42 DEVELOPMENT OF PIG EMBRYOS CLONED FROM DONOR CELLS TREATED WITH TRICHOSTATIN A
J. Li A C , Y. Du A C , P. M. Kragh A C , S. Purup B , K. Villemoes A , A. M. Pedersen A , A. L. Jørgensen C , L. Bolund C D , H. M. Yang D and G. Vajta AA Department of Genetics and Biotechnology, Tjele, DK 8830, Denmark;
B Department of Animal Health, Welfare and Nutrition, Tjele, DK 8830, Denmark;
C Department of Human Genetics, Aarhus, DK 8000, Denmark;
D Beijing Genomics Institute, Beijing 101300, China
Reproduction, Fertility and Development 20(1) 101-101 https://doi.org/10.1071/RDv20n1Ab42
Published: 12 December 2007
Abstract
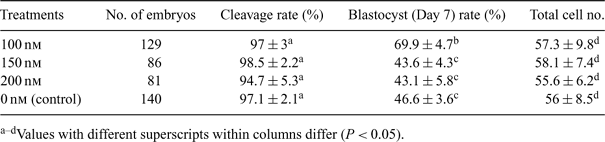
Development to the blastocyst stage following nuclear transfer is dependent on the donor cell's ability to reprogram its genome to a totipotent state. Reprogramming of the transferred somatic nuclei must be completed by the time normal activation of the embryonic genome occurs (Solter 2000 Nat. Rev. Genet. 1, 199–207). Recently, Enright et al. (2003 Biol. Reprod. 69, 896–901) reported that in vitro development of cloned cow embryos was improved by treatment of donor cells with a histone deacetylase inhibitor, TrichostatinA (TSA). So far, there are no reports available for adult pig fibroblast cells treated with TSA. The objective of this study was to investigate whether the development of handmade cloned embryos in pig could be improved by using TSA-treated donor cells. Adult pig fibroblast cells were treated with 100, 150, or 200 nm TSA for 24 h, compared to untreated controls, and were then used as donor cells. The cells were electrofused with handmade enucleated pig oocytes separately and were activated with calcium ionophore and cycloheximide. They were subsequently cultured in porcine zygote medium 3 (PZM-3; Yoshioka et al. 2002 Biol. Reprod. 66, 112–119) using the well of the well system (WOW; Vajta et al. 2000 Mol. Reprod. Dev. 55, 256–264). Experiments were repeated 4 times and the data were analyzed with AVEDEV and t-test in Excel (Microsoft Excel 2007). The cleavage rates and the total cell numbers per blastocyst were similar between groups (P > 0.05), as shown in Table 1. However, the cloned blastocyst rate using donor cells treated with 100 nm TSA was higher than in the other groups (69.9 ± 4.7% v. 43.6 ± 4.3%, 43.1 ± 5.8%, or 46.6 ± 3.6%; P < 0.05), as shown in Table 1. These data suggest that proper TSA treatment for donor cells before somatic cloning improves the rate of development of porcine handmade cloned embryos to the blastocyst stage. Further research is needed to examine the in vivo development of embryos reconstructed with TSA-treated donor cells.
|


