52 INITIATION OF PREGNANCIES IN SOUTH AFRICAN RIVERINE RABBIT (BUNOLAGUS MONTICULARES) BY INTERSPECIES NUCLEAR TRANSFER USING ADIPOSE-DERIVED SOMATIC CELLS
M. J. Sansinena A , D. Owiny B , R. S. Denniston C , D. Salamone A and D. Barry B CA Universidad de Buenos Aires, Buenos Aires, Argentina;
B University of Stellenbosch, Stellenbosch, South Africa;
C Lazaron Biotechnologies SA Ltd, Stellenbosch, South Africa
Reproduction, Fertility and Development 20(1) 106-107 https://doi.org/10.1071/RDv20n1Ab52
Published: 12 December 2007
Abstract
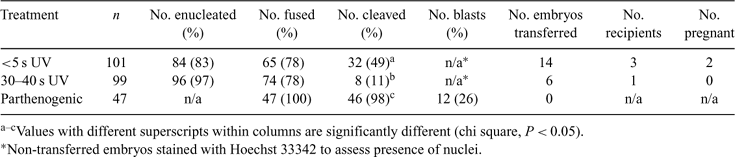
The riverine rabbit (Bunolagus monticulares), one of South Africa's most threatened mammals, with an estimated population size under 250, was upgraded from endangered to critically endangered in 2002. The low number of riverine rabbits precludes any attempts of nuclear transfer (NT) using intraspecific oocytes; therefore, the overall aim of this study was to assess the ability of the domestic rabbit (Oryctolagus cuniculus) oocyte to reprogram the somatic cell of the endangered riverine rabbit by interspecies NT. A preliminary study evaluated the effect of timing of enucleation after induction of ovulation (h post-hCG). A second study assessed the effects of two activation protocols. In addition, since the unique characteristics of the rabbit zona pellucida affect the speed of micromanipulation, different exposure periods to UV light at enucleation were evaluated. Adult domestic Californian rabbits were treated with eCG for 72 h, and ovulation was induced by hCG administration. Oocytes were collected by retrograde flushing at 12–14 h or 16–18 h post-hCG administration and stripped of cumulus investments with 0.5% hyaluronidase in Ca-Mg-free PBS. Metaphase-II oocytes were selected by visualizing the first polar body. Oocytes were stained with 2 mg mL–1 Hoechst 33342 for 5 min, and metaphase plates were removed with a 25–30 μm (O.D.) borosilicate beveled, spiked pipette after exposure to <5 or 30–40 s of UV light. Adult adipose-derived riverine rabbit fibroblasts grown to confluency in DMEM with 10% FCS were used as donor cells and fused with 2 consecutive DC pulses (3.2 kV cm–1, 45 μs). After reconstruction, couplets were randomly assigned for activation by either a second set of electrical pulses or incubation with ionomycin, followed by 1 h of incubation in 2 mm 6-DMAP. Embryos were co-cultured with a bovine oviductal cell monolayer in DMEM with 10% FCS and assessed for cleavage after 36 h of in vitro culture. There was a significant difference in the number of cleaved embryos from oocytes collected at 12–14 h post-hCG (n = 50) or 16–18 h post-hCG (n = 51) administration (57% v. 0% cleaved; P < 0.05). No significant difference was detected in embryos developing after electrofusion v. ionomycin activation treatments. However, a significantly greater number (P < 0.05) of embryos cleaved from oocytes exposed to <5 s UV than from oocytes exposed to 30–40 s UV (Table 1). A total of 20 embryos (4-cell to 16-cell stages) were surgically transferred to the oviducts of 4 adult New Zealand white synchronized recipients after 48 h of in vitro culture. Two recipients (<5 s UV exposure treatment group) were diagnosed pregnant by abdominal palpation at 15 days post-transfer; pregnancies were subsequently lost by Day 30, with placental tissues recovered. This preliminary study indicates the domestic rabbit oocyte is capable of reprogramming riverine rabbit donor cells. In addition, the time of oocyte collection after ovulation induction and the UV exposure period during enucleation have an effect on the efficiency of interspecies NT and embryo development in this species.
|


