118 ARE LIPID CONTENTS AND APOPTOSIS DETERMINANTS OF IN VITRO-PRODUCED BOVINE EMBRYO SUSCEPTIBILITY TO VITRIFICATION?
M. J. Sudano A , D. M. Paschoal A , L. C. O. Magalhães A , L. F. Crocomo A and F. D. Landim-Alvarenga ASão Paulo State University, Botucatu, Brazil
Reproduction, Fertility and Development 22(1) 217-218 https://doi.org/10.1071/RDv22n1Ab118
Published: 8 December 2009
Abstract
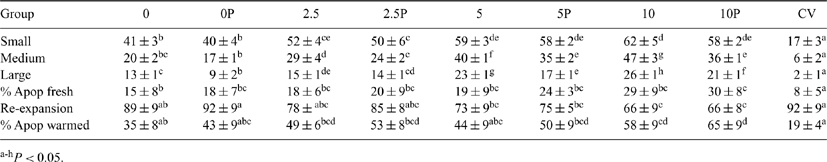
Supplementation of fetal calf serum (FCS) during culture of bovine in vitro-produced embryos (IVPE) has been correlated with lipid accumulation, representing a limiting factor on embryo cryotolerance. In the present experiment we studied lipid content and apoptosis in Nelore IVPE cultured in different concentrations of FCS. The experimental design was a 4 × 2 × 2 factorial, in which embryos, exposed or not to the metabolic regulator, phenazine ethosulfate (PES), were cultured in 4 concentrations of FCS. In 16 replicates, 560 IVPE were vitrified after cultured in SOFaa supplemented with 0, 2.5, 5 or 10% FCS. On Day 2.5 of culture, one-half of the embryos in each group were treated with 0.3 μM of PES and then returned to standard culture conditions (5% O2, 5% CO2 and 90% N2 atmosphere at 38.5°C). All embryos were vitrified on Day 7 (Campos-Chillòn LF et al. 2006 Theriogenology 65, 1200-1214). After warming, embryos were placed into culture for 12 h under standard conditions. A sample of fresh embryos was stained with Sudan Black B for quantification of small (<2 μm), medium (2-6 μm), and large (>6 μm) cytoplasmic lipid droplets. Apoptosis was analyzed, before and after vitrification, using TUNEL. As a positive control (CV), in vivo-produced embryos were collected from a superovulated Nelore cow. Data were analyzed using ANOVA followed by Tukey’s test; and Pearson’s linear correlation (P < 0.05). The results indicate that FCS concentration and PES treatment had no influence (P > 0.05) on cleavage and blastocyst formation rates (mean of 85.7 ± 2 and 36.6 ± 5, respectively). The total number of cells in fresh embryos was also similar (P > 0.05) among groups (mean of 134.1 ± 28). Additional results are shown in Table 1. Elevated concentrations of FCS increased lipid accumulation (r = 0.9) and the percentage of apoptotic cells in fresh and vitrified embryos, reducing the re-expansion rate after warming. Moreover, the number of apoptotic cells observed in fresh embryos was strongly correlated with the apoptosis observed after vitrification (r = 0.95). Although PES was able to reduce lipid accumulation, it was not efficient at preventing apoptosis in vitrified embryos. In summary, the results of this experiment indicate that the concentration of FCS during culture did not influence cleavage and blastocyst formation in bovine IVPE. We conclude that the apoptosis rate in fresh embryos is a key factor affecting survival after vitrification.
|
Acknowledgements: FAPESP (07/57766-4).


