Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction
Phillip J. Bridges A , Misung Jo B , Linah Al Alem A , Giyoun Na A , Wen Su C , Ming C. Gong C , Myoungkun Jeoung A and CheMyong Ko A D EA Division of Clinical and Reproductive Sciences, University of Kentucky, Lexington, KY 40536, USA.
B Department of Obstetrics and Gynecology, University of Kentucky, Lexington, KY 40536, USA.
C Department of Physiology, University of Kentucky, Lexington, KY 40536, USA.
D Department of Biology, University of Kentucky, Lexington, KY 40536, USA.
E Corresponding author. Email: cko2@uky.edu
Reproduction, Fertility and Development 22(5) 780-787 https://doi.org/10.1071/RD09194
Submitted: 18 August 2009 Accepted: 11 November 2009 Published: 7 April 2010
Abstract
Endothelin-2 (EDN2)-mediated contraction has been proposed as a final mechanical signal facilitating ovulation. The objectives herein were to determine (1) whether ovarian endothelins were increased before ovulation; (2) whether a specific endothelin-converting enzyme (ECE) was mediating their production; (3) which receptor was facilitating ovarian contraction; and (4) whether receptor-specific antagonism affected ovulation. Follicular development was induced in immature rats with 10 IU pregnant mare serum gonadotrophin (PMSG) and the ovulatory cascade was initiated 48 h later with 10 IU human chorionic gonadotrophin (hCG). In Experiment 1, an immunoassay revealed that the ovarian concentration of endothelin peptide was increased 7-fold 12 h after hCG when compared with 48 h after PMSG (P < 0.05). In Experiment 2, real-time PCR indicated that mRNA for Ece1, but not Ece2, was increased in granulosa cells collected 12 h after hCG when compared with those collected before the ovulatory stimulus (P < 0.05). In Experiment 3, isometric tension analysis revealed that the contractile effect of EDN2 was mediated by endothelin receptor A (EDNRA), not B (EDNRB). In Experiment 4, no effect was observed on the rate of ovulation when rats were treated with an antagonist specific to EDNRA (BQ123) or EDNRB (BQ788), or when mice were treated with BQ123, BQ788 or BQ123 + BQ788. In conclusion, endothelin peptide is produced before ovulation and the contractile action of EDN2 within the ovary is facilitated via EDNRA. In addition, findings of this study indicate synergistic interactions among contractile factors affect ovulatory outcome, while the role of EDNRB alone in the process of ovulation requires further investigation.
Additional keywords: constriction, ovulation, rupture.
Introduction
The architectural network of smooth muscle cells within the mammalian ovary has been recognised for many years as being responsive to contractile stimuli. The hypothesis that follicular rupture is the result of contraction-induced changes in intra-follicular pressure was one of several proposed to explain the mechanism of ovulation (Espey and Lipner 1963; Continho and Maia 1971; Palti and Freund 1972; Coutinho 1974; O’Shea and Phillips 1974; Virutamasen et al. 1976; Talbot and Chacon 1982). However, the physiological relevance of ovarian contraction as a facilitator of ovulation remained in question because no definitive hormonal ligand or neurological signal for this mechanical response was identified. With no conclusive ovulatory signal recognised, inconsistency among the reports of changing intra-follicular pressure during the periovulatory interval (Espey and Lipner 1963; Rondell 1964; Schroeder and Talbot 1982) and the knowledge that artificially increasing intra-follicular pressure did not induce ovulation (Rondell 1964), the focus of many researchers appeared to shift away from this working hypothesis and it became relatively dormant as a field of study.
Recently, interest has returned to the physiology of ovarian contraction and follicular pressure. Using rats as the animal model, Matousek et al. (2001) applied new techniques to the paradigm of changing intra-follicular pressure during the ovulatory cascade and we proposed that endothelin-2 (EDN2) was the final contractile signal facilitating ovulation (Ko et al. 2006). Evidence suggests that these two seemingly intertwined processes are, however, independent factors within the ovulatory cascade. Changes in intra-follicular pressure are observed as early as midway through the periovulatory cascade (Matousek et al. 2001), coincident with increases in factors affecting vascular permeability (Gómez et al. 2003; Kitajima et al. 2004; Miyabayashi et al. 2005) and the array of proteolytic enzymes known to be integral to the breakdown of the extracellular matrix before ovulation (reviewed in Curry and Smith 2006). In contrast, expression of mRNA for Edn2 is only increased very late in the periovulatory period, ~12 h after the induction of ovulation with hCG in the rat (Ko et al. 2006), well after the first observed changes in intra-follicular pressure. Therefore, we propose that the contractile action of EDN2 would be imposed at the time of ovulation itself, upon an already weakened follicular structure, resulting in no change or an actual decrease in intra-follicular pressure (as the follicle ruptures), consistent with the follicular pressure profiles described over 40 years ago by Rondell (1964).
