Equine cloning: applications and outcomes
Dirk K. Vanderwall A G , Gordon L. Woods A , Janet F. Roser B , Donald H. Schlafer C , Debra C. Sellon D , David F. Tester E and Kenneth L. White FA Northwest Equine Reproduction Laboratory, Department of Animal and Veterinary Science and Center for Reproductive Biology, University of Idaho, Moscow, ID 83844, USA.
B Department of Animal Science, University of California, Davis, CA 95616, USA.
C Department of Biomedical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA.
D Department of Clinical Sciences, College of Veterinary Medicine, Washington State University, Pullman, WA 99164, USA.
E Prairie Animal Hospital, Coeur d’Alene, ID 83814, USA.
F Center for Developmental and Molecular Biology, Biotechnology Center, Animal, Dairy and Veterinary Science Department, Utah State University, Logan, UT 84322, USA.
G Corresponding author. Email: dirkv@uidaho.edu
Reproduction, Fertility and Development 18(2) 91-98 https://doi.org/10.1071/RD05130
Submitted: 3 October 2005 Accepted: 3 October 2005 Published: 14 December 2005
Abstract
Cloning is one of several new assisted reproductive techniques being developed for clinical use in the equine industry. Potential uses of equine cloning include: (1) the preservation of genetics from individual animals that would otherwise not be able to reproduce, such as geldings; (2) the preservation of genetic material of endangered and/or exotic species, such as the Mongolian wild horse (Przewalski’s horse); and (3) because of the companion animal role that horses fill for some individuals, it is likely that some horse owners will have individual animals cloned for emotional fulfillment. Although equine cloning has been successful, like other species, it remains a very inefficient process (<3% success). In most species, the inefficiency of cloning results from a high incidence of embryonic, fetal and/or placental developmental abnormalities that contribute to extremely high rates of embryonic loss, abortion and stillbirths throughout gestation and compromised neonatal health after birth. The present review describes some of the ultrasonographic, endocrinological and histopathological characteristics of successful (produced viable offspring) and unsuccessful (resulted in pregnancy failure) cloned equine (mule and horse) pregnancies we have produced. A total of 21 cloned mule pregnancies were established using fetal fibroblast cells, whereas a total of seven cloned horse pregnancies were established using adult cumulus cells. Three of the cloned mule conceptuses were carried to term, resulting in the birth of three healthy clones. This information adds to an accumulating body of knowledge about the outcome of cloned equine pregnancies, which will help to establish when, and perhaps why, many cloned equine pregnancies fail.
Introduction
The ability to clone animals using somatic cell nuclear transfer offers tremendous potential for application in the areas of animal agriculture (e.g. genetic improvement (Lewis et al. 1998; McClintock 1998) and disease resistance (Denning et al. 2001)), conservation biology, through the preservation of endangered species (Wells et al. 1998; Loi et al. 2001), and medical biotechnology (e.g. the production of transgenic animals for ‘biopharming’ (Schnieke et al. 1997; Baguisi et al. 1999) and xenotransplantation of organs (Denning et al. 2001; Dai et al. 2002)). For the equine industry, nuclear transfer is one of several new assisted reproductive techniques (e.g. oocyte transfer, intracytoplasmic sperm injection etc.) being developed for clinical use. The potential uses of equine cloning include: (1) the preservation of genetics from individual animals that would otherwise not be able to reproduce, such as geldings; (2) the preservation of genetic material of endangered and/or exotic species, such as the Mongolian wild horse (Przewalski’s horse); and (3) because of the companion animal role that horses fill for some individuals, it is likely that some horse owners will have individual animals cloned for emotional fulfillment. Of these, cloning geldings to produce intact males for breeding purposes has been the first clinical application of equine cloning (Holden 2005). Although some breed associations (e.g. The Jockey Club, American Quarter Horse Association) do not currently allow the registration of cloned animals, for some equine sporting activities (dressage, show-jumping, cutting etc.) breed registry status is irrelevant, which eliminates that regulatory impediment to the use of cloning technology.
