Male germ cell transplantation in livestock
J. R. Hill A C and I. Dobrinski BA CSIRO Livestock Industries, New England Highway, Armidale, NSW 2350, Australia.
B Center for Animal Transgenesis and Germ Cell Research, University of Pennsylvania School of Veterinary Medicine, New Bolton Center, Kennett Square, PA 19348, USA.
C Corresponding author. Email: jon.hill@csiro.au
Reproduction, Fertility and Development 18(2) 13-18 https://doi.org/10.1071/RD05123
Submitted: 21 September 2005 Accepted: 21 September 2005 Published: 14 December 2005
Abstract
Male germ cell transplantation is a powerful approach to study the control of spermatogenesis with the ultimate goal to enhance or suppress male fertility. In livestock animals, applications can be expanded to provide an alternative method of transgenesis and an alternative means of artificial insemination (AI). The transplantation technique uses testis stem cells, harvested from the donor animal. These donor stem cells are injected into seminiferous tubules, migrate from the lumen to relocate to the basement membrane and, amazingly, they can retain the capability to produce donor sperm in their new host. Adaptation of the mouse technique for livestock is progressing, with gradual gains in efficiency. Germ cell transfer in goats has produced offspring, but not yet in cattle and pigs. In goats and pigs, the applications of germ cell transplantation are mainly in facilitating transgenic animal production. In cattle, successful male germ cell transfer could create an alternative to AI in areas where it is impractical. Large-scale culture of testis stem cells would enhance the use of elite bulls by providing a renewable source of stem cells for transfer. Although still in a developmental state, germ cell transplantation is an emerging technology with the potential to create new opportunities in livestock production.
Extra keywords: cattle, goats, pigs, stem cells, testis.
Introduction
At first glance, the germ cell transfer technique seems to have only a remote chance of success. Injection of spermatogonial stem cells taken from donor males into the tubules of a recipient testis would seem likely to induce an immune reaction or, at least, for the cells to simply flush out of the tubules into the rete testis and epididymis. However, germ cells from fertile donor mice transplanted to the testes of infertile recipient mice resulted in donor-derived spermatogenesis and sperm production by the recipient animal (Brinster and Zimmermann 1994). Following this success in mice, the technique was applied successfully in rats (Jiang and Short 1995; Ogawa et al. 1999a; Zhang et al. 2003).
Spermatogenesis is a coordinated process that begins with spermatogonial proliferation followed by differentiation, such that production of spermatozoa is continuous (Russell et al. 1990). Spermatogonial stem cells facilitate this production line via their potential for both self-renewal and the production of differentiated daughter cells that will ultimately form spermatozoa. The spermatogonial stem cell is the only adult stem cell that contributes genes to subsequent generations. This makes it a perfect target for genetic manipulations, especially if spermatogonial stem cells can be cultured and their numbers increased before transplantation into recipient testes.
The male germ cell transfer technique has provided new insights into spermatogenesis by allowing the study of the stem cell niche and by providing a functional assay to characterise putative spermatogonial stem cells (Parreira et al. 1998; Nagano et al. 1999; Ventela et al. 2002). Transplanted germ cells are able to produce ‘normal’ sperm, as demonstrated by fertilisation in vivo and in vitro (Brinster and Avarbock 1994; Goossens et al. 2003; Honaramooz et al. 2003a). Time to sperm production can also be markedly accelerated because even mouse primordial germ cells transferred to a post-natal testis were shown to support spermatogenesis (Chuma et al. 2005).