Expression of mRNA for Edn2 was localised to the granulosa cells of periovulatory follicles by in situ hybridisation and blockade of the action of EDN2 within the ovary by administration of a dual endothelin receptor antagonist (tezosentan) inhibited or delayed ovulation (Ko et al. 2006), providing strong evidence of the importance of this peptide to the process of follicular rupture. However, the increase in levels of mRNA for Edn2 during the ovulatory cascade were observed 12 h after treatment with hCG, raising the question as to whether translation of mRNA to functional peptide could occur within sufficient time for EDN2 to exert its proposed contractile effect on the periovulatory ovary. Therefore, our first objective was to quantify gonadotrophin-induced production of EDN2 within the ovary before ovulation, to confirm production of the contractile ligand proposed to facilitate follicular rupture. Expression levels of mRNA encoding endothelin-converting enzymes (ECE) 1 and 2 (Ece1 and Ece2) were then examined to begin to unravel the biochemical pathway involved in EDN2 production at ovulation. Following determination of ligand production and converting enzyme regulation within the ovary before ovulation, our final objectives were to determine which endothelin receptor subtype (EDNRA or EDNRB) facilitated the ovarian contraction induced by EDN2 and whether blockade of that receptor subtype in vivo would inhibit ovulation. Determination of EDN2 production before ovulation and identification of its action through a specific receptor subtype will increase our understanding of this final, mechanical component of the ovulatory cascade and provide new insight into the complex mechanism that is ovulation.
Materials and methods
Animals and tissue collection
Immature female Sprague Dawley rats were purchased from Harlan Inc. (Harlan, IN, USA) and maintained under controlled lighting (14 h light : 10 h dark) with continuous access to food and water. At 24–27 days of age, follicular development was induced by treatment of rats with pregnant mare serum gonadotrophin (PMSG; 10 IU, subcutaneous) and 48 h later the ovulatory cascade was initiated by treatment with human chorionic gonadotrophin (hCG; 10 IU, subcutaneous). Using this model, ovulation occurs ~14 h after treatment with hCG. Immature female CD1 mice were also purchased from Harlan Inc. and maintained as described for rats. At 23 days of age, follicular development and ovulation was induced by treatment with PMSG and hCG (5 IU, subcutaneous, 48 h apart) with ovulation expected to occur ~12 h after treatment with hCG. Animals were sacrificed by CO2 inhalation followed by cervical luxation and the ovaries collected at the times described in each experiment below. Animal use was approved by the University of Kentucky Animal Care and Use Committee.
Immunometric assay
Total ovarian concentrations of endothelins were determined using a modified, commercially available immunometric assay kit (Cayman Chemical, Ann Arbor, MI, USA). Ovaries were collected from rats (n = 4 per time point) 48 h after PMSG and at 12 h after hCG. Ovaries were individually homogenised in the assay buffer supplied with the kit and the homogenate centrifuged for 15 min at 2000g at 4°C. The supernatant was then collected, acidified with 4% acetic acid and loaded on activated SPE (C-18) cartridges. The cartridges were washed twice with water and then the bound endothelin was eluted from each cartridge with 4% acetic acid in 86% ethanol. The first 2 mL of the elution was discarded and the following 6 mL collected, dried under vacuum centrifugation and then reconstituted with the assay buffer supplied with the kit. The immunometric assay was then performed on the samples of purified homogenate following the manufacturer’s directions and absorbance determined at a wavelength of 405 nm. Because the antibody used in this assay is reported to bind endothelins 1–3 with equal specificity, total endothelin is referred to in the results.
Real-time PCR analysis
Expression of mRNA for endothelin-converting enzyme 1 (Ece1) and endothelin-converting enzyme 2 (Ece2) in granulosa cells was determined by real-time PCR. Granulosa cells were isolated from ovaries collected before rats were treated with PMSG, 48 h after PMSG and 12 h after hCG (n = 3 animals per time point), as described previously (Ko et al. 2006). Total RNA was extracted from granulosa cells with TRIzol (Invitrogen Corp., Carlsbad, CA, USA), according to the manufacturer’s directions and the integrity verified by visualisation of distinct 18S and 28S rRNA bands after ethidium bromide staining in an agarose gel. Complementary DNA was reverse-transcribed from each sample of total RNA as described previously (Ko et al. 2006). Oligonucleotide primers corresponding to cDNA for rat Ece1 (accession number BC072504; forward 5′-TTT GGG TGG TTG TGA GCA-3′, reverse 5′-ACA AGC TCC TTT CTC GAC CA-3′), Ece2 (accession number NM_001002815; forward 5′-TGA CGC CTC AGA CTG TGA AC-3′, reverse 5′-GCC CAG GTT GTT TTC TGT GT-3′) and L32 as an endogenous reference gene (accession number BC061562; forward 5′-GAA GCC CAA GAT CGT CAA AA-3′, reverse 5′-AGG ATC TGG CCC TGG CCC TTG AAT CT-3′) were designed using PRIMER3 software and the specificity for each primer set was confirmed by both running the PCR products on a 2.0% agarose gel and analysing the melting (dissociation) curve in the MxPro real-time PCR analysing program (Stratagene, La Jolla, CA, USA) after each PCR reaction. The real-time PCR reactions contained 10% of the RT reaction product, 0.4 μM of forward and reverse primers, 0.3 μL of 1 : 10 diluted ROX reference dye (provided with SYBR Green ER qPCR SuperMix Universal kit; Invitrogen), and SYBR Green SuperMix. PCR reactions were performed on Mx3000P QPCR System (Stratagene). The thermal cycling steps included 2 min at 50°C to permit optimal AmpErase uracil-n-glycosylase activity, 10 min at 95°C for initial denaturation, and then each cycle 15 s at 95°C, 30 s at 55°C, and 45 s at 72°C for 40 cycles, followed by 1 min at 95°C, 30 s at 55°C, and then 30 s at 95°C for ramp dissociation. The relative amount of each transcript was calculated using the 2–ΔΔCT method (Livak and Schmittgen 2001) and normalised to the endogenous reference gene L32.