Equine cloning was first successful in 2003, when we reported the live birth of three mule foals cloned from a fetal fibroblast cell line (Woods et al. 2003) and Galli et al. (2003) reported the live birth of a horse foal cloned from an adult fibroblast cell line. Since then, several more cloned horses have been produced (Hinrichs 2005; Holden 2005). Despite these successes, like other species, the current efficiency of equine cloning is very low, as evidenced by the fact that only between 0.7% (Hinrichs 2005) and 2.7% (Woods et al. 2003) of reconstructed cloned equine embryos result in the birth of live offspring. During the course of our work, we found that increasing the calcium concentration in the medium used for oocyte handling and activation significantly increased the pregnancy rates with the cloned embryos, which led to the birth of the three cloned mules (Woods et al. 2003). In addition, similar to other species (DeLegge et al. 2004), there is evidence that the individual cell donor can influence the success of equine cloning (Galli et al. 2003; Vanderwall et al. 2004a). Clearly, further work is needed to increase the efficiency of equine cloning. The present review describes some of the ultrasonographic, endocrinological and histopathological characteristics of successful (produced viable offspring) and unsuccessful (resulted in pregnancy failure) cloned equine (mule and horse) pregnancies that we have produced. Details of the specific procedures used to produce all the cloned pregnancies have been reported previously (Woods et al. 2003; Vanderwall et al. 2004a). A total of 21 cloned mule pregnancies were established using fetal fibroblast cells, whereas a total of seven cloned horse pregnancies were established using adult cumulus cells.
Ultrasonographic characteristics
Immediately following reconstruction and activation of cloned embryos, the embryos were transferred surgically to the oviduct of recipient mares (n = 2–5 embryos per mare) that had ovulated within 24 h prior to the transfer. An initial pregnancy examination was performed using transrectal ultrasonography between Days 12 and 16 (Day 0 = surgery); subsequent examinations were then performed approximately every 7–10 days until Day 60 and then approximately every 2–4 weeks throughout gestation (or until pregnancy loss occurred). End-points evaluated at each examination during early gestation (<60 days) were as follows: (1) the size and location of the embryonic vesicle(s); (2) the presence of an embryo proper within the vesicle; and (3) the presence of an embryonic heartbeat. Conceptuses were considered small for their gestational age if they were greater than or equal to two standard deviations less than the mean value for viable non-cloned horse conceptuses reported previously (Vanderwall et al. 2000). The primary end-point evaluated at each examination during mid-gestation (60–270 days) was ultrasonographic and/or manual detection of fetal movement. End-points evaluated during late gestation (>270 days) were fetal movement and ultrasonographic assessment of the combined thickness of the uterus and placenta (CTUP), as described previously (Renaudin et al. 1997).
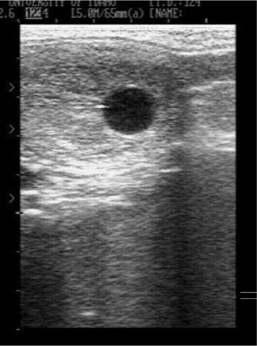
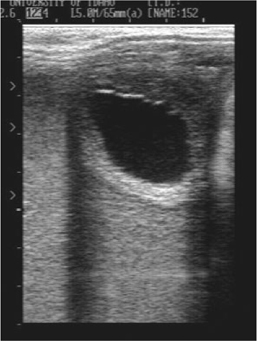
