207 IN VITRO MATURATION TREATMENT AFFECTS DEVELOPMENTAL COMPETENCE OF LAPAROSCOPIC OVUM PICKUP-DERIVED OOCYTES IN FOLLICLESTIMULATING HORMONE-STIMULATED GOATS
Y. Locatelli A B , N. Poulin A , G. Baril A , J.-L. Touzé A , A. Fatet A , J.-F. Beckers C and P. Mermillod AA INRA, Unité de Physiologie de la Reproduction et des Comportements, Nouzilly, France;
B MNHN, Laboratoire de la Réserve Animalière de la Haute Touche, Obterre, France;
C Université de Liège, Faculté de Médecine Vétérinaire, Liège, Belgique
Reproduction, Fertility and Development 20(1) 182-183 https://doi.org/10.1071/RDv20n1Ab207
Published: 12 December 2007
Abstract
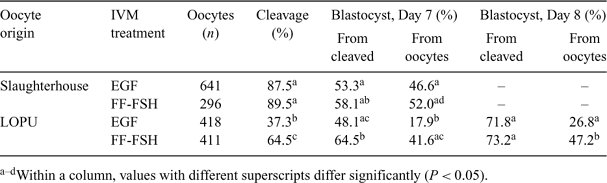
The aim of the present study was to assess the effect of IVM treatment on the developmental competence of oocytes recovered from repeated laparoscopic ovum pickukp (LOPU) in goats. A total of 94 LOPU sessions were performed on 33 adult goats of the Saanen and Alpine breeds. Females were synchronized (Day 0) during the nonbreeding season by inserting vaginal sponges (45 mg of fluorogestone acetate, Intervet, Boxmeer, The Netherlands). At Day 8, an i.m. injection of 50 μg of cloprostenol (Estrumate; Schering-Plough Animal Health, Pointe-Claire, Quebec, Canada) was administered. Porcine FSH (Stimufol, Merial, Brussels, Belgium, 160 mg/goat) was administered in 5 injections at 12-h intervals, starting on Day 8. The LOPU took place under general anesthesia on Day 11, and follicles ≥2 mm were aspirated with an 18-gauge needle connected to a controlled vacuum system. Vaginal sponges were removed at the time of LOPU. Treatments were repeated 2 times in a 2-week interval scheme (2 goats and 1 goat were excluded from the experiment during the second and third LOPU sessions, respectively). Cumulus–oocyte complexes were washed and evaluated for quality (graded from 1 to 3). Oocytes recovered from unstimulated slaughterhouse-derived ovaries served as a control. Cumulus–oocytes complexes from Grades 1 and 2 were submitted to IVM in TCM-199, supplemented with 100 μm of cysteamine and either 10 ng mL–1 of epidermal growth factor (EGF) or 10% follicular fluid and 100 ng mL–1 of ovine FSH (FF-FSH). Matured oocytes were then submitted to IVF and in vitro development as described by Cognié et al. (2004 Reprod. Fertil. Dev. 16, 437–445). Over the 94 LOPU sessions, 20.4 ± 0.9 follicles were aspirated (mean ± SEM), allowing the recovery of 12.3 ± 0.7 COC per goat and per session, of which 80.1% were suitable for IVM (Grades 1 and 2). Results of in vitro production are detailed in the table. The IVM treatment did not significantly affect cleavage or blastocyst development rates in oocytes derived from slaughterhouse ovaries. Cleavage rates were significantly decreased in LOPU-derived oocytes when compared with control oocytes. For LOPU-derived oocytes, cleavage and final blastocyst development rates were increased significantly and kinetics of embryo development were accelerated when FF-FSH was used during IVM as compared with EGF. The IVM with FF-FSH allowed us to produce 4.1 blatocysts per goat per LOPU session. These results demonstrate the interest in LOPU for goat embryo production once appropriate IVM treatment is used. The difference observed between LOPU and slaughterhouse oocytes in terms of response to IVM treatments may be related to FSH stimulation prior to the LOPU session or to postmortem changes in oocyte responsiveness in the slaughterhouse group.
|


