Perceptions of barriers to the management of respiratory tract infections in general practice settings in Australia
Stephanie Fletcher-Lartey A C and Rabia Khan BA Public Health Unit, South Western Sydney Local Health District, PO Box 38, Liverpool, NSW 1871, Australia.
B Organisation for Economic Co-operation and Development, 2, rue André Pascal, Cedex 16, F-75775 Paris, France.
C Corresponding author. Email: stephanie.fletcher@sswahs.nsw.gov.au
Australian Journal of Primary Health 23(5) 471-475 https://doi.org/10.1071/PY17017
Submitted: 15 February 2017 Accepted: 9 July 2017 Published: 21 September 2017
Journal Compilation © La Trobe University 2017 Open Access CC BY-NC-ND
Abstract
Inappropriate prescribing of antibiotics for the management of respiratory tract infections (RTIs) has contributed to increased prevalence of antibiotic resistance, and this remains a challenge. The aim of this study was to evaluate the effect of general practitioners’ (GPs) participation in the Antibiotics: Clinical e-Audit, a quality-improvement activity, on GP self-reported knowledge and practice change, and explored barriers encountered in the management of respiratory tract infections (RTIs). Participants completed a survey at the end of the activity to assess the usefulness of the audit, any reported changes made and barriers encountered to their clinical practice. More than half of the 872 participants reported the audit assisted them in reviewing patients with RTIs. The majority of GP registrars (48.2%, N = 66) indicated that the clinical e-Audit had changed their practice in terms of identifying patients for whom an antibiotic was recommended. GPs identified several barriers to achieving best practice in the management of RTIs, including patient or carer expectations for an antibiotic prescription and non-adherence to symptomatic management by patients. Empowering GPs to overcome these barriers should be the aim of future education and behaviour change programs.
Additional keywords: antibiotic prescribing, antimicrobial stewardship, Clinical e-Audit, quality improvement activity, symptomatic management.
| What is known about the topic? |
• Antibiotics used for non-specific upper respiratory tract infections (URTIs) of viral origin and prescribing broad-spectrum antibiotics when a narrow-spectrum antibiotic is required has contributed to the epidemic of antimicrobial resistance. |
| What does this paper add? |
• Patient or carer expectations for an antibiotic prescription and non-adherence to symptomatic management remain persistent barriers to achieving best practice in the management of respiratory tract infections in general practice. |
Introduction
The prescribing of antibiotics for non-specific upper respiratory tract infections (URTIs) including prescribing broad-spectrum antibiotics when a narrow-spectrum antibiotic is required (Wong et al. 2006; Essack and Pignatari 2013), remains common. These factors have contributed to the increased prevalence of antibiotic resistance (Coxeter et al. 2015); with a growing level of antimicrobial resistance in the healthcare and community settings a major concern in Australia (Daley 2012; Del Mar et al. 2012; Earnshaw et al. 2013). There has been a global focus on reducing antibiotic resistance among respiratory tract infections in primary care (World Health Organization 2001, 2011; Essack and Pignatari 2013; Earnshaw et al. 2013; van der Velden et al. 2013). Reducing inappropriate prescribing of antibiotics is difficult to achieve as prescribing behaviour is multi-faceted, and appears to be driven by both clinician- and patient-related factors (Del Mar et al. 2012). Both physician-associated (Doust and Del Mar 2004; White 2004; Del Mar et al. 2012) and patient-associated factors (Little et al. 2004; Del Mar et al. 2012; Lewis and Tully 2011) have been well described. Further understanding of the prescribing practice in the Australian setting is necessary to inform the development of educational and behaviour change interventions aimed at promoting best practice in antibiotic stewardship in primary care settings.
NPS MedicineWise is undertaking a 5-year national program to encourage judicious use of antibiotics in the community. The program targets both health professionals and the broader community, with a major focus on the management of URTIs. As part of this program, one of the activities for general practitioners (GPs) and GP registrars (doctors training to be GPs) is a Clinical e-Audit, a quality-improvement activity whereby GPs review their current prescribing methods for patients with certain conditions and compares it to current best practice guidelines. The antibiotic Clinical e-Audit was designed to increase GP knowledge of and adherence to national guidelines for the management of RTIs. The Clinical e-Audit was provided free-of-charge and was open to all Australian GPs and GP registrars, who could claim for continuing professional development points upon completion of the activity. The Clinical e-Audit was also recognised for the Quality Prescribing Incentive of the Practice Incentives Program (PIP QPI).