Six of 18 (33%) cloned mule embryos and one of seven (14%) cloned horse embryos that failed spontaneously were small for their gestational age at the first and/or second examination at which they were detected (Fig. 1). Most remained small for age until they were lost. All three successful mule conceptuses were of normal size at each examination during early gestation. Another characteristic noted in several failed pregnancies was the absence of the development of the embryo proper, which generally becomes evident by Day 25 of gestation (Vanderwall et al. 2000). Six of 14 (43%) of the cloned mule embryos that were maintained past Day 25 of gestation failed to develop an embryo proper and appeared to be developing as trophoblastic vesicles (Fig. 2). Three of the six apparent trophoblastic vesicles were small for their gestational age. All four cloned horse embryos that were maintained past Day 25 developed an embryo proper.
|
|
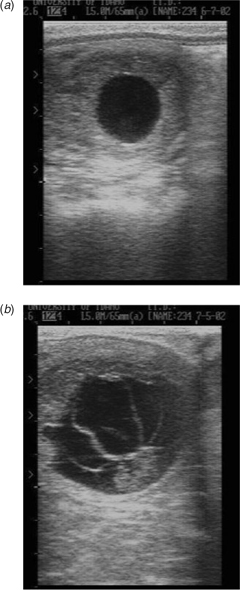
There were generally no premonitory signs of impending embryonic loss in the conceptuses that did not develop an embryo proper; the conceptus was simply not evident at a subsequent examination. Signs of impending embryonic loss were generally observed in conceptuses in which an embryo proper was observed and included the following: (1) the absence of an embryonic heartbeat; (2) disorganisation of the conceptus membranes; and (3) increased echogenicity of conceptus fluids. One or more of those signs were typically observed in conceptuses before pregnancy loss (Fig. 3). Of the 18 cloned mule pregnancies that failed spontaneously, 10 (56%) were lost between Days 14 and 30, whereas the remaining eight (44%) were lost between Days 30 and 60 of gestation. Similarly, of the seven cloned horse pregnancies that failed spontaneously, four (57%) were lost between Days 14 and 30, whereas the remaining three (43%) were lost between Days 30 and 80 of gestation.
|
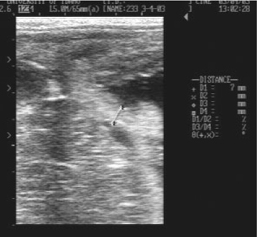
During the 9th, 10th and 11th months of gestation, CTUP measurements of the three successful cloned mule pregnancies were compared with CTUP measurements obtained from commercial broodmares carrying non-cloned horse pregnancies (Troedsson et al. 1997). At each examination at which it was measured (Fig. 4), the CTUP of the cloned pregnancies was within the normal reference range for non-cloned pregnancies.
|
Endocrinological characteristics
Jugular blood samples were collected from the mares at the time of each pregnancy examination and the serum was recovered and stored at −20°C until further analysis. Equine chorionic gonadotropin (eCG) was determined with a radioimmunoassay, as described previously (Roser and Lofstedt 1989). The sensitivity of the eCG assay was 1.0 ng mL−1 and the intra- and interassay coefficients of variation were 6.5 and 13.9%, respectively. Levels of conjugated oestrogens were determined with a radioimmunoassay, as described previously (Roser and Hughes 1991). The sensitivity of the conjugated oestrogen assay was 0.2 ng mL−1 and the intra- and interassay coefficients of variation were 4.5% and 9.2%, respectively.
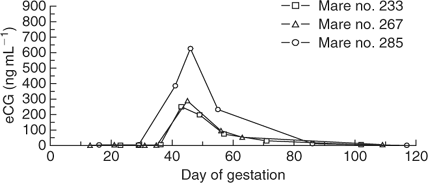
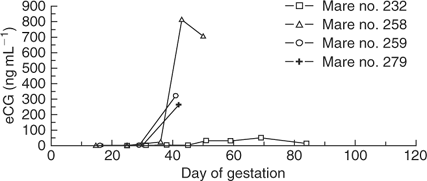
Equine chorionic gonadotropin levels in the three successful mule pregnancies and four unsuccessful cloned mule pregnancies are shown in Figs 5 and 6, respectively. All three successful cloned mule pregnancies developed peak eCG levels between 250 and 650 ng mL−1 between Days 40 and 50 of gestation, which then declined to baseline levels again by Day 100 of gestation. The eCG level in three of four unsuccessful cloned mule pregnancies reached levels similar to those observed in successful pregnancies; however, in one mare (no. 232) the eCG level remained less than 50 ng mL−1, despite the fact that a conceptus with a heartbeat was evident on Day 45 of gestation. When that mare (no. 232) was examined one week later on Day 51, the embryo proper was evident, but there was no cardiac activity.
|
|
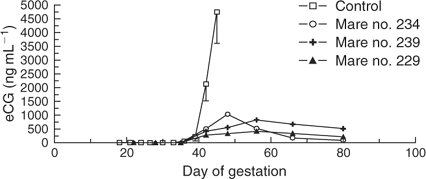
Figure 7 shows eCG levels in three unsuccessful cloned horse pregnancies and the mean (± s.e.m.) eCG levels through Day 45 of gestation in six control mares carrying viable non-cloned horse conceptuses. The three unsuccessful cloned horse pregnancies developed peak eCG levels between 400 and 1050 ng mL−1 between Days 40 and 60 of gestation; in contrast, the mean concentration of eCG in the control non-cloned horse pregnancies was greater than 4500 ng mL−1 on Day 45 of gestation.
