., Bracks, J. H., Davies, N., and Baxter, G. S. (2015). Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingoes. Australasian Journal of Environmental Management 22, 197–215.
| Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingoes.Crossref | GoogleScholarGoogle Scholar |
Appleby, R., and Jones, D. (2011). Analysis of preliminary dingo capture–mark–recapture experiment on Fraser Island. Final Report to Queensland Parks and Wildlife Service. Griffith University, Brisbane.
Ballou, J.D., Breitenmoser-Würsten, C., Rohner, C., Breitenmoser, U. (1998). General problems of small (carnivore) populations: minimum viable population size and inbreeding. Council of Europe Publishing, Environmental Encounters 38, 27–40.
Bartholomew, K., and Gaffney, A. (2016). Fraser Island dingo deaths confirms baiting suspicion says animal protection group. ABC Sunshine Coast. Available at: http://www.abc.net.au/news/2016-06-22/fraser-island-dingo-deaths-confirms-baiting-suspicion-says-group/7533714 [accessed 24 August 2016].
Baxter, G., and Davies, N. (2013). Tracking dingoes on Fraser Island: final report on Stage 2 of the Dingo Population Study. The University of Queensland, Brisbane.
Behrendorff, L., Leung, L. K. P., McKinnon, A., Hanger, J., Belonje, G., Tapply, J., Jones, D., and Allen, B. L. (2016). Insects for breakfast and whales for dinner: the diet and body condition of dingoes on Fraser Island (K’gari). Scientific Reports 6, 23469.
| Insects for breakfast and whales for dinner: the diet and body condition of dingoes on Fraser Island (K’gari).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XkvVCgtL4%3D&md5=14a561a125264ec28abb94b1d42347c7CAS |
Bekoff, M. (2007). ‘The Emotional Lives of Animals: A Leading Scientist Explores Animal Joy, Sorrow, and Empathy – and Why They Matter.’ (New World Library: California.)
Bradshaw, G. A., Schore, A. N., Brown, J. L., Poole, J. H., and Moss, C. J. (2005). Elephant breakdown. Nature 433, 807–807.
| Elephant breakdown.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsFOrt78%3D&md5=32a23481351b0a1a169a3531799f16bdCAS |
Brainerd, S. M., Andrén, H., Bangs, E. E., Bradley, E. H., Fontaine, J. A., Hall, W., Iliopoulos, Y.,, Jimenez, M. D., Jozwiak, E. A., Liberg, O., Mack, C. M., Meier, T. J., Niemeyer, C. C., Pedersen, H. C., Sand, H., Schultz, R. N., Smith, D. W., Wabakken, P., and Wydeven, A. P. (2008). The effects of breeder loss on wolves. Journal of Wildlife Management 72, 89–98.
| The effects of breeder loss on wolves.Crossref | GoogleScholarGoogle Scholar |
Brait, E. (2015). Canine PTSD: how the US military’s use of dogs affects their mental wellbeing. Available at: http://www.theguardian.com/society/2015/nov/11/canine-ptsd-us-military-working-dogs [accessed September 2015].
Brendgen, M., Boivin, M., and Vitaro, F. (2008). Linkages between children’s and their friends’ social and physical aggression: evidence for a gene–environment interaction? Child Development 79, 13–29.
| Linkages between children’s and their friends’ social and physical aggression: evidence for a gene–environment interaction?Crossref | GoogleScholarGoogle Scholar |
Bryan, H. M., Smits, J. E., Koren, L., Paquet, P. C., Wynne‐Edwards, K. E., and Musiani, M. (2015). Heavily hunted wolves have higher stress and reproductive steroids than wolves with lower hunting pressure. Functional Ecology 29, 347–356.
| Heavily hunted wolves have higher stress and reproductive steroids than wolves with lower hunting pressure.Crossref | GoogleScholarGoogle Scholar |
Cai, N., Chang, S., and Li, Y. (2015). Molecular signatures of major depression. Current Biology 25, 1146–1156.
| Molecular signatures of major depression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXntFKjsbk%3D&md5=7bf909ec9befbd8910b37542cfa479feCAS |
Cairns, K. M. (2015). Population differentiation in the dingo: biogeography and molecular ecology of the Australian native dog using maternal, paternal and autosomal genetic markers. Ph.D. Thesis, University of New South Wales, Sydney.
