161 EFFECTS OF LIPOSOMES ON SPERM MOTILITY AND DNA-BINDING EFFICIENCY
S. N. Lotti A , M. Rubessa B , R. V. Knox A and M. B. Wheeler A BA Department of Animal Science University of Illinois, Urbana-Champaign, IL, USA;
B Institute for Genomic Biology, Urbana-Champaign, IL, USA
Reproduction, Fertility and Development 29(1) 189-189 https://doi.org/10.1071/RDv29n1Ab161
Published: 2 December 2016
Abstract
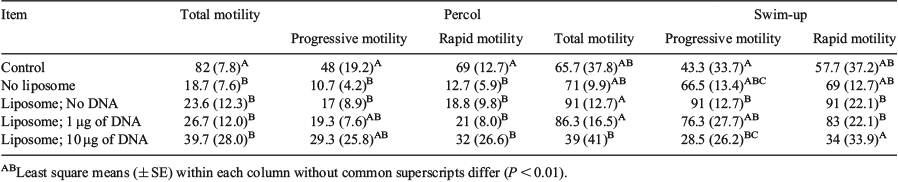
In mice, microinjection is the most common gene transfer method used. Unfortunately, this strategy does not translate as well to livestock. Another potential method is sperm-mediated gene transfer, which takes advantage of sperm’s natural ability to bind to naked DNA. Gene transfer using sperm-mediated gene transfer has been shown in pigs (Gandolfi et al. 1989 J. Reprod. Fert. Abstr. Ser. 4) and cattle (Perez et al. 1991 Biotecnol. Apl. 8, 90–94). Based on these observations, we examined the efficiency of exogenous DNA binding to sperm using liposomes. In this experiment, we analysed methods to select thawed bovine sperm for DNA binding and evaluated the binding of exogenous DNA to those sperm. To determine the optimal sperm-selection method, the sperm were analysed using a computer-assisted sperm analyzer (CASA), the parameters selected were: total motility, rapid motility, and progressive motility. To measure the binding of DNA we used an indirect analysis using NanoDrop technology (Thermo Scientific, Wilmington, DE, USA) to compare the different DNA concentrations among groups. Liposome preparation was done using a cationic lipid, 3-(trimethyl ammonium iodide) 1,2 dimystryl-propanediate and a neutral lipid, l-a Dioleoyl phosphatidyl-ethanolamine prepared according to the protocol of Russell (1997). Percoll or swim-up methods were used to select sperm after thawing (Rubessa et al. 2016), followed by incubation (3 h) with the liposome-DNA complexes according to liposome preparation protocol (Russell, 1997). We used enhanced green fluorescent protein in combination with the liposomes as a marker for exogenous DNA binding. Five treatments per selection method were analysed: 1) immediately after processing (Control), 2) After 3 h of incubation with no liposomes, 3) incubation with liposomes and no DNA, 4) incubation with 1 ng of DNA, and 5) incubation with 10 ng of DNA. This was repeated five times. The CASA results for total motility and rapid motility showed a greater amount of significant differences (P < 0.01) between the control and the other treatments in the Percol group as opposed to swim-up. These results confirm that the sperm selected with swim-up is more stable. Following CASA analysis, sperm was washed with PBS twice and collected in tubes. The DNA from all samples was extracted to determine the quantity of attaching varying amounts of DNA to sperm. The results showed a general increase in DNA concentrations with the increase of DNA added for both methods, but the statistical variation was too large to draw any definite conclusion. In future studies, real-time PCR will be used to determine the quantity of enhanced green fluorescent protein bound to the sperm.
|