|
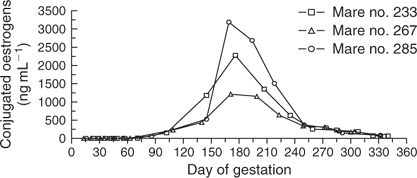
Conjugated oestrogen levels in the three successful mule pregnancies are shown in Fig. 8. All three successful cloned mule pregnancies exhibited an initial rise in conjugated oestrogens after Day 80 of gestation, which then rose to peak levels greater than 1000 ng mL−1 between Days 150 and 180 of gestation, and then underwent a continual decline back towards baseline throughout the remainder of gestation. The level of conjugated oestrogens remained at baseline levels in all unsuccessful cloned mule and horse pregnancies (all lost before Day 80 of gestation).
|
Histopathological characteristics
Two mares carrying unsuccessful cloned mule conceptuses were killed on Days 44 (mare no. 259) and 45 (mare no. 279) and the entire reproductive tract containing the conceptus was removed from each mare. An embryo proper and heartbeat had been visualised in both mares on Days 29 (no. 259) and 30 (no. 279); however, on Days 41 (no. 259) and 42 (no. 279) the embryo proper was still evident, but cardiac activity was not detected. Therefore, the conceptuses were recovered for gross and microscopic evaluation. Gross examination did not reveal any abnormalities in either conceptus (Fig. 9). Similarly, histopathological evaluation of each fetus and associated placental membranes did not reveal any pathological lesions that may have contributed to their demise. Evaluation of sections of endometrium containing endometrial cups showed that there was a heavy infiltration of inflammatory cells in and around the endometrial cups. The endometrial cups in both mares had begun to secrete eCG, as shown in Fig. 6.
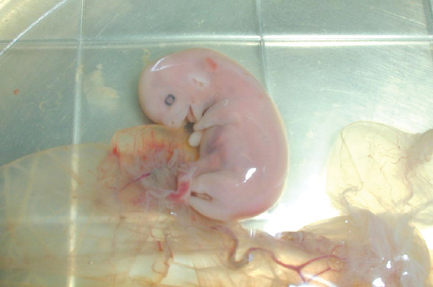
|
The three mares that delivered live cloned mules spontaneously expelled the placenta within 90 min of parturition. The physical characteristics of each placenta were evaluated (Fig. 10) and compared with previously published data (Whitwell and Jeffcott 1975) for thoroughbred horses; of note was the finding that the non-pregnant horn was longer than the pregnant horn in all three cloned placentas, whereas the pregnant horn is typically longer than the non-pregnant horn in equine placentas. The clinical significance (if any) of this finding is not known. In addition, the placenta of one mare showed gross and microscopic evidence of mild, focal microcotyledonary necrosis in three areas (4.0 × 5.0, 3.0 × 1.5 and 2.0 × 2.0 cm) that was associated with minimal inflammatory changes (lymphocytic placentitis) and that yielded growth of moderate numbers of Aeromonas and Klebsiella organisms. There was no evidence that these areas of focal placentitis compromised the fetal and/or neonatal health of this mare’s foal. Although the inciting cause of the placentitis is unknown, it is unlikely that it was related to the cloning procedure. Other than the focal placentitis in this one mare, gross and histological evaluation of all three placentas was unremarkable (Fig. 11).
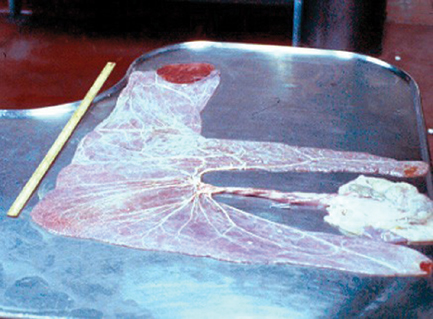
|
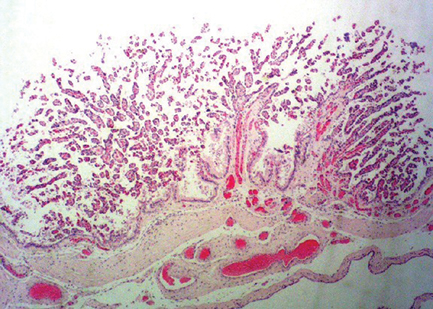
|
Neonatal health
Immediately after birth, all three foals were weighed and underwent a complete physical examination. Within 36 h of birth, blood samples were collected from each foal for routine haematology and blood chemistry analyses, which have been reported previously (Vanderwall et al. 2004b). All three foals exhibited a mild to moderate thrombocytopenia, which resolved spontaneously and was not associated with any clinical signs. The underlying cause of the thrombocytopenia is not known, but may have been the result of the absorption of alloantibodies in the colostrum that recognised foal platelets (Ramirez et al. 1999). Clinically, the foals remained healthy throughout the neonatal period and continue to be healthy and vigorous at the time of writing (Fig. 12).