Isometric tension measurement
The receptor subtype mediating EDN2-induced ovarian contraction was determined by isometric tension analysis. Ovaries were collected 10 h after hCG and maintained in Kreb’s solution throughout the duration of each recording. Each ovary was cut into 2–3 sections and, using silk threads, individual sections were then tied to the arms of a force transducer, as described previously (Guo et al. 2003; Ko et al. 2006). Sections were allowed to equilibrate then treated with K+ to induce depolarisation and establish a baseline response. In the first experiment, the contractile response to treatment with PBS (vehicle) or 50 nM EDN2 (American Peptide Co., Sunnyvale, CA, USA) was determined after the sections were pre-treated for 15 min with 3 μM of the specific EDNRA or EDNRB antagonists, BQ123 and BQ788, respectively (American Peptide Co.). In the second experiment, the ability of 10 μM BQ123 or BQ788 to overcome EDN2-induced ovarian contraction was determined. Antagonists were administered 10 to 15 min after contraction was induced with EDN2 and doses were chosen based on other studies (Tirapelli et al. 2006; Chester et al. 2007) and our own preliminary experiments (data not shown). Each treatment was replicated with 4–5 different ovarian sections. Isometric tension was recorded and the results expressed relative to K+-induced depolarisation (first experiment) or EDN2-induced contraction (second experiment), as described further in the results.
Ovulation assay: rats
The effect of EDNRA and EDNRB inactivation on ovulation rate was examined by intra-peritoneal (i.p.) and intra-ovarian (i.o.) administration of BQ123 and BQ788 in vivo. Immature rats were treated with 10 IU PMSG to induce follicular development and 48 h later with 10 IU hCG to induce the ovulatory cascade. At 9–10 h after hCG, injections of 10 mg per kg body weight BQ123, BQ788 or ~450 μL vehicle (PBS) were administered by i.p. injection or 10 μL of a 1 mM solution of BQ123, BQ788 or 10 μL vehicle (PBS) was injected into the stroma of the right ovary (i.o.; Ko et al. 2006). A dose of 10 mg per kg body weight was chosen for i.p. treatments based upon the use of these antagonists in mice (Palanisamy et al. 2006), whereas 10 μL of a 1 mM solution of each antagonist was chosen for i.o. treatments to give an estimated total ovarian concentration ~10-fold higher than that used for the isometric tension experiments in vitro. Animals (n = 5–7 per treatment) were sacrificed 18–20 h after hCG and cumulus–oocyte complexes (COCs) dissected from each oviduct and counted, as described previously (Al-Alem et al. 2007). The results are expressed as the mean rate of ovulation per rat ± s.e.m. (intra-peritoneal injections) or the mean rate of ovulation per ovary ± s.e.m. (intra-ovarian injections).
Ovulation assay: mice
The effect of inactivation of EDNRA, EDNRB and both receptors on ovulation rate was then reexamined in mice by administration of BQ123, BQ788 or BQ123 plus BQ788 in vivo. Immature CD1 mice were treated with 5 IU PMSG to induce follicular development and 48 h later with 5 IU hCG to induce the ovulatory cascade. At 9 h after hCG, injections of 10 mg per kg body weight BQ123, BQ788 or vehicle (Experiment 1) or 10 mg per kg body weight BQ123 plus BQ788 or vehicle (Experiment 2) were administered by i.p. injection. Animals (n = 4–5 per treatment) were sacrificed 18 h after hCG and cumulus–oocyte complexes dissected from each oviduct and counted. For each in vivo treatment, the results are expressed as the mean rate of ovulation per mouse ± s.e.m.
Statistical analysis
The Student’s t-test was used to determine differences in the isometric tension and immunometric assay analysis. One-way ANOVA using SigmaStat 3.5 (Systat Software Inc., Point Richmond, CA, USA) was used to determine differences in the real-time PCR analysis and the ovulation assays. If differences were detected, Tukey’s test was used to determine which means differed.