An evaluation of the Clinical e-Audit was conducted to determine its usefulness to participants, self-reported behaviour change, and the barriers and strategies identified to achieving best practice in the management of URTIs. This paper does not present the Clinical e-Audit results; instead it focuses on participants’ perception of the extent of change in their knowledge and prescribing practice associated with participation in the Clinical e-Audit. It also describes participants’ perceptions of any barriers in the management of RTIs in general practice settings.
Methods
An online feedback and evaluation survey was available to all GPs who completed the audit. The evaluation survey was open at the same time as the Clinical e-Audit from February 2012 until October 2013. The survey comprised multi-choice and open-ended questions, which used Likert scales to assess the usefulness of the audit to the clinical management of patients with URTIs; the extent to which the audit met the learning objectives; and asked participants to report on any self-reported changes made to clinical practice; as well as open-ended questions about any barriers to best practice and planned actions to overcome these barriers.
Descriptive analysis of the multi-choice responses was conducted using SPSS, ver. 22 (SPSS Inc., Chicago, IL, USA). Open-ended responses were analysed using a thematic approach and then grouped based on emerging themes and subthemes.
Human Research Ethics Committee (HREC) approval was not sought for the evaluation as it was an integral component of participating in the Clinical e-Audit, which was a quality-improvement activity. However, the audit process was governed by strict ethical processes in keeping with National Health and Medical Research Council (NHMRC’s) guidelines for ethical considerations in quality assurance and evaluation activities (National Health and Medical Research Council 2014) and in keeping with the organisation’s privacy and confidentiality policy (NPS MedicineWise 2016).
Results
The evaluation survey was completed by 80% of the 1088 participants from all over Australia who had submitted the Clinical e-Audit; 735 identified themselves as GPs and 137 identified themselves as GP registrars. Two-thirds of participants, both GPs and GP registrars (66%) felt that the five learning objectives and their overall learning needs were entirely met by participating in the Clinical e-Audit. The majority (85%) indicated that the activity was entirely relevant to their practice and 86% reported that their overall opinion of the Clinical e-Audit was either excellent or good.
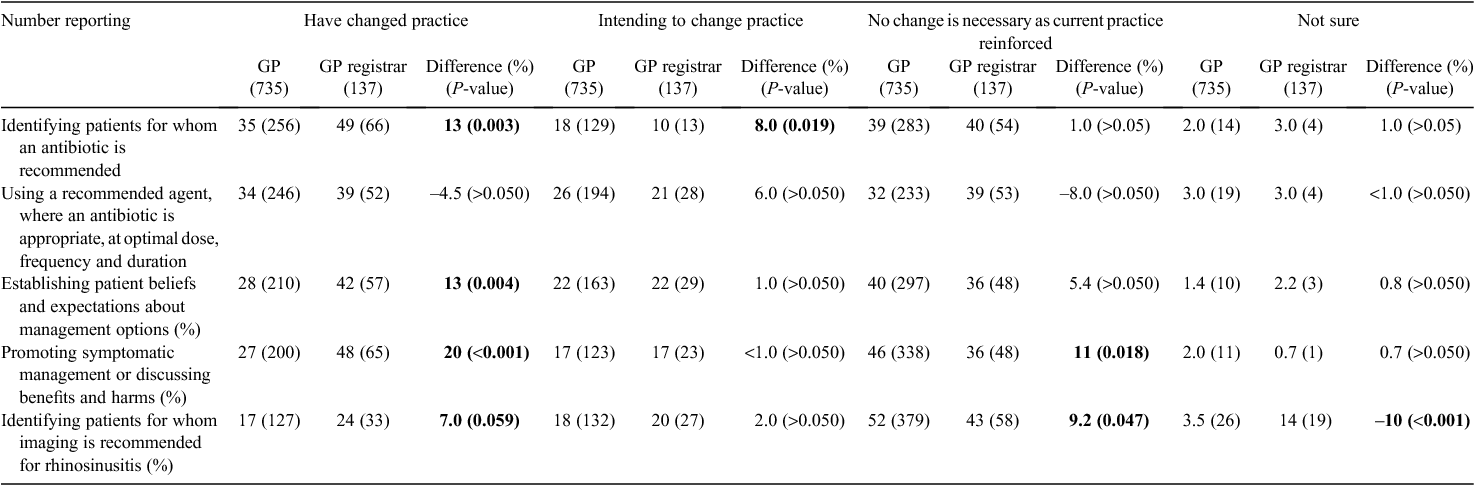
More than half (59%) of the participants agreed or strongly agreed that participation in this Clinical e-Audit assisted their review of the clinical management of patients with URTIs. However, a larger proportion of GP registrars indicated that involvement in the Clinical e-Audit had changed their practice compared with GPs (Table 1). The main areas where a change in practice was reported by GP registrars was in terms of identifying patients for whom an antibiotic is recommended (48%, n = 66), establishing patient beliefs and expectations about management options (42%, N = 57) and promoting symptomatic management and discussing the benefits and harms of antibiotics (47%, N = 65). Over one-third of GPs reported that they had changed their practice in terms of identifying patients for whom an antibiotic is recommended and using a recommended agent, where an antibiotic is appropriate, at an optimal dose, frequency and duration.