Cairns, K. M., and Wilton, A. N. (2016). New insights on the history of canids in oceania based on mitochondrial and nuclear data. Genetica 144, 553–565.
| New insights on the history of canids in oceania based on mitochondrial and nuclear data.Crossref | GoogleScholarGoogle Scholar |
Carbyn, L. N. (1989). Coyote attacks on children in western North America. Wildlife Society Bulletin 17, 444–446.
Cariappa, C. A., Oakleaf, J. K., Ballard, W. B., and Breck, S. W. (2011). A reappraisal of the evidence for regulation of wolf populations. Journal of Wildlife Management 75, 726–730.
| A reappraisal of the evidence for regulation of wolf populations.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C., Corbett, L. K., and Newsome, A. E. (1992). Reproduction in captive and wild dingoes (Canis familiaris dingo) in temperate and arid environments of Australia. Wildlife Research 19, 195–209.
| Reproduction in captive and wild dingoes (Canis familiaris dingo) in temperate and arid environments of Australia.Crossref | GoogleScholarGoogle Scholar |
Conner, M. M., Jacger, M. M., Weller, T. J., and McCullough, D. R. (1998). Effect of coyote removal on sheep depredation in northern California. Journal of Wildlife Management 62, 690–699.
| Effect of coyote removal on sheep depredation in northern California.Crossref | GoogleScholarGoogle Scholar |
Cooley, H. S., Wielgus, R. B., Koehler, G., Robinson, H. S., and Maletzke, B. (2009). Does hunting regulate cougar populations? A test of the compensatory mortality hypothesis. Ecology 90, 2913–2921.
| Does hunting regulate cougar populations? A test of the compensatory mortality hypothesis.Crossref | GoogleScholarGoogle Scholar |
Corbett, L. K. (1988). Social dynamics of a captive dingo pack: population regulation by dominant female infanticide. Ethology 78, 177–198.
| Social dynamics of a captive dingo pack: population regulation by dominant female infanticide.Crossref | GoogleScholarGoogle Scholar |
Corbett, L. K. (1998). Management of dingoes on Fraser Island. Consultancy report for the Queensland Government. ERA Environmental Services.
Corbett, L. K. (2001). ‘The Dingo in Australia and Asia.’ (University of NSW Press: Sydney.)
Corbett, L. K. (2009). Audit of Fraser Island Dingo Management Strategy for Queensland Parks and Wildlife Service. Job No: 0809-3.
Creel, S., and Rotella, J. J. (2010). Meta-analysis of relationships between human offtake, total mortality and population dynamics of grey wolves (Canis lupus). PLoS One 5, e12918.
| Meta-analysis of relationships between human offtake, total mortality and population dynamics of grey wolves (Canis lupus).Crossref | GoogleScholarGoogle Scholar |
Crooks, K. R., and Soulé, M. E. (1999). Mesopredator release and avi-faunal extinctions in a fragmented system. Nature 400, 563–566.
| Mesopredator release and avi-faunal extinctions in a fragmented system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltFKrsrw%3D&md5=377d6c2cd3878ef0c34b1d28dc8bba9eCAS |
Crowther, M. S., Fillios, M., Colman, N., and Letnic, M. (2014). An updated description of the Australian dingo (Canis dingo Meyer, 1793). Journal of Zoology 293, 192–203.
| An updated description of the Australian dingo (Canis dingo Meyer, 1793).Crossref | GoogleScholarGoogle Scholar |
Darimont, C. T., Carlson, S. M., Kinnison, M. T., Paquet, P. C., Reimchen, T. E., and Wilmers, C. C. (2009). Human predators outpace other agents of trait change in the wild. Proceedings of the National Academy of Sciences of the United States of America 106, 952–954.
| Human predators outpace other agents of trait change in the wild.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht12htrw%3D&md5=ac8f194ab94c02c48ff978841c6e53d3CAS |
Dasgupta, S. (2015). Many animals can become mentally ill. BBC Earth. Available at: http://www.bbc.com/earth/story/20150909-many-animals-can-become-mentally-ill [accessed September 2015].
EPA (Environmental Protection Agency) (2001). Fraser Island dingo management strategy. Queensland Parks and Wildlife Service, Brisbane.
EPA (Environmental Protection Agency) (2013). Fraser Island dingo conservation and risk management strategy. Ecosystem Services, Department of Environment and Heritage Protection, Brisbane.