Cross-species germ cell transfer
Cross-species (xenogeneic) spermatogonial transplantation from rats to mice (Clouthier et al. 1996) has produced rat sperm in mice testes and the reverse also was achieved (Ogawa et al. 1999a; Zhang et al. 2003). Using cross-species germ cell transfer, Franca et al. (1998) demonstrated that control of the spermatogenic cycle is predominantly via the germ cell rather than the Sertoli cell. Transplantation of hamster germ cells into mouse testes also resulted in donor-derived spermatogenesis (Ogawa et al. 1999b); however, transfer of germ cells from non-rodent donors into rodents resulted in colonisation of the stem cell niche in the seminiferous tubules, but not in complete spermatogenesis (Dobrinski et al. 1999, 2000; Nagano et al. 2001a, 2002a). Thus, transplanted cells are able to interact with host Sertoli cells to permit localisation to the basement membrane and initial proliferation. However, the next steps in spermatogenesis appear to be more conserved between species (possibly controlled by paracrine factors), so that differentiation and meiosis are not supported in the mouse testis. This incompatibility of donor germ cells and recipient testicular environment between phylogenetically distant species could, perhaps, be overcome by cotransplantation of germ cells and Sertoli cells, so far only reported in the mouse (Shinohara et al. 2003), or by testis tissue transplantation (Honaramooz et al. 2002a). Although not resulting in complete donor-derived spermatogenesis, spermatogonial transplantation into rodents nonetheless provides a bioassay for the stem cell potential of germ cells isolated from different mammalian species (Dobrinski et al. 1999, 2000; Izadyar et al. 2002a).
Applications of germ cell transplantation in livestock
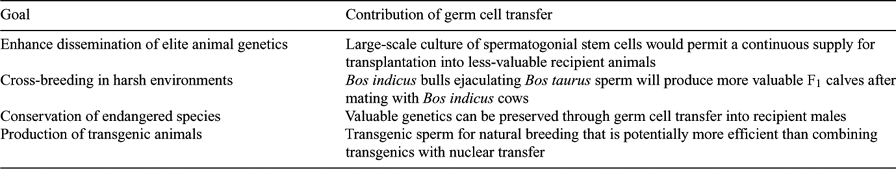
Successful germ cell transplantation in rodents, and perhaps cattle, requires that recipient animals are either closely related to the donor or immunosuppressed (Izadyar et al. 2003a; Kanatsu-Shinohara et al. 2003a; Zhang et al. 2003), whereas germ cell transplantation in pigs and goats was also successful between unrelated individuals (Honaramooz et al. 2002b, 2003a, 2003b). The results obtained from this latter research indicate that successful germ cell transplantation is feasible between immunocompetent, unrelated animals and that this process can produce live offspring in non-rodent species. This opens the door to a wide range of research opportunities and developments in this novel technology in livestock. Some potential applications are listed in Table 1. For example, germ cell transplantation provides an opportunity to preserve the genetic material of valuable males. Germ cell transplantation has an advantage over the only currently available approach, cryopreservation of sperm. Germ cell transplantation can be applied to prepubertal animals, from which sperm cannot be obtained, or even to adults rendered azoospermic or teratozoospermic by disease. Another potential application would be the delivery of genetic material to a closed production herd through resident recipient animals.
|
Initial transplantation experiments in livestock animals
The germ cell transfer technique used in pigs and goats is readily adapted to cattle (Honaramooz et al. 2002b, 2003a, 2003b; Izadyar et al. 2003a; Joerg et al. 2003; Hill et al. 2005) and the approach in cattle is schematically summarised in Fig. 1. Ultrasound-guided placement of the injection catheter or needle into the rete testis can be accomplished in prepubertal bull calves and rams. General anaesthesia is required for the injection into the rete testis. This approach provides access into the network of tubules that can be confirmed by visualisation of the spread of fluid into the tubules. A successful injections has a characteristic ultrasonographic appearance and can be seen when a small amount of air is present in the needle tip, which then flows quickly into the tubules causing a characteristic echogenic appearance. Alternatively, injection of a commercial ultrasound opaque fluid can highlight the path of the fluid through the tubules (Izadyar et al. 2003a). Thus, the ‘quality’ of a cell transfer injection can be assessed immediately. In addition, the most promising candidate recipient animals are identified early on in the process, thereby minimising the cost associated with maintenance of recipient animals through to breeding age and mating.