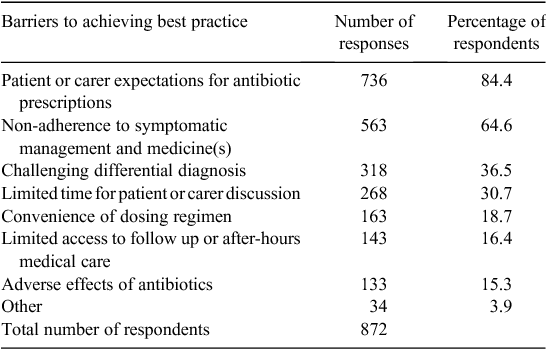
Several barriers to achieving best practice in the management of URTIs were identified by participants (Table 2). Participants could choose more than one response from the barriers listed. There was also an open-ended option for other barriers. Patient or carer expectation for antibiotics was the barrier most frequently identified by the participants. Non-adherence to symptomatic management and medicines was the second most common barrier identified by GPs and GP registrars. Challenging differential diagnosis and multiple co-morbidities were also cited as barriers, as well as limited time for patient or carer discussion and limited access to or use of patient educational material. Some doctors reportedly felt that they had limited access to educational material, which could be a result of not having enough time ‘to print out information’ or due to the fact that they ‘use different consulting rooms, so sometimes [it is] difficult to find brochures [patient leaflets] easily to hand out to patients’ [Survey respondent GP]. In contrast, some doctors found it difficult ‘in keeping up with and or following current guidelines or applying best practice’ [Survey respondent GP].
|
Respondents were asked to indicate several strategies that they had implemented or planned to implement to overcome the identified barriers detailed in an open-ended question. The responses were analysed by the authors to identify common themes. Five thematic areas emerging from the open-ended responses (in descending order) are discussed (Box 1).
| Box 1. Actions implemented or planned as reported by GPs and GP registrars to overcome the barriers to best practice in the management of upper respiratory tract infections |
| Provide educational interventions for patients or carers including, providing educational material and better communication. |
| Identify patient or carer’s beliefs and expectations about antibiotics for acute upper respiratory tract infections (URTIs); for example, discussing patient expectations early in an appointment so there is adequate time to address and focus on them; spend more time to discuss the natural course of the disease, explaining antibiotic resistance. |
| Access and utilise evidence-based clinical resources follow current guidelines for antibiotic use to treat a URTI; for example, using therapeutic guidelines to ensure antibiotics are used when needed. |
| Create opportunities for follow-up appointments and spend more time to review cases; for example, working with Medicare local to improve out-of-hours access; making time to explore patients’ expectations; and offering easy access to follow-up reviews if needed. |
| Encourage self-management of acute respiratory tract infections (RTIs) and explain to patients why antibiotics may not be appropriate; for example, reinforcing with patients the importance of symptomatic relief, and staying away from work and reducing infection transfer; discussing conservative management options for viral infections; and allaying patients’ need for antibiotics and GPs and GP registrars’ urge to prescribe something. |
Discussion
Main findings
Most GPs reported that participating in the Clinical e-Audit was useful as it met their learning needs and resulted in a change in prescribing behaviour for some respondents. Significantly more GP registrars than GPs indicated they had changed their practice in terms of identifying patients for whom an antibiotic is recommended (Difference: 13% P < 0.01) and establishing patient beliefs and expectations about management options (Difference: 13% P < 0.01) after participating in the activity. Although self-reported estimations of actual behaviour and intention-to-change behaviour are not objective measures, these can serve as useful indicators of areas where translation into practice might be achieved through educational means.