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., Bond, W. J., Carpenter, S. R., Essington, T. E., Holt, R. D., Jackson, J. B., and Marquis, R. J. (2011). Trophic downgrading of planet Earth. Science 333, 301–306.
| Trophic downgrading of planet Earth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXos1ylur0%3D&md5=7b59fe3f4e27f18a39a9a0b502fe8640CAS |
Fedriani, J. M., Fuller, T. K., and Sauvajot, R. M. (2001). Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24, 325–331.
| Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California.Crossref | GoogleScholarGoogle Scholar |
Ferdowsian, H. R., Durham, D. L., Kimwele, C., Kranendonk, G., Otali, E., Akugizibwe, T., Mulcahy, J. B., Ajarova, L., and Johnson, C. M. (2011). Signs of mood and anxiety disorders in chimpanzees. PLoS One 6, e19855.
| Signs of mood and anxiety disorders in chimpanzees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotVWhsr0%3D&md5=5abbcacb69dcdf00e2e106f5ec53081fCAS |
Fernández-Gil, A., Naves, J., Ordiz, A., Quevedo, M., Revilla, E., and Delibes, M. (2016). Conflict misleads large carnivore management and conservation: brown bears and wolves in Spain. PLoS One 11, e0151541.
| Conflict misleads large carnivore management and conservation: brown bears and wolves in Spain.Crossref | GoogleScholarGoogle Scholar |
Fleming, P., Corbett, L. K., Harden, R., and Thomson, P. (2001). ‘Managing the Impacts of Dingoes and Other Wild Dogs.’ (Bureau of Rural Sciences: Canberra.)
Franklin, T. B., Russig, H., Weiss, I. C., Gräff, J., Linder, N., Michalon, A., Vizi, S., and Mansuy, I. M. (2010). Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry 68, 408–415.
| Epigenetic transmission of the impact of early stress across generations.Crossref | GoogleScholarGoogle Scholar |
Glen, A. S., Dickman, C. R., Soule, M. E., and Mackey, B. G. (2007). Evaluating the role of the dingo as a trophic regulator in Australian ecosystems. Austral Ecology 32, 492–501.
Gross, L. (2008). No place for predators? PLoS Biology 6, e40.
| No place for predators?Crossref | GoogleScholarGoogle Scholar |
Haber, G. C. (1996). Biological, conservation, and ethical implications of exploiting and controlling wolves. Conservation Biology 10, 1068–1081.
| Biological, conservation, and ethical implications of exploiting and controlling wolves.Crossref | GoogleScholarGoogle Scholar |
Hayes, R. D., Baer, A. M., and Larsen, D. G. (1991). Population dynamics and prey relationships of an exploited and recovering wolf population in the southern Yukon. Yukon Fish and Wildlife Branch.
Healy, S. (2007). Deadly dingoes; wild or simply requiring due process? Social Studies of Science 37, 443–471.
| Deadly dingoes; wild or simply requiring due process?Crossref | GoogleScholarGoogle Scholar |
Hervieux, D., Hebblewhite, M., Stepnisky, D., Bacon, M., and Boutin, S. (2014). Managing wolves (Canis lupus) to recover threatened woodland caribou (Rangifer tarandus caribou) in Alberta. Canadian Journal of Zoology 92, 1029–1037.
| Managing wolves (Canis lupus) to recover threatened woodland caribou (Rangifer tarandus caribou) in Alberta.Crossref | GoogleScholarGoogle Scholar |
Hone, J., Duncan, R. P., and Forsyth, D. M. (2010). Estimates of maximum annual population growth rates (rm) of mammals and their application in wildlife management. Journal of Applied Ecology 47, 507–514.
| Estimates of maximum annual population growth rates (rm) of mammals and their application in wildlife management.Crossref | GoogleScholarGoogle Scholar |
Jablonka, E., and Raz, G. (2009). Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. The Quarterly Review of Biology 84, 131–176.
| Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., and Wallach, A. D. (2016). The virtuous circle: predator‐friendly farming and ecological restoration in Australia. Restoration Ecology , .
| The virtuous circle: predator‐friendly farming and ecological restoration in Australia.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N., Isaac, J. L., and Fisher, D. O. (2007). Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proceedings of the Royal Society of London. Series B, Biological Sciences 274, 341–346.
| Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia.Crossref | GoogleScholarGoogle Scholar |
Johnson, L. E. (1991). ‘A Morally Deep World; An Essay on Moral significance and Environmental Ethics.’ (Cambridge University Press.)