Results
Quantification of endothelin peptide in the ovary
Microarray analysis previously revealed that expression of mRNA for Edn2, but not Edn1, was dramatically increased by hCG in the ovaries of PMSG-primed immature rats (Ko et al. 2006). This dramatic induction of mRNA for Edn2 in granulosa cells 12 h after hCG was confirmed by real-time PCR analysis (Fig. 1a, adapted from Al-Alem et al. 2007) and is now quantified at the protein level (Fig. 1b). Since no currently available assay system can fully distinguish EDN2 from EDN1 peptide, an immunoassay 100% inclusive to all the isoforms was utilised. The concentration of endothelin peptide present in the ovaries of rats 48 h after treatment with PMSG was low and likely reflects basal production of EDN1. In contrast, the total ovarian concentration of endothelins 12 h after treatment with hCG was 7-fold higher (P < 0.05). Because only mRNA for Edn2 is induced by hCG, this simultaneous increase in total endothelins is likely to represent EDN2 and indicates functional translation of mRNA to peptide before ovulation.
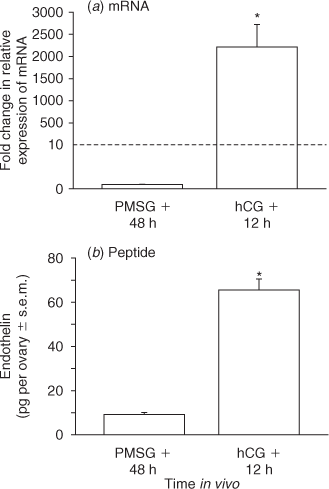
|
Expression of mRNA for endothelin-converting enzymes
Real-time PCR analysis indicated that expression of mRNA for Ece1, but not Ece2, was increased in granulosa cells before ovulation (Fig. 2). Expression of mRNA for Ece1 did not differ among granulosa cells from untreated ovaries and those collected 48 h after treatment with PMSG. However, in comparison to these earlier time points, mRNA for Ece1 increased ~2-fold in granulosa cells from ovaries collected 12 h after hCG (Fig. 2a, P < 0.05). In contrast, expression of mRNA for Ece2 in granulosa cells from ovaries collected 48 h after PMSG and 12 h after hCG decreased when compared with untreated (0 h) controls (Fig. 2b, P < 0.05). Analysis of mRNA levels alone would suggest regulation by Ece1, but not Ece2, in the overall production of EDN2 by granulosa cells before ovulation.
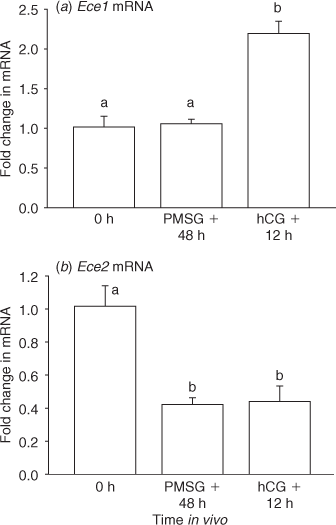
|
Endothelin receptor-specific contraction of the ovary
Treatment of sections of ovarian tissue with PBS does not induce any contractile response, whereas treatment with 50 nM EDN2 induces a sustained contractile response (Ko et al. 2006). Hence, receptor subtype-specific antagonists were utilised to determine whether EDNRA, EDNRB or both mediated the contractile effect of EDN2 on the ovary. In the first isometric tension experiment, sections of the ovary were pretreated for 15 min with 3 μM of the EDNRA or EDNRB antagonists and the contraction induced by 50 nM EDN2 determined. EDN2 induced a sustained contraction of sections of ovarian tissue in the presence of the EDNRB antagonist (BQ788), but not in the presence of BQ123, the EDNRA antagonist (Fig. 3, P < 0.05). In the second isometric tension experiment, sections of the ovary were induced to contract with 50 nM EDN2 and the ability of the antagonists to overcome EDN2-induced contraction was determined. Treatment with 10 μM BQ123, but not 10 μM BQ788 successfully reduced the contraction induced by EDN2 (Fig. 4, P < 0.05). Taken together, the results demonstrate that EDNRA mediates EDN2-induced ovarian contraction.