|
Discussion
The high incidence of pregnancy loss (89% overall) in the cloned equine pregnancies described herein is similar to results obtained with other species (for a review, see Trounson 2001). The primary problem contributing to the inefficiency of cloning has been referred to as ‘cloned offspring syndrome’ (Cross 2001), which is characterised by a high incidence of embryonic, fetal and/or placental developmental abnormalities that result in extremely high rates of embryonic loss, abortion and stillbirths throughout gestation and compromised neonatal health after birth (Renard et al. 2002). The embryonic/fetal and/or placental developmental abnormalities that result in abortion, stillbirth or compromised neonatal health are thought to reflect incomplete or abnormal genetic reprogramming of the donor nucleus, specifically related to critically important imprinted genes (Mitalipov and Wolf 2000; Young and Fairburn 2000; Han et al. 2003). It seems likely that reprogramming errors contributed to the high incidence of early embryonic loss in these cloned pregnancies but, unlike cattle and sheep, in which pregnancy losses occur throughout gestation (Renard et al. 2002; Edwards et al. 2003), the losses were confined to early gestation.
Overall, 28% of the unsuccessful cloned pregnancies were small for their gestational age, whereas all three successful cloned pregnancies were of normal size during early gestation. This is consistent with several studies that have documented higher pregnancy loss rates in mares carrying undersized non-cloned equine conceptuses (Chevalier and Palmer 1982; Ginther et al. 1985; Adams et al. 1987; Bergfelt et al. 1992). The finding that three of the six undersized cloned pregnancies appeared to be developing as trophoblastic vesicles is not surprising, because it has been reported that non-cloned horse conceptuses that were small for their gestational age were 50-fold more likely to develop as a trophoblastic vesicle compared with normal sized conceptuses (Vanderwall et al. 2000). It seems likely that these ultrasonographically detectable characteristics (small for age and trophoblastic vesicle formation) of the unsuccessful cloned pregnancies reflect genetic reprogramming errors, as described above.
Although one mare developed a focal placentitis, there was no evidence of any cloning-related change in placental development or function during late gestation in the three mares that gave birth to cloned mules. The CTUP measurements were within the normal reference range and gross and morphological assessment of each placenta did not reveal any changes that could be ascribed to the fact they were from cloned pregnancies. This is in marked contrast with the high incidence of developmental and/or functional abnormalities of the placenta in cloned cattle and sheep, which contributes to the high incidence of pregnancy failure and neonatal loss in these species (Hill et al. 2000; De Sousa et al. 2001; Edwards et al. 2003). The underlying reason for this difference is not known.
The three mares that successfully carried cloned mule conceptuses to term developed peak eCG levels between 250 and 650 ng mL−1 between Days 40 and 50 of gestation, which then declined to baseline levels again by Day 100 of gestation. Three of four mares carrying unsuccessful cloned mule conceptuses developed similar levels of eCG between Days 40 and 50 of gestation, despite the fact that the pregnancies were failing at that time; therefore, there was no evidence that endometrial cup formation or function was different (or contributed to pregnancy loss) in those three unsuccessful pregnancies. In contrast, the eCG level in one mare carrying an unsuccessful cloned mule conceptus increased to only 50 ng mL−1, despite the presence of a viable conceptus on Day 45. It seems likely the altered eCG secretion in this mare was due to abnormal development and/or function of the endometrial cups; however, whether the altered eCG secretion contributed directly to the failure of this cloned pregnancy, or was simply an indication of its impending demise, is unknown. Mares carrying (non-cloned) mule conceptuses develop eCG levels that are approximately 80–90% lower than mares carrying horse conceptuses (Clegg et al. 1962; Allen et al. 1993); therefore, the levels of eCG in the successful cloned mule pregnancies appeared to be within the expected normal range for non-cloned mule pregnancies based on comparison with the eCG levels in the control non-cloned horse pregnancies in the present report (Fig. 7).