Jones, E., and Stevens, P. L. (1988). Reproduction in wild canids Canis familiaris from the eastern highlands of Victoria, Australia. Australian Wildlife Research 15, 385–394.
| Reproduction in wild canids Canis familiaris from the eastern highlands of Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Keller, L. F., and Waller, D. M. (2002). Inbreeding effects in wild populations. Trends in Ecology & Evolution 17, 230–241.
| Inbreeding effects in wild populations.Crossref | GoogleScholarGoogle Scholar |
Knowlton, F. F., Gese, E. M., and Jaeger, M. M. (1999). Coyote depredation control: an interface between biology and management. Journal of Range Management 52, 398–412.
| Coyote depredation control: an interface between biology and management.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Koch, F., Gordon, C., Crowther, M. S., and Dickman, C. R. (2009). Keystone effects of an alien top-predator stem extinctions of native mammals. Proceedings of the Royal Society of London. Series B, Biological Sciences 276, 3249–3256.
| Keystone effects of an alien top-predator stem extinctions of native mammals.Crossref | GoogleScholarGoogle Scholar |
McManus, J. S., Dickman, A. J., Gaynorm, D., Smuts, B. H., and MacDonald, D. W. (2015). Dead or alive? Comparing costs and benefits of lethal and non-lethal human–wildlife conflict mitigation on livestock farms. Oryx 49, 687–695.
| Dead or alive? Comparing costs and benefits of lethal and non-lethal human–wildlife conflict mitigation on livestock farms.Crossref | GoogleScholarGoogle Scholar |
McMillan, F. D., Duffy, D. L., Zawistowski, S. L., and Serpell, J. A. (2015). Behavioral and psychological characteristics of canine victims of abuse. Journal of Applied Animal Welfare Science 18, 92–111.
| Behavioral and psychological characteristics of canine victims of abuse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1ehs7%2FP&md5=4a134efe7533cc059dc3f6c24ec23edaCAS |
Moussalli, A. (1994). ‘A preliminary study of dingoes inhabiting the Waddy Point region, north-east Fraser Island: diet, social organisation and behaviour.’ BSc Thesis. Griffith University, Brisbane.
Newsome, T. M., Ballard, G.-A., Crowther, M. S., Dellinger, J. A., Fleming, P. J. S., Glen, A. S., Greenville, A. C., Johnson, C. N., Letnic, M., Moseby, K. E., Nimmo, D. G., Nelson, M. P., Read, J. L., Ripple, W. J., Ritchie, E. G., Shores, C. R., Wallach, A. D., Wirsing, A. J., and Dickman, C. R. (2015). Resolving the value of the dingo in ecological restoration. Restoration Ecology 23, 201–208.
| Resolving the value of the dingo in ecological restoration.Crossref | GoogleScholarGoogle Scholar |
O’Neill, A. (2002). ‘Living with the Dingo.’ (Envirobook: Sydney.)
Ordiz, A., Bischof, R., and Swenson, J. E. (2013). Saving large carnivores, but losing the apex predator? Biological Conservation 168, 128–133.
| Saving large carnivores, but losing the apex predator?Crossref | GoogleScholarGoogle Scholar |
Packer, C., Kosmala, M., Cooley, H. S., Brink, H., Pintea, L., Garshelis, D., Purchase, G., Strauss, M., Swanson, A., Balme, G., Hunter, L., and Nowell, K. (2009). Sport hunting, predator control and conservation of large carnivores. PLoS One 4, e5941.
| Sport hunting, predator control and conservation of large carnivores.Crossref | GoogleScholarGoogle Scholar |
Parkhurst, J. (2010). ‘Vanishing Icon; The Fraser Island Dingo.’ (Grey Thrush Publishing: Melbourne.)
