87 ONE-STEP VERSUS TWO-STEP VITRIFICATION AND IN-STRAW DILUTION OF IN VITRO-PRODUCED BOVINE EMBRYOS
L.F. Campos-Chillon A , D.J. Walker B and J.F. De La Torre-Sanchez BA CVMBS, Colorado State University. email: LFCampos@colostate.edu;
B Animal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO, USA.
Reproduction, Fertility and Development 16(2) 165-165 https://doi.org/10.1071/RDv16n1Ab87
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
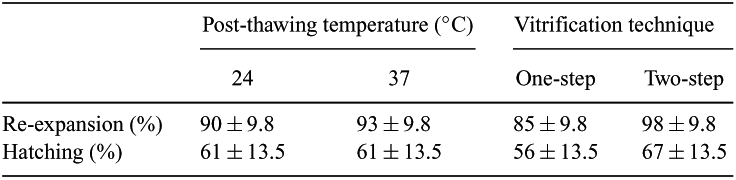
Slow-cooling techniques are widely used in cryopreservation of bovine embryos. We have previously developed a simple, two-step vitrification technique for direct transfer in the field; however, simplification to one-step vitrification would be attractive. Therefore, factorial combinations of two techniques (one-step and two-step) and two post-thaw temperatures until culture (24 and 37°C) were studied. Blastocysts (n = 220) sired by two bulls were obtained in vitro in four replicates. Briefly, oocytes were aspirated from 2–8-mm follicles of ovaries obtained at a slaughterhouse, matured, fertilized and cultured in vitro with standard procedures using chemically defined media (CDM1/2 or G1/2). Two-step embryos were transferred in 1 μL into 1 mL of V1-CDM (5 M ethylene glycol (EG) in HEPES-buffered holding medium (HCDM2)), and one-step embryos into a 7-μL droplet of V2-CDM (7 M EG, 0.5 M galactose and 18% w/v Ficoll 70 in HCDM2) for 3 min at 24°C. Next, embryos for the two-step method were moved in 1 μL into a 7 μL droplet of V2-CDM at 24°C. Droplets containing embryos (one or two-step) were loaded into 0.25-mL straws preloaded with a 1-cm column of D-HCDM (0.5 M galactose in HCDM2), then 0.5 cm air, and then 7 cm of D-HCDM followed by 0.5 cm air. The column containing the embryos (0.5 cm (7 μL)) was followed with 0.5 cm air and 1 cm of D-HCDM. Straws were heat-sealed and plunged vertically, sealed end first, into liquid nitrogen just covering the embryo, and the rest of the straw was then slowly immersed. The time from loading to plunging was 40–50 s. Straws were thawed in air (24°C) for 10 s and then in water horizontally at 37°C until ice disappeared. Straws were gently shaken to mix the columns; then, after 5 min at 24 or 37°C, embryos were expelled and cultured in CDM2 + 5% FCS. Re-expansion and hatching rates were evaluated 48 h post thaw. Data (Table 1) were calculated as a percentage of non-vitrified controls for respective replicates (control means: re-expansion 87%; hatching 74%) and analyzed by ANOVA. There were no main effects of post-thawing temperature (P > 0.1), indicating that, after thawing, embryos can be kept at room or body temperature. Also, main effect means for re-expansion and hatching for one-step or two-step addition of cryoprotectant were similar (P > 0.1), but there was a tendency for higher survival for the two-step procedure. Further refinements of the one-step technique including EG concentrations, embryological stages and equilibration times should be studied.
|