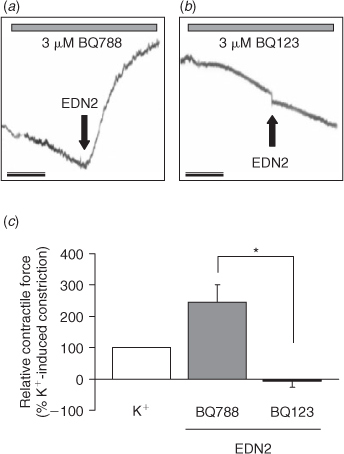
|
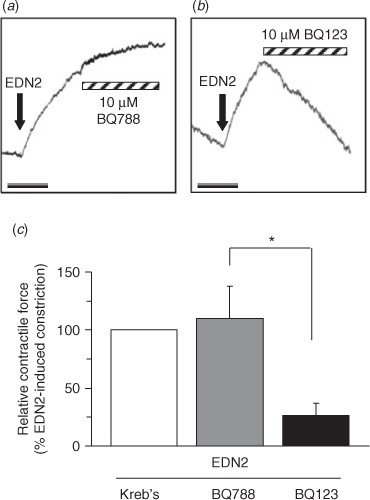
|
Effect of receptor-specific antagonists on ovulation rate
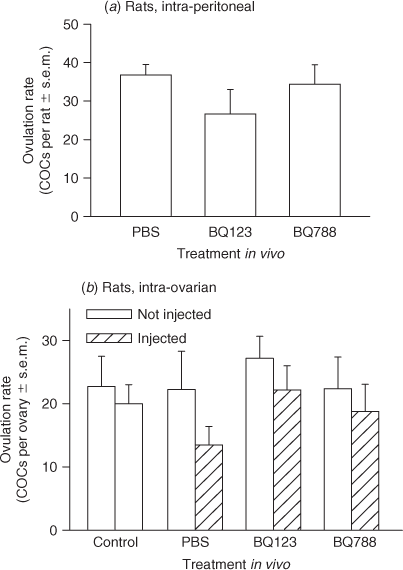
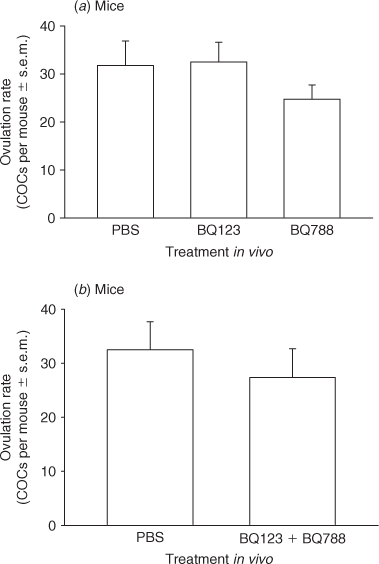
Because the isometric tension analysis revealed that EDN2 mediated ovarian contraction via EDNRA, we tested the hypothesis that EDN2 binding to EDNRA was required for ovulation. However, no effect of treatment with either the EDNRA antagonist (BQ123) or the EDNRB antagonist (BQ788) was observed on ovulation rate in rats by either i.p. or i.o. injection (P > 0.05, Fig. 5a, b). This was an unexpected finding given that (1) intra-ovarian treatment of rats with the dual endothelin receptor antagonist tezosentan decreases ovulation rate (Ko et al. 2006) and (2) intra-peritoneal treatment of mice with BQ788, but not JKC-301, is also reported to reduce the rate of ovulation (Palanisamy et al. 2006). We therefore sought to reexamine the effectiveness of using peptide-based receptor-specific antagonists on the rate of ovulation using mice as the experimental model. First, the effect of treatment with each antagonist alone was determined. No effect of treatment with either BQ123 or BQ788 was observed on the ovulation rate of gonadotrophin-primed CD1 mice (P > 0.05, Fig. 6a). This was an unexpected finding and suggested that either inactivation of both endothelin receptors was required to inhibit or delay ovulation or that these peptide-based antagonists do not retain sufficient bioactivity in vivo to effect ovulation. A second experiment was therefore performed to determine the rate of ovulation after treatment of mice with BQ123 plus BQ788. There was no effect on the rate of ovulation when mice were treated with BQ123 plus BQ788 (P > 0.05, Fig. 6b), suggesting that these peptide-based antagonists do not retain sufficient bioactivity in vivo to affect ovulation.
|
|
Discussion
The experiments described herein indicate that EDN2, the contractile ligand previously proposed as a mechanical signal that would induce ovulation (Ko et al. 2006), is produced by the periovulatory ovary before follicular rupture. Furthermore, the contractile response of EDN2 is mediated by binding to the EDNRA, not the EDNRB, subtype. When considered with the report that EDN2 also affects oviductal contractility via the same receptor subtype (Al-Alem et al. 2007) it appears that production and binding of EDN2 to EDNRA may play an integral role in both facilitating oocyte release and subsequent transport of the cumulus–oocyte complex within the oviduct.
Consistent with the profile of expression of mRNA for Edn2, total ovarian concentrations of endothelin peptide were increased in ovaries collected 12 h after hCG. Although this assay was not able to distinguish among EDN1, EDN2 and EDN3 peptides, mRNA for Edn1 remains at a low basal level throughout the ovulatory cascade and mRNA for Edn3 is not detectable within the ovary (Ko et al. 2006). Therefore, it is highly likely that the increased endothelin measured by immunometric assay 12 h after hCG represents production of EDN2 by granulosa cells of the periovulatory ovary. The low level of total endothelin that was detected in ovaries collected 48 h after PMSG is also consistent with the production and function of EDN1 described during earlier stages of follicular development (Tedeschi et al. 1992; Acosta et al. 1999; Flores 2000).
