285 ISOLATION AND CHARACTERIZATION OF BOVINE ADIPOSE-DERIVED SOMATIC STEM CELLS
A. A. Picou A , R. A. MacLean B , B. Dresser B C , R. A. Godke A and K. R. Bondioli AA Embryo Biotechnology Laboratory, School of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA, USA;
B Audubon Nature Institute Center for Research of Endangered Species, New Orleans, LA, USA;
C Department of Biological Sciences, University of New Orleans, New Orleans, LA, USA
Reproduction, Fertility and Development 21(1) 239-240 https://doi.org/10.1071/RDv21n1Ab285
Published: 9 December 2008
Abstract
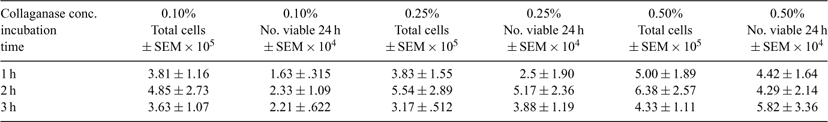
Adipose tissue has proven to serve as an abundant and rich source of adult stem cells with pluripotent properties. It has been suggested that pluri potent cell could serve as en efficient source of donor cells for nuclear transfer. The objective of this study was to develop an isolation and culture protocol for bovine adipose tissue-derived stem cells Tissue was collected post-mortem from the brisket of cows at a local abattoir. For cell isolation, the tissue was finely minced and weighed. The tissue was washed two times with PBS plus 2% penicillin-streptomycin (P/S) and enzymatically digested in a collagenase type 1 solution. Isolation conditions were determined by using a 3 × 3 factorial design. The tissue was digested in PBS with 0.1%, 0.25%, or 0.50% collagenase type 1 and 1% BSA, 2% P/S, and 2% Fungazone. Each enzyme concentration was incubated for 1, 2, or 3 hours in a shaking incubator at 39°C. The cellular mixtures were centrifuged at 300g for 5 minutes, floating adipocytes removed, and the remaining cell suspension passed through filter apparatus consisting of 180 and 20 μm nylon meshes. Cells were resuspended in PBS plus 1% BSA and centrifuged again for 5 minutes at 300g. Pelleted stromal cells were washed and nucleated cells were counted using Hoechst stain at a concentration of 2 μg mL–1. Primary cell cultures were plated in a 12.5 cm2 culture flask in DMEM (high glucose) supplemented with 10%FBS and 1% P/S. Cells were cultured for 48 h in a 38°C incubator in a humidified atmosphere of 5% CO2 in air. Nonadherent cells were removed by washing with PBS. For some cultures adherent cells were removed by trypsinization to determine the proportion of cell adhering. On average 23.38% of cells remained adherent, and the average cell cycle length was 1.85 days. Table 1 displays the average number of nucleated cells that were released per gram of fat for the various collagenase concentrations and exposure times. Analysis of data by one-way ANOVA show no differences. Growth factor supplementation is currently being evaluated on the effects of culture duration and passage number. Isolation conditions of 0.25% collagenase for 2 hours incubation has been consistently successful at obtaining viable ADAS cultures from cattle and common eland (Taurotragus oryx).
|
Funding provided by LSU/ACRES collaborative research program.


