333 IN VIVO PREMATURATION AFFECTS PROTEIN SYNTHESIS AT THE BEGINNING OF IN VITRO MATURATION
H. M. Knijn A , P. L. A. M. Vos A , S. J. Dieleman A and P. J. M. Hendriksen AFaculty of Veterinary Medicine, Department of Farm Animal Health, Utrecht, The Netherlands
Reproduction, Fertility and Development 18(2) 274-274 https://doi.org/10.1071/RDv18n2Ab333
Published: 14 December 2005
Abstract
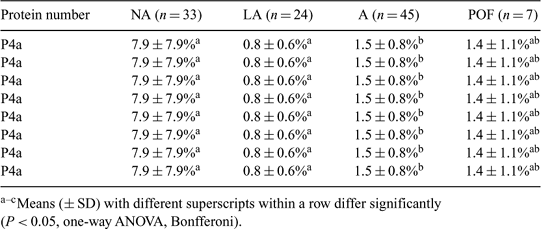
The cumulus–oocyte complex (COC) from a preovulatory follicle is better equipped than a COC from a small healthy follicle to develop into a blastocyst. This might be due to the presence of mRNAs and proteins in the oocyte and/or cumulus cells from which the expression is increased or induced during the prematuration period. There are several reports that beginning atresia enhances in vitro oocyte competence and the processes of atresia show similarities with prematuration on an ultrastructural level. Therefore, we investigated the effect of in vivo prematuration and atresia on the protein synthesis in bovine oocytes collected from preovulatory and small follicles during the first period of IVM. To study the follicular population of an undisturbed cycle we synchronized 14 cows with a norgestomet ear implant. We performed progesterone and LH analysis (Hendriksen et al. 2003 Biol. Reprod. 69, 2036–2044) to determine the start of luteolysis and the LH surge. At 48–62 h after the start of luteolysis, 10 cows were ovariectomized (OVX) and were considered to be shortly before the LH surge. At 1.5–3 days after the LH surge, at the beginning of a new cycle, four cows were OVX. From the ovaries collected before the LH surge the preovulatory follicle (POF) and all follicles ≥3 mm were dissected. From the ovaries collected at the start of a new cycle all follicles ≥3 mm were dissected. All follicles were classified for degree of atresia as non-atretic (NA), light atretic (LA), and atretic (A) by the proportion of apoptotic granulosa cells and levels of estradiol and progesterone, and of small IGF-binding proteins (IGFBPs) in the follicular fluid (Hendriksen et al. 2003). The COCs were individually incubated for 4 h in maturation medium containing 35S-labeled methionine/cysteine (Amersham-Pharmacia, Sweden). After separation of oocyte and cumulus cells, the proteins produced in the oocyte were analyzed by 1-D electrophoresis (SDS-PAGE, ExcelGel-12.5%, Amersham-Pharmacia). Gels were dried and digitized images were obtained by use of a Phosphor Imaging System (Molecular Imager, Bio-Rad, Hercules, CA, USA). Twenty-eight different proteins were distinguished. To compare different oocytes, the 35S incorporation per protein band was calculated as proportion of the 35S incorporation of all bands of the oocyte. The total amount of newly synthetized proteins per oocyte was normalized to the oocyte on the gel with the highest incorporation of 35S. In all POF oocytes (n = 7), the total amount of newly synthetized protein was lower than that in the oocytes from the NA and LA follicles (Table 1). This suggests that already during in vivo prematuration new proteins were synthesized in POFs and therefore less protein synthesis occurs during maturation. We can speculate that oocytes from small follicles have to synthesize many proteins during the beginning of IVM to make up for what is normally produced during the prematuration period. In atretic oocytes fewer proteins were synthesized, which suggests that these oocytes have either undergone prematuration-like processes or are less viable. Nevertheless, some proteins remain to be synthesized in POFs, such as, for example, protein number ‘p 23’.
|