GPs also identified several barriers to achieving best practice in the management of RTIs, mainly patient or carer expectation and non-adherence to symptomatic management. They have also identified several measures that they themselves can implement in order to overcome the barriers they have identified. Educating patients, identifying and managing patient expectations of antibiotic treatment and encouraging them to adhere to symptom management were among the top interventions suggested by the participants.
The barrier to achieving best practice in the management of RTIs most frequently identified by GPs and GP registrars was patient or carer expectations for obtaining a prescription for antibiotics. Several studies have described patient expectations as a common barrier to antimicrobial stewardship for clinicians in both general practice and emergency care (Ong et al. 2007). Patient expectations are important to GPs, as studies have shown that GPs are more likely to prescribe antibiotics when their patients are perceived to be expecting them (Ong et al. 2007; Fletcher-Lartey et al. 2016). However, these self-reported challenges may not reflect actual practice. Although GP feedback on their practice is an important part of behaviour change, a greater change in prescribing behaviour would occur from actually challenging values or beliefs, which was not included in the scope of this investigation (Meeker et al. 2016). There is evidence that doctors commonly misinterpret the reasons for a patient’s consultation and their expectations for antibiotic treatment, and that the doctors might be overestimating these expectations. Approximately 10–20% of patients presenting with an URTI expect an antibiotic prescription from their GP (McNulty et al. 2013; Gaarslev et al. 2016); this is much lower than what is perceived by GPs.
Non-adherence to symptomatic management and medicines was the second most common barrier identified by GPs and GP registrars. URTIs are generally self-limiting, hence symptom relief is generally recommended (Wong et al. 2006; Antibiotic Expert Group 2010), as antibiotics do not necessarily shorten the time to cure URTIs (Tashima and Piccirillo 2014). Symptomatic management usually involves use of non-prescription, over-the-counter medicines (Huston et al. 2010). Although significantly more GP registrars than GPs indicated this to be the area in which their behaviour had changed, some participants felt the ‘expense of symptomatic management i.e. it is not on the PBS’ [Survey Respondent GP], prevented patients from adhering to symptomatic management recommendations.
Challenging differential diagnoses due to multiple co-morbidities, degenerative disorders and other chronic diseases such as diabetes, asthma and chronic obstructive pulmonary disease were also cited as a barrier to prescribing. Participants associated the presence of co-morbidities and chronic illnesses with increased risk of negative outcomes from acute illness, hence their justification for prescribing antibiotics for those patients. Other authors have suggested that physicians may feel obliged to prescribe antibiotics in order to safeguard themselves against any potentially negative clinical outcomes (Radyowijati and Haak 2003). It must be noted, however, that there are exceptional situations where antibiotics might be beneficial; for example, where there is increased risk of bacterial infection and those with clinical signs of serious complications (Tashima and Piccirillo 2014).
Patient education was the most common intervention identified by GPs and GP registrars to overcome the barriers to managing URTIs, which is similar to the results of other studies (Yox and Scudder 2014). Recognising the need for educational interventions for patients or carers, such as providing patients with educational material, improved communication, using a health professional mediated tool is a move in the right direction to start the conversation between the clinician and the patients. Educational efforts that target both physicians and patients have been described as being most promising in decreasing unnecessary antibiotic use (Shapiro 2002). However, there is evidence that educational modules alone are inadequate for guidelines to be transferred into practice, and socially motivated behavioural interventions that consider both established practice and clinician values, such as accountable justification and peer comparison, are effective to lower rates of inappropriate antibiotic prescribing for URTIs (Meeker et al. 2016). Further understanding of how these models can be applied to existing educational programs in Australia should be explored.