Typical to the synthesis of many peptide hormones, EDN2 is derived from selective proteolysis of larger proteins. Biologically inactive ‘big endothelins’ are cleaved from a precursor protein and then converted to active endothelins by hydrolysis catalysed by endothelin-converting enzymes (reviewed in Turner and Murphy 1996). Immunoreactive ECE1 is detected in luteinising granulosa cells collected 36 h after the administration of hCG in women previously treated with human menopausal gonadotrophin for in vitro fertilisation purposes (Yoshioka et al. 1998) and we observed regulation of mRNA for Ece1 in granulosa cells collected from pre- v. periovulatory follicles. Although modest, the increase in expression of mRNA for Ece1 12 h after treatment with hCG would suggest regulation of this converting enzyme as part of the mechanism to increase total EDN2 production at ovulation; however, alternative roles should be considered. ECE1 is reported to hydrolyse peptides including bradykinin, neurotensin and substance P (Hoang and Turner 1997; Johnson et al. 1999), which are all reported to affect ovarian function or ovulation itself (Alexander et al. 1989; Hellberg et al. 1991; Debeljuk 2006). Hence, future studies are needed to delineate the precise role(s) of this converting enzyme within the periovulatory follicle during the ovulatory cascade.
Two G-protein-coupled receptors (EDNRA and EDNRB) are believed to mediate the many actions of endothelins (Masaki et al. 1999). In the rat ovary, gene expression profiles for Ednra and Ednrb generated after microarray analysis were inconclusive in suggesting which receptor subtype facilitated ovarian contraction, as was immunohistochemical staining (Ko et al. 2006). In contrast, functional isometric analysis clearly indicated that the contractile effect of EDN2 within the ovary was mediated by EDNRA, which is consistent with EDNRA-mediated contraction of the oviduct (Al-Alem et al. 2007) as well as of smooth muscle cells in the vasculature (Masaki et al. 1999). However, the results herein suggest that (1) the role of each receptor subtype in the process of ovulation and (2) the effectiveness of these commonly used peptide-based antagonists on analysis of the ovulatory cascade, need to be re-evaluated. Furthermore, the role of alternative contractile factors such as prostaglandins (Priddy and Killick 1993), histamines (Schmidt et al. 1987) and bradykinin (Smith and Perks 1984) should be considered inclusive to the process of ovulation.
Palanisamy et al. (2006) reported that a dose of 10 mg per kg bodyweight of the EDNRB antagonist, BQ788, administered intra-peritoneally to immature gonadotrophin-primed mice resulted in a dramatic decrease in ovulation. They treated mice with BQ788 at 4, 6 or 8 h after hCG and reported increasing effectiveness of BQ788 in the ability to inhibit ovulation the closer the antagonist was administered towards the time of ovulation. Greater than 85% inhibition of ovulation was reported when BQ788 was administered at hCG + 8 h. In contrast, the isometric tension analysis led us to hypothesise that treatment with the EDNRA antagonist BQ123, but not BQ788, would inhibit EDN2-induced ovarian contraction and reduce or delay ovulation. We used the same dose (by bodyweight) as Palanisamy et al. (2006) when administering the antagonists by intra-peritoneal injection and a dose of 10 μL of 1 mM antagonists (~90 μg per kg bodyweight) when administering the antagonists directly into the ovarian stroma. We chose to use this alternative technique as it (1) reduced the potential for non-specific effects of treatment with these vaso-regulatory antagonists and (2) was successful in reducing the rate of ovulation when tezosentan was administered in a previous experiment (Ko et al. 2006). We observed no effect of treatment with either antagonist via either route of administration on the rate of ovulation in rats. We then sought to test the possibility that the reported EDNRB dependence in ovulation was specific to mice and treated gonadotrophin-primed mice with 10 mg per kg bodyweight BQ788 or BQ123. Again, neither antagonist affected the rate of ovulation. Because tezosentan, a dual-receptor antagonist, was successful in reducing or delaying the rate of ovulation (Ko et al. 2006), we then sought to test the possibility that endothelin-mediated ovulation was dependent upon signalling through both EDNRA and EDNRB. Mice were treated with 10 mg per kg bodyweight BQ788 plus BQ123. Again, no effect on the rate of ovulation was observed. Because there are a multitude of proteolytic enzymes present within the ovary during the periovulatory period (Chun et al. 1992; Liu et al. 1997; Hasan et al. 2002), it is likely that the lack of any effect of these antagonists on oocyte release is due to rapid degradation of these peptide-based antagonists in vivo. However, given the results reported herein, the requirement for EDNRB-mediated signalling in the process of ovulation needs to be re-evaluated.