Selection of recipient animals
After an initial report exploring the feasibility of ultrasound-guided cannulation of the rete testis in an isolated bovine testis (Schlatt et al. 1999), Izadyar et al. (2003a) first reported the technique of germ cell transfer in cattle. The study confirmed the usefulness of ultrasound-guided cannulation of the rete testis in vivo and showed that transplanted autologous germ cells can initiate spermatogenesis in the recipient testis. The study did not succeed in efficient donor cell colonisation using homologous germ cells. These results are in contrast with our findings in cattle, pigs and goats and have to be interpreted with caution because only a small number of relatively old recipient animals were analysed, as acknowledged by the authors. Joerg et al. (2003) used an infertile Kleinfelter bull as a recipient for germ cell transfer. Ejaculates collected for 6 months following transfer contained donor germ cells, but not sperm from the donor bull. We have shown that heterologous transplantation of bovine germ cells can succeed when we assessed the outcome of male germ cell transfer between breeds of cattle (Hill et al. 2005). Testis cells from three Bos taurus (Angus) bull calves labelled with a fluorescent dye (PKH26) were used as donor cells for transfer into five Bos indicus cross (predominantly Brahman bloodline) bull calves. Each of the calves was prepubertal (between 5 and 7 months of age). These recipients were castrated at 2, 4, 6 and 8 weeks after transfer. Following castration, PKH26-positive donor cells were found in freshly isolated tubules of each of the five recipients. In the freshly isolated tubules, groups of PKH26-positive donor cells were observed, which indicated either cell division or substantial local colonisation of certain areas of the tubules. Frozen sections of seminiferous tubules were used to further localise the PKH26-positive donor cells. Donor-derived cells were located on the seminiferous tubule basement membrane, which indicates these cells had successfully migrated from the tubule lumen and were likely to be spermatogonia. There was a variation in the amount of fluorescence for individual cells, which indicated either cell division or variable uptake of the stain during the staining procedure.
Each of the recipients that received PKH26-stained cells retained these cells in the tubule epithelium for at least 8 weeks after transfer, which suggests that transfer between different animals, and, indeed, even between animals of different breeds of cattle, can be achieved. Further studies will aim to demonstrate whether donor cells are able to undergo spermatogenesis in the recipient animals.
Preparation of recipient animals
The efficiency of colonisation of seminiferous tubules by the transplanted germ cells can be improved if the recipient testes have little or no endogenous spermatogonia. Izadyar et al. (2003a) used recipient animals in which spermatogenesis had been abolished by irradiation. This approach requires specialised radiotherapy equipment, which, in its present form, is not very practical for field application. This emphasises the necessity to find more practical ways of suppressing endogenous spermatogenesis in recipient bulls or, alternatively, choosing an optimised donor age that will result in adequate donor cell colonisation without the need for further intervention.
Busulfan, a DNA-alkylating agent that destroys proliferating cells, is frequently used in rodents to deplete recipient germ cells before germ cell transplantation. However, the stem cell-depleting dose of busulfan is species and strain specific and treatment can be lethal due to severe bone marrow depression (Ogawa et al. 1999a; Brinster et al. 2003). Treatment of pigs and rams with busulfan between 5 and 17 weeks of age resulted not only in a significant reduction of testis weight and germ cell number, but also in systemic toxicity. In litter-bearing animals, such as pigs, in utero treatment is effective, as described previously in the mouse (Brinster et al. 2003; Honaramooz et al. 2005).
