28 CLONED EMBRYOS CAN BE PRODUCED USING DONOR CELLS OBTAINED FROM A 72-HOUR COOLED CARCASS
S. Arat A , H. Bagis A , H. Odaman Mercan A and A. Dinnyes BA Research Institute for Genetic Engineering and Biotechnology, TUBITAK, Kocaeli Turkey
B Research Group for Applied Animal Genetics and Biotechnology, Hungarian Academy of Sciences. Email: sezen@rigeb.gov.tr
Reproduction, Fertility and Development 17(2) 164-164 https://doi.org/10.1071/RDv17n2Ab28
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
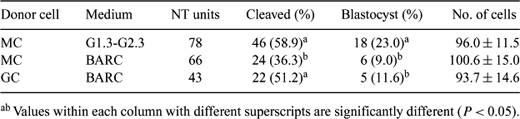
There are few reports on the use of cells from a dead mammal for nuclear transfer (NT). So far, most calves have been cloned from live adult cows or fresh fetal samples. The ability to produce cloned animals using postmortem tissue can provide an additional application to the field of NT. This study was conducted to investigate whether viable cells could be obtained from tissues chilled for 72 h and whether these cells could be used for NT. Bovine oocytes isolated from slaughterhouse ovaries were matured in TCM199 supplemented with 10% fetal calf serum (FBS), 50 μg/mL sodium pyruvate, 1% v:v penicillin-streptomycin (10,000 U/mL penicillin G, 10,000 μg/mL streptomycin), 10 ng/mL EGF, 0.5 μg/mL FSH, and 5 μg/mL LH. A cell line (MC) was established from leg muscle of a cow carcass stored at 0°C for 72 h. Tissues from muscle were cut into small pieces. Tissue explants were cultured in DMEM-F12 supplemented with 10% FBS at 37°C in 5% CO2 in air. Bovine granulosa cells (GC) were isolated from ovarian follicles and used for NT as control cells. Prior to NT, all somatic cells were allowed to grow to confluency (G1/G0) in DMEM-F12 medium supplemented with 10% FBS. Cumulus cells were removed by vortexing with hyaluronidase at 18 h after the start of maturation. Matured oocytes labeled with DNA fluorochrome Hoechst 33342 were enucleated under UV to ensure full removal of the chromatin. A single cell was inserted into the perivitelline space of the enucleated oocyte. Oocyte-cell couples were fused by a DC pulse of 133V/500 μm for 25 μs. After fusion, NT units were activated using a combination of calcium ionophore (5 μM), cytochalasin D (2.5 μg/mL) and cycloheximide (10 μg/mL) and cultured for 7 days in BARC or G1.3-G2.3 medium. Differences (developmental potential and cell numbers) among groups were analyzed by one-way ANOVA after arcsin square transformation. The results are summarized in Table 1. The results suggest that viable cells can be obtained from muscle of a cow carcass stored at cold temperature for 72 h and that these cells have ability to generate NT blastocysts at rates similar to those obtained with fresh GCs. In addition, G1.3 and G2.3 culture medium supported embryo development better than BARC medium.
|
This study was supported by a grant from TUBITAK, Turkey (VHAG-1908 and Turkey-Hungary bilateral project VHAG-2022).


