304 SHORT-PERIOD SPERM–OOCYTE INCUBATION AND FERTILIZATION MEDIA EFFECTS ON THE IN VITRO PRODUCTION OF SWINE EMBRYOS
M. G. Marques A , A. B. Nascimento A , L. F. Martins A , F. R. O. Barros A , M. D. Goissis A , M. E. O. D'Avila Assumpção A and J. A. Visintin AADepartment of Animal Reproduction, FMVZ, São Paulo University, São Paulo, SP, Brazil
Reproduction, Fertility and Development 19(1) 267-267 https://doi.org/10.1071/RDv19n1Ab304
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
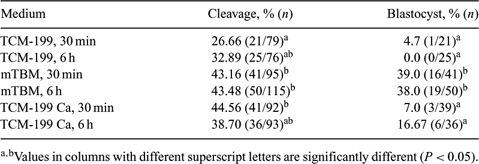
The aim of this study was to evaluate the effect of 2 sperm–oocyte incubation periods (30 min and 6 h) and 3 IVF media (TCM-199, TCM-199 with Ca-lactate, or mTBM). Cumulus–oocyte complexes (COCs) from abattoir-derived swine ovaries were matured in TCM-199 supplemented with 3.05 mM glucose, 50µg mL−1 gentamycin, 0.91 mM sodium pyruvate, 10% swine follicular fluid, 0.57 mM cysteine, 10 ng mL−1 of epidermal growth factor, 10 IU mL−1 eCG, and 10 IU mL−1 of hCG for 22 h, followed by a 22-h incubation without hormones. After in vitro maturation, oocyteswere allocated into 3 IVF media: TCM-199 (supplemented with 3.05 mM glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 50µg mL−1 gentamycin, 1 mg mL−1 BSA, and 3.06 mM mL−1 caffeine-standard medium);TCM-199 Ca (standard medium with 2.92 mM Ca lactate); or mTBM (supplemented with 5 mM mL−1 sodium pyruvate, 0.57 mM cysteine, 50µg mL−1 entamycin, 1 mg mL−1 BSA, and 2 mM mL−1 caffeine). The refrigerated semen at 15°C was centrifuged and capacitated for 2 h at 38.5°C in an atmosphere of 5% CO2 in air and under high humidity conditions in the respective IVF media. Oocytes were denuded, washed with the respective IVF media, and inseminated with the capacitated spermatozoa at a concentration of 6 × 105 sperm mL−1 in 90-µL microdroplets. After 30 min, a group of oocytes from each fertilization medium was gently washed to remove nonbound spermatozoa and returned to a new medium droplet for another 5.5 h (30-min group). The other group of oocytes remained in the same droplet with spermatozoa for 6 h (6-h group). After IVF, oocytes were cultured in porcine zygote medium-3 for 7 days. Data were analyzed by chi-squared test (P < 0.05).With regard to cleavage rates on the third day of embryo development (Day 3), no significant difference was observed among sperm–oocyte incubation periods; however, there was a difference in the TCM-199 group, compared with the mTBM and TCM-199 Ca groups, at the 30-min incubation period. With 6 h of incubation, no differences were observed among groups. When blastocyst (Day 7) rates were evaluated, no significant differences were observed between the 2 TCM-199 media groups; however, the mTBM groups showed higher blastocyst rates. The sperm–oocyte incubation period of 30 min or 6 h of IVF did not interfere with the blastocyst rate. In conclusion, the IVF medium mTBM was more efficient than the TCM-199 media when considering embryo production. Moreover, 30 min of IVF was sufficient for the spermatozoa responsible for oocyte fertilization to bind to the zona pellucida.
|
This work was supported by the FAPESP 05/01420-7.