The challenge of limited access to educational material and inadequate time to print out information has implications for patient management and shared decision-making. These challenges faced by GPs can affect the creation of a supportive environment required for patient autonomy, through building trusting and respectful relationships (Elwyn et al. 2012; Fletcher-Lartey et al. 2016), and is an area that could be targeted by GP education. Health professionals require proficient communication and rapport-building skills, access to and awareness of current clinical evidence to facilitate best practice shared decision-making (Coxeter et al. 2015).
This study has some limitations in terms of generalisability to the broader GP and GP registrar population as it reflects the barriers and strategies identified by the audit participants only. However, the high response rate of 80% would suggest that the potential for bias due to non-response is low. Additionally, only self-reported behaviour change, which is less convincing than actual documented behaviour change, is presented because data from the actual audits was not available. The results should be interpreted in this context. However, these can serve as useful indicators of areas where translation into practice might be achieved through educational means.
Implications for general practice
Educational interventions should seek to assist GPs and GP registrars in developing skills and sourcing appropriate educational materials to support shared decision-making and encourage patient adherence to treatment and symptomatic management. These educational interventions should focus on building the confidence and skills of prescribers to manage patients with URTIs without recourse to antibiotics, unless indicated (Fletcher-Lartey et al. 2016). Educating patients is useful in setting the stage for GPs and GP registrars to be able to engage with patient and carers to identify their beliefs and expectations (a commonly identified barrier), and to further manage these expectations in keeping with clinical best practice (Gaarslev et al. 2016). Participants’ description of the Clinical e-Audit as a relevant tool with a good or excellent process is an important indicator of a change in historical engagement in both antibiotic prescribing as a topic and in audits in the primary healthcare setting. This paper demonstrates that there is scope for more intensive research into what are the critical drivers of change in prescribing habits and antibiotic stewardship in the general practice setting, particularly in the context of an ageing population.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This research was funded by the Australian Department of Health through NPS MedicineWise. We also acknowledge the contribution of Sheena O’Riordan for her advice during project development, and Suzanne Blogg and Lynn Weekes for their thoughtful contribution to editing this article.
References
Antibiotic Expert Group (2010) Therapeutic Guidelines: Antibiotic. Version 14. Therapeutic Guidelines Limited, Melbourne, Vic., Australia.Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC (2015) Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2015, CD010907
| Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care.Crossref | GoogleScholarGoogle Scholar |
Daley D (2012) Staphylococcus aureus programme 2012 (SAP 2012), community survey antimicrobial susceptibility report. PhD dissertation, University of Adelaide, Adelaide, SA, Australia.
Del Mar C, Glasziou P, Lowe JB, Van Driel ML, Hoffmann T, Beller E (2012) Addressing antibiotic resistance: focusing on acute respiratory infections in primary care. Australian Family Physician 41, 839–840.
Doust J, Del Mar C (2004) Why do doctors use treatments that do not work? For many reasons – including their inability to stand idle and do nothing. British Medical Journal 328, 474–475.
| Why do doctors use treatments that do not work? For many reasons – including their inability to stand idle and do nothing.Crossref | GoogleScholarGoogle Scholar |
Earnshaw S, Mendez A, Monnet DL, Hicks L, Cruickshank M, Weekes L, Njoo H, Ross S (2013) Global collaboration to encourage prudent antibiotic use. The Lancet Infectious Diseases 13, 1003–1004.
| Global collaboration to encourage prudent antibiotic use.Crossref | GoogleScholarGoogle Scholar |
Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, Edwards A (2012) Shared decision making: a model for clinical practice. Journal of General Internal Medicine 27, 1361–1367.
| Shared decision making: a model for clinical practice.Crossref | GoogleScholarGoogle Scholar |
Essack S, Pignatari AC (2013) A framework for the non-antibiotic management of upper respiratory tract infections: towards a global change in antibiotic resistance. International Journal of Clinical Practice 67, 4–9.
| A framework for the non-antibiotic management of upper respiratory tract infections: towards a global change in antibiotic resistance.Crossref | GoogleScholarGoogle Scholar |
Fletcher-Lartey S, Yee M, Gaarslev C, Khan R (2016) Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open 6, e012244
| Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study.Crossref | GoogleScholarGoogle Scholar |
Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R (2016) A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrobial Resistance and Infection Control 5, 39–48.
| A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection.Crossref | GoogleScholarGoogle Scholar |
Huston SA, Porter KB, Clements T, Shepherd G (2010) Pharmacists’ attitudes towards pediatric cough and cold products and behind the counter status. The Journal of Pediatric Pharmacology and Therapeutics : JPPT : the Official Journal of PPAG 15, 126–137.