All three mares carrying cloned horse conceptuses in which eCG was measured developed levels markedly lower than control mares carrying non-cloned horse conceptuses; however, the viability of all three cloned conceptuses had become questionable (i.e. loss of cardiac activity) between Day 35 and 42 of gestation. Therefore, it is not known whether the apparently lower (compared with control) level of eCG secretion was due to a generalised loss of viability or a primary result of abnormal embryonic/placental development. It seems unlikely that the altered secretion of eCG contributed directly to pregnancy loss in these three mares because their conceptuses appeared to be losing viability coincidentally with the expected start of eCG secretion.
The pattern of conjugated oestrogen secretion in the three successful cloned mule pregnancies was consistent with that observed in non-cloned equine pregnancies (Cox 1975). A rise in conjugated oestrogens of placental origin typically begins between Days 70 and 80 of gestation, which generally reaches peak levels between Days 200 and 240 of gestation (Allen 2000). The change in conjugated oestrogen levels observed throughout gestation in the successful cloned mule pregnancies is further evidence of the apparent normality of placental development and function in these cloned equine pregnancies.
Gross and histological examination of two unsuccessful cloned mule conceptuses did not identify any conclusive pathological changes, so the underlying reason(s) for their demise remains unknown. Given that some of the conceptuses showed ultrasonographically detectable derangements of the fetal membranes (as in Fig. 3), specific lesions may have been identified had more unsuccessful conceptuses been examined. Further work will be needed to determine whether specific developmental abnormalities can be identified that may contribute to the high rate of pregnancy loss in cloned equine conceptuses.
In summary, like most species, there was a very high incidence of pregnancy failure in the cloned equine pregnancies described herein; however, unlike some species (most notably sheep and cattle), all pregnancy losses occurred during early gestation (<60–80 days). The unsuccessful cloned equine pregnancies appeared to be more likely to develop conceptuses that were small for their gestational age and/or failed to develop an embryo proper. Although four of seven of the unsuccessful cloned pregnancies appeared to have decreased eCG secretion, only one conceptus appeared to have decreased eCG secretion at a time when the conceptus was clearly still viable and, in the other three unsuccessful pregnancies, conceptus viability was waning at the time when eCG secretion should have been increasing. Therefore, we did not see conclusive evidence that altered eCG secretion contributed to the pregnancy failures that were observed. Similarly, gross and microscopic examination of two conceptuses did not reveal evidence of an underlying reason for pregnancy failure. Further work will be necessary to more fully understand the underlying and/or inherent defects in cloned equine conceptuses that contribute to the high rate of failure.
Acknowledgments
The authors’ work reported herein was supported by the Idaho Equine Education Bill and by D. W. Jacklin and the Jacklin Family Foundation, Inc. The authors thank Jennifer Dodge and Lillian Sibley for technical assistance.
Adams, G. P. , Kastelic, J. P. , Bergfelt, D. R. , and Ginther, O. J. (1987). Effect of uterine inflammation and ultrasonically-detected uterine pathology on fertility in the mare. J. Reprod. Fertil. 35(Suppl.), 445–454.