Pavlov, K., Chistiakov, D., and Chekhonin, V. (2012). Genetic determinants of aggression and impulsivity in humans. Journal of Applied Genetics 53, 61–82.
| Genetic determinants of aggression and impulsivity in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVKnsbo%3D&md5=a8c097eeb8e1eb8c7dd911f637e7c585CAS |
Peace, A. (2001). Dingo discourse: constructions of nature and contradictions of capital in an Australian eco-tourist location. Anthropological Forum 11, 175–194.
| Dingo discourse: constructions of nature and contradictions of capital in an Australian eco-tourist location.Crossref | GoogleScholarGoogle Scholar |
Peebles, K. A., Wielgus, R. B., Maletzke, B. T., and Swanson, M. E. (2013). Effects of remedial sport hunting on cougar complaints and livestock depredations. PLoS One 8, e79713.
| Effects of remedial sport hunting on cougar complaints and livestock depredations.Crossref | GoogleScholarGoogle Scholar |
Popova, N. K. (2006). From genes to aggressive behavior: the role of serotonergic system. BioEssays 28, 495–503.
| From genes to aggressive behavior: the role of serotonergic system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvVyjs7Y%3D&md5=89aef690aaac637019d2116e866f17adCAS |
Promislow, D. E. L., and Harvey, P. H. (1990). Living fast and dying young: a comparative analysis of life history variation among mammals. Journal of Zoology 220, 417–437.
| Living fast and dying young: a comparative analysis of life history variation among mammals.Crossref | GoogleScholarGoogle Scholar |
Purcell, B. (2010). ‘Dingo.’ (CSIRO Publishing: Melbourne.)
Ripple, W. J., Wirsing, A. J., Wilmers, C. C., and Letnic, M. (2013). Widespread mesopredator effects after wolf extirpation. Biological Conservation 160, 70–79.
| Widespread mesopredator effects after wolf extirpation.Crossref | GoogleScholarGoogle Scholar |
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., Berger, J., Elmhagen, B., Letnic, M., Nelson, M. P., Schmitz, O. J., Smith, D. W., Wallach, A. D., and Wirsing, A. J. (2014). Status and ecological effects of the world’s largest carnivores. Science 343, .
| Status and ecological effects of the world’s largest carnivores.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktVyrsQ%3D%3D&md5=19af5eca5da31f7a8dd59a2c9a02a5e0CAS |
Rogers, L. J., and Kaplan, G. (2003). ‘Spirit of the Wild Dog: the World of Wolves, Coyotes, Foxes, Jackals and Dingoes.’ (Allen and Unwin: Australia.)
Rose, D. B. (2000). ‘Dingo Makes Us Human; Life and Land in an Australian Aboriginal Culture.’ (Cambridge University Press: United Kingdom.)
Rutledge, L. Y., Patterson, B. R., Mills, K. J., Loveless, K. M., Murray, D. L., and White, B. N. (2010). Protection from harvesting restores the natural social structure of eastern wolf packs. Biological Conservation 143, 332–339.
| Protection from harvesting restores the natural social structure of eastern wolf packs.Crossref | GoogleScholarGoogle Scholar |
Schmidt, R. H., and Timm, R. M. (2007). Bad dogs: why do coyotes and other canids become unruly? In ‘Proceedings of the 12th Wildlife Damage Management Conference’. (Eds D. L. Nolte, W. M. Arjo, and D. H. Stalman.) pp. 287–302. (University of Nebraska.)
Slotow, R., and van Dyk, G. (2001). Role of delinquent young “orphan” male elephants in high mortality of white rhinoceros in Pilanesberg National Park, South Africa. Koedoe 44, 85–94.
| Role of delinquent young “orphan” male elephants in high mortality of white rhinoceros in Pilanesberg National Park, South Africa.Crossref | GoogleScholarGoogle Scholar |
Smith, B. (2015). ‘The Dingo Debate, Origins, Behaviour and Conservation.’ (CSIRO Publishing: Melbourne.)
Stephens, D., Wilton, A. N., Fleming, P. J. S., and Berry, O. (2015). Death by sex in an Australian icon: a continent-wide survey reveals extensive hybridization between dingoes and domestic dogs. Molecular Ecology 24, 5643–5656.
| Death by sex in an Australian icon: a continent-wide survey reveals extensive hybridization between dingoes and domestic dogs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvVyitbbN&md5=d4d719a93c79f975b2adaae7060ecbcbCAS |
Swartz, J. R., Hariri, A. R., and Williamson, D. E. (2016). An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Molecular Psychiatry , .
| An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C. (1986). The effectiveness of aerial baiting for the control of dingoes in north-western Australia. Wildlife Research 13, 165–176.
| The effectiveness of aerial baiting for the control of dingoes in north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C. (1992). The behavioural ecology of dingoes in north-western Australia. IV. Social and special organization, and movements. Wildlife Research 19, 519–530.
| The behavioural ecology of dingoes in north-western Australia. IV. Social and special organization, and movements.Crossref | GoogleScholarGoogle Scholar |
Timm, R. M. (2006). Coyotes nipping at our heels: a new suburban dilemma. In ‘Proceedings of the 11th Triennial National Wildlife and Fisheries Extension Specialists Conference’. pp. 139–145. (Big Sky: Montana.)
