115 DIMETHYLSULPHOXIDE AND 1,2-PROPANEDIOL DIFFERENTIALLY CRYOPROTECT BOVINE AND PORCINE PRIMORDIAL, PRIMARY AND SECONDARY FOLLICLES
E. Papasso Brambilla A , A. Paffoni B , T.A.L. Brevini A , M. De Eguileor C , G. Ragni B and F. Gandolfi AA Department of Anatomy of Domestic Animals, University of Milan, Italy. email: fulvio.gandolfi@unim.it;
B Department of Obstetrics and Gynaecology, Infertility Unit. University of Milan, Italy;
C Department of Structural and Functional Biology, University of Insubria, Varese, Italy
Reproduction, Fertility and Development 16(2) 180-180 https://doi.org/10.1071/RDv16n1Ab115
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
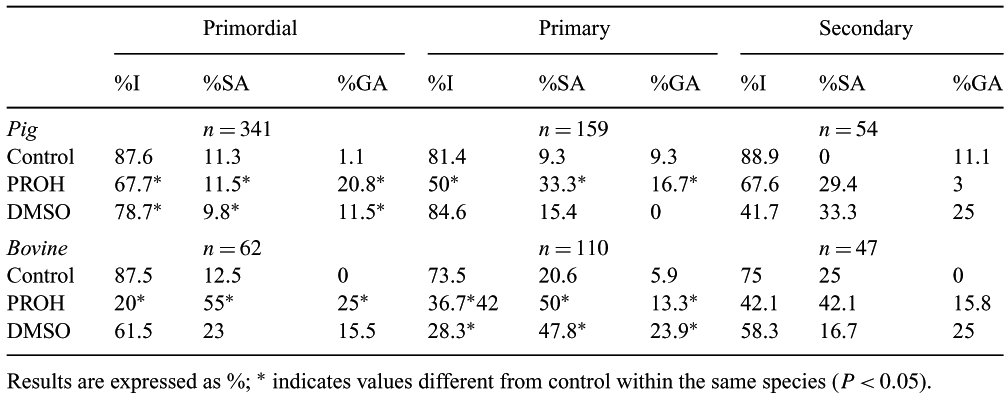
Ovarian tissue cryopreservation is of interest for many areas of assisted reproduction. Normal structure and intra- as well as intercellular organization have to be maintained in order to preserve follicle viability. In the present work we studied the effects of two largely used cryoprotectants, namely, dimethylsulphoxide (DMSO) and 1,2-propanediol (PROH), on follicle morphology. Experiments were carried out on bovine and porcine ovaries and the two cryoprotectants were assessed for their ability to preserve structural integrity of primordial, primary and secondary follicles, in order to investigate the protective effects of these molecules on specific developmental stages. Fragments from each ovary were divided in three groups: a) immediately fixed (control); b) cryopreserved in 1.5 M DMSO; c) cryopreserved in 1.5 M PROH. To allow equilibration with the cryoprotectant, samples were held for 30 min at 4°C in order to minimize toxic effects. Cryopreservation was carried out in a controlled rate freezer (Planer Planerple, Sunbury, Middlesex, UK), with the following protocol: precooling (4°C); cooling at −2°C/min to 9°C; seeding at 9°C and standby for 10 minutes; cooling at −0.3°C/min to 40°C and 10°C/min to 140°C; plunging into liquid nitrogen. Samples were rapidly thawed. Dilution of cryoprotectants was carried out in 3 steps of ten minutes each at 4°C (1 M; 0.5 M; 0 M). Samples were then fixed, paraffin embedded, serially sectioned and evaluated with a Nikon TE200 inverted microscope. Follicles of each developemental stage were scored in three categories using the criteria previously described by Paynter et al. (1999, Cryobiology 38, 301–309) and presented in the table below as I = Intact; SA = Small Abnormalities; GA = Great Abnormalities. Statistical differences were assessed by the chi-square test (P < 0.05). The results obtained showed that both cryoprotectants are effective for the preservation of secondary follicles. The use of PROH was unable to protect primary and primordial follicles in both species. Conversely, DMSO showed a satisfying cryoprotecting effect for pig primary and bovine primordial follicles but had a poor protecting capability for pig primordial and bovine primary follicles. Altogether the present results suggest that the choice of the cryoprotectant needs to be carefully targeted in relation to the follicular stage and the species of interest. This work was funded by Industria Farmaceutica Serono SpA.
|