Lewis PJ, Tully MP (2011) The discomfort caused by patient pressure on the prescribing decisions of hospital prescribers. Research in Social & Administrative Pharmacy 7, 4–15.
| The discomfort caused by patient pressure on the prescribing decisions of hospital prescribers.Crossref | GoogleScholarGoogle Scholar |
Little P, Dorward M, Warner G, Stephens K, Senior J, Moore M (2004) Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. British Medical Journal 328, 444–446.
| Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study.Crossref | GoogleScholarGoogle Scholar |
McNulty CAM, Nichols T, French DP, Joshi P, Butler CC (2013) Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg. The British Journal of General Practice 63, 429–436.
| Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg.Crossref | GoogleScholarGoogle Scholar |
Meeker D, Linder JA, Fox CR, Friedberg MW, Persell SD, Goldstein NJ, Knight TK, Hay JW, Doctor JN (2016) Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. Journal of the American Medical Association 315, 562–570.
| Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtFKqtrjJ&md5=73218789f62290a682ac8f29833e4396CAS |
National Health and Medical Research Council (2014) Ethical considerations in quality assurance and evaluation activities. Available at https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e111_ethical_considerations_in_quality_assurance_140326.pdf [Verified 23 November 2016]
NPS MedicineWise (2016) Clinical e-audits: privacy and confidentiality. Available at https://activities.nps.org.au/ClinicalAudit/Home/Privacy [Verified 23 November 2016]
Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA, EMERGEncy ID NET Study Group (2007) Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Annals of Emergency Medicine 50, 213–220.
| Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction.Crossref | GoogleScholarGoogle Scholar |
Radyowijati A, Haak H (2003) Improving antibiotic use in low-income countries: an overview of evidence on determinants. Social Science & Medicine 57, 733–744.
| Improving antibiotic use in low-income countries: an overview of evidence on determinants.Crossref | GoogleScholarGoogle Scholar |
Shapiro E (2002) Injudicious antibiotic use: an unforeseen consequence of the emphasis on patient satisfaction? Clinical Therapeutics 24, 197–204.
| Injudicious antibiotic use: an unforeseen consequence of the emphasis on patient satisfaction?Crossref | GoogleScholarGoogle Scholar |
Tashima L, Piccirillo JF (2014) Are antibiotics indicated for acute sinusitis? The Laryngoscope 124, 1979–1980.
| Are antibiotics indicated for acute sinusitis?Crossref | GoogleScholarGoogle Scholar |
van der Velden A, Duerden MG, Bell J, Oxford JS, Altiner A, Kozlov R, Sessa A, Pignatari AC, Essack SY (2013) Prescriber and patient responsibilities in treatment of acute respiratory tract infections – essential for conservation of antibiotics. Antibiotics 2, 316–327.
| Prescriber and patient responsibilities in treatment of acute respiratory tract infections – essential for conservation of antibiotics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVSktbrM&md5=65f4a69e8ae5f97f3a5ed029331c6657CAS |
White C (2004) If it doesn’t work, stop it: summary of rapid responses. British Medical Journal 328, 1016
| If it doesn’t work, stop it: summary of rapid responses.Crossref | GoogleScholarGoogle Scholar |
Wong DM, Blumberg DA, Lowe LG (2006) Guidelines for the use of antibiotics in acute upper respiratory tract infections. American Family Physician 74, 956–966.
World Health Organization (2001) World Health Organisation Global Strategy for Containment of Antimicrobial Resistance. WHO, Geneva, Switzerland.
World Health Organization (2011) World Health Day – 7 April 2011. Antimicrobial resistance and its global spread. WHO, Geneva, Switzerland.
Yox S, Scudder L (2014) Too many antibiotics! Patients and prescribers speak up. WebMD/Medscape Special Report. (WebMD/Medscape) Available at http://www.medscape.com/features/slideshow/public/antibiotic-misuse [Verified 15 August 2017]