In conclusion, endothelin peptide is produced before ovulation and the total production of this peptide may be regulated by the coordinate action of endothelin-converting enzymes. The contractile response to EDN2 within the rat ovary appears to be mediated via EDNRA in a receptor subtype-specific manner. These results are consistent with the hypothesis that EDN2 acts as a final mechanical signal to facilitate rupture of the periovulatory follicle. However, our inability to affect the rate of ovulation using either antagonist in both rats and mice suggests that these pathways and antagonists need to be examined in more detail. Overall, contraction as a final, requisite mediator of ovulation bears further investigation in relation to both assisted reproductive technologies and the possible development of strategies to overcome anovulation-based ovarian diseases.
Acknowledgements
This work was supported by grant RO1HD052694 (C.K.), K12 DA014040 (P.B.) and P20 RR15592 (P.B. and C.K.) from the National Institutes of Health.
Acosta, T. J. , Berisha, B. , Ozawa, T. , Sato, K. , Schams, D. , and Miyamoto, A. (1999). Evidence for a local endothelin–angiotensin–atrial natriuretic peptide system in bovine mature follicles in vitro: effects on steroid hormones and prostaglandin secretion. Biol. Reprod. 61, 1419–1425.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Al-Alem, L. , Bridges, P. J. , Su, W. , Gong, M. C. , Iglarz, M. , and Ko, C. (2007). Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J. Endocrinol. 193, 383–391.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Alexander, M. J. , Mahoney, P. D. , Ferris, C. F. , Carraway, R. E. , and Leeman, S. E. (1989). Evidence that neurotensin participates in the central regulation of the preovulatory surge of luteinizing hormone in the rat. Endocrinology 124, 783–788.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Chester, A. H. , Azam, R. , Felkin, L. E. , George, R. , and Brand, N. (2007). Correlation between vascular responsivensss and expression of novel transcripts of the ETA-receptor in human vascular tissue. Vascul. Pharmacol. 46, 181.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Chun, S. Y. , Popliker, M. , Reich, R. , and Tsafriri, A. (1992). Localization of preovulatory expression of plasminogen activator inhibitor type-1 and tissue inhibitor of metalloproteinase type-1 mRNAs in the rat ovary. Biol. Reprod. 47, 245–253.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Continho, E. M. , and Maia, H. S. (1971). The contractile response of the human uterus, fallopian tubes, and ovary to prostaglandins in vivo. Fertil. Steril. 22, 539–543.
| PubMed |

Coutinho, E. M. (1974). Ovarian contractility and ovulation. Res. Reprod. 6, 3–4.
| PubMed | CAS |

Curry, T. E. , and Smith, M. F. (2006). Impact of extracellular matrix remodeling on ovulation and the folliculo–luteal transition. Semin. Reprod. Med. 24, 228–241.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Debeljuk, L. (2006). Tachykinins and ovarian function in mammals. Peptides 27, 736.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Espey, L. L. , and Lipner, H. (1963). Measurements of intrafollicular pressures in the rabbit ovary. Am. J. Physiol. 205, 1067–1072.
| PubMed | CAS |

Flores, J. A. (2000). Gene expression of endothelin-1 in the porcine ovary: follicular development. Biol. Reprod. 63, 1377–1382.
| PubMed | CAS |

Gómez, R. , Simón, C. , Remohí, J. , and Pellicer, A. (2003). Administration of moderate and high doses of gonadotropins to female rats increases ovarian vascular endothelial growth factor (VEGF) and VEGF Receptor-2 expression that is associated to vascular hyperpermeability. Biol. Reprod. 68, 2164–2171.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guo, Z. , Su, W. , Ma, Z. , Smith, G. M. , and Gong, M. C. (2003). Ca2+-independent phospholipase A2 is required for agonist-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 278, 1856–1863.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Hasan, S. , Hosseini, G. , Princivalle, M. , Dong, J.-C. , Birsan, D. , Cagide, C. , and de Agostini, A. I. (2002). Coordinate expression of anticoagulant heparan sulfate proteoglycans and serine protease inhibitors in the rat ovary: a potent system of proteolysis control. Biol. Reprod. 66, 144–158.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Hellberg, P. , Larson, L. , Olofsson, J. , Hedin, L. , and Brannstrom, M. (1991). Stimulatory effects of bradykinin on the ovulatory process in the in vitro-perfused rat ovary. Biol. Reprod. 44, 269–274.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Hoang, M. V. , and Turner, A. J. (1997). Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem. J. 327(Pt 1), 23–26.