Allen, W. R. (2000). The physiology of early pregnancy in the mare. Proc. Annu. Conv. Am. Assoc. Equine Practnr 46, 338–354.

Allen, W. R. , Skidmore, J. A. , Stewart, F. , and Antczak, D. F. (1993). Effects of fetal genotype and uterine environment on placental development in equids. J. Reprod. Fertil. 97, 55–60.

Baguisi, A. , Behboodi, E. , Melican, D. T. , Pollock, J. S. , and Destrempes, M. M. , et al. (1999). Production of goats by somatic cell nuclear transfer. Nat. Biotechnol. 17, 456–461.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bergfelt, D. R. , Woods, J. A. , and Ginther, O. J. (1992). Role of the embryonic vesicle and progesterone in embryonic loss in mares. J. Reprod. Fertil. 95, 339–347.
| PubMed |

Chevalier, F. , and Palmer, E. (1982). Ultrasonic echography in the mare. J. Reprod. Fertil. 32(Suppl.), 423–430.

Clegg, M. T. , Cole, H. H. , Howard, C. B. , and Pigon, H. (1962). The influence of foetal genotype on equine gonadotrophin secretion. J. Endocrinol. 25, 245–248.
| PubMed |

Cox, J. E. (1975). Oestrone and equilin in the plasma of the pregnant mare. J. Reprod. Fertil. 23(Suppl.), 463–468.

Cross, J. C. (2001). Factors affecting the developmental potential of cloned mammalian embryos. Proc. Natl Acad. Sci. USA 98, 5949–5951.
| Crossref | GoogleScholarGoogle Scholar |

Dai, Y. , Vaught, T. D. , Boone, J. , Chen, S. H. , and Phelps, C. J. , et al. (2002). Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 20, 251–255.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

DeLegge, K. , Maserati, M. , Kieser, N. , Delanski, D. , Henderson, B. , Dobbie, T. , Middour, J. , Balladares, J. , and Page, R. (2004). Effect of genotype and cell line on the efficiency of live calf production by somatic cell nuclear transfer. Reprod. Fertil. Dev. 16, 139–140.[Abstract]
| Crossref | GoogleScholarGoogle Scholar |

Denning, C. , Burl, S. , Ainslie, A. , Bracken, J. , and Dinnyes, A. , et al. (2001). Deletion of the alpha(1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat. Biotechnol. 19, 559–562.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

De Sousa, P. A. , King, T. , Harkness, L. , Young, L. E. , Walker, S. K. , and Wilmut, I. (2001). Evaluation of gestational deficiencies in cloned sheep fetuses and placentae. Biol. Reprod. 65, 23–30.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Edwards, J. L. , Schrick, F. N. , McCracken, M. D. , van Amstel, S. R. , Hopkins, F. M. , Welborn, M. G. , and Davies, C. J. (2003). Cloning adult farm animals: a review of the possibilities and problems associated with somatic cell nuclear transfer. Am. J. Reprod. Immunol. 50, 113–123.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Galli, C. , Lagutina, I. , Crotti, G. , Colleoni, S. , Turini, P. , Ponderato, N. , Duchi, R. , and Lazzari, G. (2003). Pregnancy: a cloned horse born to its dam twin. Nature 424, 635.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ginther, O. J. , Bergfelt, D. R. , Leith, G. S. , and Scraba, S. T. (1985). Embryonic loss in mares: incidence and ultrasonic morphology. Theriogenology 24, 73–86.
| Crossref | GoogleScholarGoogle Scholar |

Han, Y. M. , Kang, Y. K. , Koo, D. B. , and Lee, K. K. (2003). Nuclear reprogramming of cloned embryos produced in vitro. Theriogenology 59, 33–44.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hill, J. R. , Burghardt, R. C. , Jones, K. , Long, C. R. , Looney, C. R. , Shin, T. , Spencer, T. E. , Thompson, J. A. , Winger, Q. A. , and Westhusin, M. E. (2000). Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 63, 1787–1794.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hinrichs, K. (2005). Update on equine ICSI and cloning. Theriogenology 64, 535–541.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Holden, C. (2005). Champion racer cloned. Science 308, 628.