Cryopreservation of spermatogonial stem cells
In 1996, Brinster’s group showed that mouse spermatogonial stem cells, cryopreserved for prolonged periods of time before transplantation, still established spermatogenesis in the recipient testis (Avarbock et al. 1996). Subsequently, it was shown that live offspring could result from spermatozoa produced after transplantation of frozen–thawed mouse germ cells (Kanatsu-Shinohara et al. 2003b). Using frozen–thawed germ cells in cattle will make large-scale field application of the technique significantly easier from a logistic point of view (Izadyar et al. 2002b). Donor animals could be selected at convenient times and their germ cells preserved until transfer.
Stem cell identification and culture
Positive identification of bovine spermatogonial stem cells is required to enrich the population of male germ cells so as to improve the success of male germ cell transplantation. Identifying the correct conditions required for male germ line stem cell culture would likely lead towards in vitro spermatogenesis, which has major applications for the study of male infertility and, in particular, the effect of environmental toxins on male fertility. It has been estimated that there are only approximately 2 × 104 stem cells in 108 cells of a mouse testis (Tegelenbosch and De Rooij 1993). Enrichment steps aimed at increasing the low proportion of germ cells in the cell population isolated from donor seminiferous tubules include elimination of the interstitial cells, induction of cryptorchidism to deplete differentiated germ cells, active enrichment via sedimentation methods or immunosorting. In the mouse, selection of germ cells for expression of α6- and β1-integrin in the absence of c-kit receptor, as well as the collection of cells from experimentally induced cryptorchid testes, resulted in a significant enrichment for spermatogonial stem cells (Shinohara et al. 1999, 2000). Subsequently, expression of either Thy-1, CD9, Egr3 (zinc-finger protein early growth response factor) or GFRA-1 (GDNF family receptor alpha-1) surface proteins was used to enrich mouse germ line stem cell populations (Kubota et al. 2003; Hamra et al. 2004; Kanatsu-Shinohara et al. 2004; Kubota et al. 2004a; Buageaw et al. 2005). However, for germ cell transplantation in livestock species, large numbers of cells are required. This limits the usefulness of the technology. Although, in theory, a small number of stem cells could eventually repopulate the recipient tubules, the length of the tubules in large domestic animals would markedly increase the time required for repopulation.
In cattle, we are harvesting donor germ cells from animals just before puberty when spermatogonia and Sertoli cells are the only cell types present in the seminiferous tubules. This will ensure that the relative number of germ cells collected from the donor testis will be maximised. Differential plating using adhesion to surface coatings or plastic is also being investigated, as reported previously in rodents (Shinohara et al. 1999; Orwig et al. 2002a).
Nagano et al. (1998) were the first to show that mouse germ line stem cells could be maintained in culture for a long period of time. Improving culture conditions for male germ line stem cells is still under intense study, as evidenced by recent reports of improved culture systems for mouse germ cells (Jeong et al. 2003; Kanatsu-Shinohara et al. 2003c), including an efficient long-term culture system for mouse spermatogonial stem cells (Kubota et al. 2004a, 2004b; Kanatsu-Shinohara et al. 2005). In cattle, type A spermatogonia have been cultured for up to 1 month (Izadyar et al. 2003b).
A key aspect to culturing male germ line stem cells in vitro will be understanding the stem cell niche and how it regulates proliferation and differentiation of the stem cell. Spermatogenesis is a complex and precise process that is ultimately dependent on Sertoli cells for physical support, nutrient supply, hormonal control and signals required for spermatogonial stem cell renewal (Griswold 1998). In the testis, stem spermatogonia are located in niches on the basement membrane of the seminiferous tubule, enclosed by the basal lamina, the Sertoli cells and the tight junctions between Sertoli cells. The number of these niches is a critical determinant of stem germ cell number and function. When a stem germ cell divides, only one daughter cell remains in the niche as a stem cell and the other must differentiate unless another niche is available. Because the Sertoli cells provide the niches for the germ cells, the number and health of Sertoli cells is the key factor that decides the rate of sperm production. Therefore, understanding the physical and molecular determinants of the stem cell niche will provide the basis for designing a suitable microenvironment for the efficient in vitro culture of germ line stem cells.