Treves, A., and Karanth, K. U. (2003). Human–carnivore conflict and perspectives on carnivore management worldwide. Conservation Biology 17, 1491–1499.
| Human–carnivore conflict and perspectives on carnivore management worldwide.Crossref | GoogleScholarGoogle Scholar |
Treves, A., Kapp, K. J., and MacFarland, D. M. (2010). American black bear nuisance complaints and hunter take. Ursus 21, 30–42.
| American black bear nuisance complaints and hunter take.Crossref | GoogleScholarGoogle Scholar |
Twyford, K. (1995). ‘Investigations into the dietary ecology of dingoes on Fraser Island: third interim report.’ Queensland Parks and Wildlife Service, Brisbane.
Tyrka, A. R., Parade, S. H., Price, L. H., Kao, H.-T., Porton, B., Philip, N. S., Welch, E. S., and Carpenter, L. L. (2016). Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biological Psychiatry 79, 78–86.
| Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjvFCju7c%3D&md5=e2735af47a0b0ae65435585457951c93CAS |
Van Meter, P. E., French, J. A., Dloniak, S. M., Watts, H. E., Kolowski, J. M., and Holekamp, K. E. (2009). Fecal glucocorticoids reflect socio-ecological and anthropogenic stressors in the lives of wild spotted hyenas. Hormones and Behavior 55, 329–337.
| Fecal glucocorticoids reflect socio-ecological and anthropogenic stressors in the lives of wild spotted hyenas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSluro%3D&md5=e26c2d168c216cd8853135f4cf07443dCAS |
vonHoldt, B., Pollinger, J. P., Lohmueller, K. E., Han, E., Parker, H. G., Quignon, P., Degenhardt, J. D., Boyko, A. R., Earl, D. A., Auton, A., Reynolds, A., Bryc, K., Brisbin, A., Knowles, J. C., Mosher, D. S., Spady, T. C., Elkahloun, A., Geffen, E., Pilot, M., Jedrzejewski, W., Greco, C., Randi, E., Bannasch, D., Wilton, A., Shearman, J., Musiani, M., Cargill, M., Jones, P. G., Qian, Z., Huang, W., Ding, Z. L., Zhang, Y. P., Bustamante, C. D., Ostrander, E. A., Novembre, J., and Wayne, R. K. (2010). Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464, 898–902.
| Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjsFSqu70%3D&md5=7fa1a34b540b1ddfb53d68130b97f62fCAS |
Wallach, A. D., Ritchie, E. G., Read, J., and O’Neill, A. J. (2009). More than mere numbers: the impact of lethal control on the social stability of a top-order predator. PLoS One 4, e6861.
| More than mere numbers: the impact of lethal control on the social stability of a top-order predator.Crossref | GoogleScholarGoogle Scholar |
Wallach, A. D., Johnson, C. N., Ritchie, E. G., and O’Neill, A. J. (2010). Predator control promotes invasive dominated ecological states. Ecology Letters 13, 1008–1018.
Wallach, A. D., Izhaki, I., Toms, J. D., Ripple, W. J., and Shanas, U. (2015). What is an apex predator? Oikos , .
| What is an apex predator?Crossref | GoogleScholarGoogle Scholar |
White, L. A., and Gehrt, S. D. (2009). Coyote attacks on humans in the United States and Canada. Human Dimensions of Wildlife 14, 419–432.
Wielgus, R. B., Sarrazin, F., Ferriere, R., and Clobert, J. (2001). Estimating effects of adult male mortality on grizzly bear population growth and persistence using matrix models. Biological Conservation 98, 293–303.
| Estimating effects of adult male mortality on grizzly bear population growth and persistence using matrix models.Crossref | GoogleScholarGoogle Scholar |
Williams, F. (2002). ‘Princess K’Gari’s Fraser Island.’ (Self-published: Brisbane.)
Woodall, P. F., Pavlov, P., and Twyford, K. L. (1996). Dingoes in Queensland, Australia: skull dimensions and the identity of wild canids. Wildlife Research 23, 581–587.
| Dingoes in Queensland, Australia: skull dimensions and the identity of wild canids.Crossref | GoogleScholarGoogle Scholar |