| PubMed | CAS |

Johnson, G. D. , Stevenson, T. , and Ahn, K. (1999). Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J. Biol. Chem. 274, 4053–4058.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Kitajima, Y. , Endo, T. , Manase, K. , Nishikawa, A. , Shibuya, M. , and Kudo, R. (2004). Gonadotropin-releasing hormone agonist administration reduced vascular endothelial growth factor (VEGF), VEGF receptors, and vascular permeability of the ovaries of hyperstimulated rats. Fertil. Steril. 81, 842.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Ko, C. , Gieske, M. C. , Al-Alem, L. , Hahn, Y. , Su, W. , Gong, M. C. , Iglarz, M. , and Koo, Y. (2006). Endothelin-2 in ovarian follicle rupture. Endocrinology 147, 1770–1779.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Liu, Y. X. , Peng, X. R. , Chen, Y. J. , Carrico, W. , and Ny, T. (1997). Prolactin delays gonadotrophin-induced ovulation and down-regulates expression of plasminogen-activator system in ovary. Hum. Reprod. 12, 2748–2755.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Livak, K. J. , and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Masaki, T. , Ninomiya, H. , Sakamoto, A. , and Okamoto, Y. (1999). Structural basis of the function of endothelin receptor. Mol. Cell. Biochem. 190, 153–156.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Matousek, M. , Carati, C. , Gannon, B. , and Brannstrom, M. (2001). Novel method for intrafollicular pressure measurements in the rat ovary: increased intrafollicular pressure after hCG stimulation. Reproduction 121, 307–314.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Miyabayashi, K. , Shimizu, T. , Kawauchi, C. , Sasada, H. , and Sato, E. (2005). Changes of mRNA expression of vascular endothelial growth factor, angiopoietins and their receptors during the periovulatory period in eCG/hCG-treated immature female rats. J. Exp. Zool. A Comp. Exp. Biol. 303A, 590–597.
| Crossref | GoogleScholarGoogle Scholar | CAS |

O’Shea, J. D. , and Phillips, R. E. (1974). Contractility in vitro of ovarian follicles from sheep, and the effect of drugs. Biol. Reprod. 10, 370–379.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Palanisamy, G. S. , Cheon, Y.-P. , Kim, J. , Kannan, A. , Li, Q. , Sato, M. , Mantena, S. R. , Sitruk-Ware, R. L. , Bagchi, M. K. , and Bagchi, I. C. (2006). A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol. Endocrinol. 20, 2784–2795.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Palti, Z. , and Freund, M. (1972). Spontaneous contractions of the human ovary in vitro. J. Reprod. Fertil. 28, 113–115.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Priddy, A. R. , and Killick, S. R. (1993). Eicosanoids and ovulation. Prostaglandins Leukot. Essent. Fatty Acids 49, 827–831.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Rondell, P. (1964). Follicular pressure and distensibility in ovulation. Am. J. Physiol. 207, 590–594.
| PubMed | CAS |

Schmidt, G. , Kannisto, P. , Owman, C. , and Walles, B. (1987). Characterization of histamine receptors mediating contraction and relaxation of the ovarian follicle wall. Int. J. Fertil. 32, 399–406.
| PubMed | CAS |

Schroeder, P. C. , and Talbot, P. (1982). Intrafollicular pressure decreases in hamster preovulatory follicles during smooth muscle cell contraction in vitro. J. Exp. Zool. 224, 417–426.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Smith, C. , and Perks, A. M. (1984). The effects of bradykinin on the contractile activity of the isolated rat ovary. Acta Endocrinol. (Copenh.) 106, 387–392.
| PubMed | CAS |

Talbot, P. , and Chacon, R. S. (1982). In vitro ovulation of hamster oocytes depends on contraction of follicular smooth muscle cells. J. Exp. Zool. 224, 409–415.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Tedeschi, C. , Hazum, E. , Kokia, E. , Ricciarelli, E. , Adashi, E. Y. , and Payne, D. W. (1992). Endothelin-1 as a luteinization inhibitor: inhibition of rat granulosa cell progesterone accumulation via selective modulation of key steroidogenic steps affecting both progesterone formation and degradation. Endocrinology 131, 2476–2478.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Tirapelli, C. R. , Casolari, D. A. , Montezano, A. C. , Yogi, A. , Tostes, R. C. , Legros, E. , D’Orleans-Juste, P. , Lanchote, V. L. , Uyemura, S. A. , and de Oliveira, A. M. (2006). Ethanol consumption enhances endothelin-1-induced contraction in the isolated rat carotid. J. Pharmacol. Exp. Ther. 318, 819–827.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Turner, A. J. , and Murphy, L. J. (1996). Molecular pharmacology of endothelin-converting enzymes. Biochem. Pharmacol. 51, 91.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |

Virutamasen, P. , Smitasiri, Y. , and Fuchs, A. R. (1976). Intraovarian pressure changes during ovulation in rabbits. Fertil. Steril. 27, 188–196.
| PubMed | CAS |

Yoshioka, S. , Fujiwara, H. , Yamada, S. , Tatsumi, K. , Nakayama, T. , Higuchi, T. , Inoue, T. , Maeda, M. , and Fujii, S. (1998). Endothelin-converting enzyme-1 is expressed on human ovarian follicles and corpora lutea of menstrual cycle and early pregnancy. J. Clin. Endocrinol. Metab. 83, 3943–3950.
| Crossref | GoogleScholarGoogle Scholar | PubMed | CAS |