Transgenesis
Transgenic animals can be produced via the transfer of transgenic donor germ cells (Honaramooz et al. 2003a). The advantage over inducing transgenesis using nuclear transfer technology is the reduced interval to producing ‘founder’ transgenic sires, because the recipient animals will be capable of mating and sperm production in a much shorter time than animals generated by nuclear transfer. Although, to date, the lack of pure starting populations of germ line stem cells and optimised culture systems have made this a difficult task, some success has been reported in generating transgenic mice and rats by retroviral or lentiviral transduction of germ cells before transplantation (Nagano et al. 2001b, 2002b; Hamra et al. 2002; Orwig et al. 2002b). An alternative vector that integrates into non-replicating cells, namely recombinant adeno-associated virus, has shown promising results (Honaramooz et al. 2003c) and is a topic of current investigation. Genetic manipulation of male germ line stem cells may provide an approach to generate males that produce unisex sperm by introducing a sex-linked mutation that selectively prevents formation of haploid cells carrying either an X or a Y chromosome. The production of all male or all female offspring is of tremendous economic interest to livestock production systems.
Conclusions
The adaptation of the germ cell transfer technique from rodents to livestock has great value in preserving and disseminating desirable genetics. Significant progress has been made in key aspects of the technique. Donor cell isolation, enrichment, delivery to recipient testes and recipient preparation has been established in goats and pigs and, to a large extent, in cattle. Other aspects, such as long-term culture and large-scale culture of germ cells, are under investigation. Long-term culture and multiplication of these stem cells will provide a renewable resource for transfer into multiple recipients.
The commercialisation end-point requires the germ cell transfer to be developed as an alternative to artificial insemination. Application of germ cell transplantation technology to cattle will enable dissemination of selected genetics through a ‘natural’ delivery system. Although currently still technically challenging, germ cell transplantation does have the potential to transform the cattle-breeding industry.
Acknowledgments
Work from the authors’ laboratories was supported by CSIRO Flagship project ‘Engineering new breeding systems’ (to JRH) and R01 RR017359–04 (NCRR/NIH) and R41 HD044780–01 (NICHD/NIH) (to ID).
Avarbock, M. R. , Brinster, C. J. , and Brinster, R. L. (1996). Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nat. Med. 2, 693–696.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Schlatt, S. , Rosiepen, G. , Weinbauer, G. F. , Rolf, C. , Brook, P. F. , and Nieschlag, E. (1999). Germ cell transfer into rat, bovine, monkey and human testes. Hum. Reprod. 14, 144–150.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Shinohara, T. , Avarbock, M. R. , and Brinster, R. L. (1999). beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl Acad. Sci. USA 96, 5504–5509.
| Crossref | GoogleScholarGoogle Scholar |

Shinohara, T. , Orwig, K. E. , Avarbock, M. R. , and Brinster, R. L. (2000). Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc. Natl Acad. Sci. USA 97, 8346–8351.
| Crossref | GoogleScholarGoogle Scholar |

Shinohara, T. , Orwig, K. E. , Avarbock, M. R. , and Brinster, R. L. (2003). Restoration of spermatogenesis in infertile mice by sertoli cell transplantation. Biol. Reprod. 68, 1064–1071.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tegelenbosch, R. A. , and De Rooij, D. G. (1993). A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 290, 193–200.
| PubMed |

Ventela, S. , Ohta, H. , Parvinen, M. , and Nishimune, Y. (2002). Development of the stages of the cycle in mouse seminiferous epithelium after transplantation of green fluorescent protein-labeled spermatogonial stem cells. Biol. Reprod. 66, 1422–1429.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang, Z. , Renfree, M. B. , and Short, R. V. (2003). Successful intra- and interspecific male germ cell transplantation in the rat. Biol. Reprod. 68, 961–967.
| Crossref | GoogleScholarGoogle Scholar | PubMed |