Lewis, I. M. , Peura, T. T. , and Trounson, A. O. (1998). Large-scale applications of cloning technologies for agriculture: an industry perspective. Reprod. Fertil. Dev. 10, 677–681.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Loi, P. , Ptak, G. , Barboni, B. , Fulka, J. , Cappai, P. , and Clinton, M. (2001). Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 19, 962–964.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

McClintock, A. E. (1998). Impact of cloning on cattle breeding systems. Reprod. Fertil. Dev. 10, 667–669.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Mitalipov, S. M. , and Wolf, D. P. (2000). Mammalian cloning: possibilities and threats. Ann. Med. 32, 462–468.
| PubMed |

Ramirez, S. , Gaunt, S. D. , McClure, J. J. , and Oliver, J. (1999). Detection and effects on platelet function of anti-platelet antibody in mule foals with experimentally induced neonatal alloimmune thrombocytopenia. J. Vet. Intern. Med. 13, 534–539.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Renard, J. P. , Zhou, Q. , LeBourhis, D. , Chavatte-Palmer, P. , Hue, I. , Heyman, Y. , and Vignon, X. (2002). Nuclear transfer technologies: between successes and doubts. Theriogenology 57, 203–222.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Renaudin, C. D. , Troedsson, M. H. T. , Gillis, C. L. , King, V. L. , and Bodena, A. (1997). Ultrasonographic evaluation of the equine placenta by transrectal and transabdominal approach in the normal pregnant mare. Theriogenology 47, 559–573.
| Crossref | GoogleScholarGoogle Scholar |

Roser, J. F. , and Lofstedt, R. M. (1989). Urinary eCG patterns in the mare during pregnancy. Theriogenology 32, 607–622.
| Crossref | GoogleScholarGoogle Scholar |

Roser, J. F. , and Hughes, J. P. (1991). Prolonged pulsatile administration of gonadotrophin-releasing hormone (GnRH) to fertile stallions. J. Reprod. Fertil. 44(Suppl.), 155–168.

Schnieke, A. E. , Kind, A. J. , Ritchie, W. A. , Mycock, K. , Scott, A. R. , Ritchie, M. , Wilmut, I. , Colman, A. , and Campbell, K. H. (1997). Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science 278, 2130–2133.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Troedsson, M. H. T. , Renaudin, C. D. , Zent, W. W. , and Steiner, J. V. (1997). Transrectal ultrasonography of the placenta in normal mares and mares with pending abortion: a field study. Proc. Annu. Conv. Am. Assoc. Equine Practnr 43, 256–258.

Trounson, A. (2001). Nuclear transfer in human medicine and animal breeding. Reprod. Fertil. Dev. 13, 31–39.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vanderwall, D. K. , Squires, E. L. , Brinsko, S. P. , and McCue, P. M. (2000). Diagnosis and management of abnormal embryonic development characterized by formation of an embryonic vesicle without an embryo in mares. J. Am. Vet. Med. Assoc. 217, 58–63.
| PubMed |

Vanderwall, D. K. , Woods, G. L. , Aston, K. I. , Bunch, T. D. , Li, G. , Meerdo, L. N. , and White, K. L. (2004a). Cloned horse pregnancies produced using adult cumulus cells. Reprod. Fertil. Dev. 16, 675–679.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Vanderwall, D. K. , Woods, G. L. , Sellon, D. C. , Tester, D. F. , Schlafer, D. H. , and White, K. L. (2004b). Present status of equine cloning and clinical characterization of embryonic, fetal, and neonatal development of three cloned mules. J. Am. Vet. Med. Assoc. 225, 1694–1699.
| PubMed |

Wells, D. N. , Misica, P. M. , Tervit, H. R. , and Vivanco, W. H. (1998). Adult somatic cell nuclear transfer is used to preserve the last surviving cow of the Enderby Island cattle breed. Reprod. Fertil. Dev. 10, 369–378.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Whitwell, K. E. , and Jeffcott, L. B. (1975). Morphological studies on the fetal membranes of the normal singleton foal at term. Res. Vet. Sci. 19, 44–55.
| PubMed |

Woods, G. L. , White, K. L. , Vanderwall, D. K. , Li, G. P. , Aston, K. I. , Bunch, T. D. , Meerdo, L. N. , and Pate, B. J. (2003). A mule cloned from fetal cells by nuclear transfer. Science 301, 1063.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Young, L. E. , and Fairburn, H. R. (2000). Improving the safety of embryo technologies: possible role of genomic imprinting. Theriogenology 53, 627–648.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



