Placentation in the Amazonian manatee (Trichechus inunguis)
A. M. Carter A , M. A. Miglino B F , C. E. Ambrosio B , T. C. Santos B , F. C. W. Rosas C , J. A. d’Affonseca Neto C , S. M. Lazzarini D , A. F. Carvalho E and V. M. F. da Silva CA University of Southern Denmark, J. B. Winsloewsvej 21, DK 5000 Odense, Denmark.
B University of Sao Paulo, Faculty of Veterinary Medicine and Animal Science, Av. Prof. Dr. Orlando Marques de Paiva 87, Cidade Universitária, 05580-270 Sao Paulo, SP, Brazil.
C National Institute of Amazonian Research, Av. André Araújo, 2936, 69083-000 Manaus, AM, Brazil.
D Centre of Protection and Research of Aquatic Mammals, Rodovia Manaus-Caracarai Km 122, Balbina, 69736-000 Presindente Figueiredo, AM, Brazil.
E School of Veterinary Medicine, Av. Octávio Bastos, Nova São João, 13870-040 Sao João da Boa Vista, SP, Brazil.
F Corresponding author. Email: miglino@usp.br
Reproduction, Fertility and Development 20(4) 537-545 https://doi.org/10.1071/RD08009
Submitted: 18 January 2008 Accepted: 4 March 2008 Published: 11 April 2008
Abstract
Evidence from several sources supports a close phylogenetic relationship between elephants and sirenians. To explore whether this was reflected in similar placentation, we examined eight delivered placentae from the Amazonian manatee using light microscopy and immunohistochemistry. In addition, the fetal placental circulation was described by scanning electron microscopy of vessel casts. The manatee placenta was zonary and endotheliochorial, like that of the elephant. The interhaemal barrier comprised maternal endothelium, cytotrophoblasts and fetal endothelium. We found columnar trophoblast beneath the chorionic plate and lining lacunae in this region, but there was no trace in the term placenta of haemophagous activity. The gross anatomy of the cord and fetal membranes was consistent with previous descriptions and included a four-chambered allantoic sac, as also found in the elephant and other afrotherians. Connective tissue septae descended from the chorionic plate and carried blood vessels to the labyrinth, where they gave rise to a dense capillary network. This appeared to drain into shorter vessels near the chorionic plate. The maternal vasculature could not be examined in the same detail, but maternal capillaries ran rather straight and roughly parallel to the fetal ones. Overall, there is a close resemblance in placentation between the manatee and the elephant.
Additional keywords: Afrotheria, haemophagous organ, phylogeny, Sirenia.
Introduction
The order Sirenia comprises three manatee species, the dugong and the recently extinct Steller’s sea cow (Shoshani 2005). All are aquatic mammals with a herbivorous diet. It has long been recognised that sirenians are closely related to elephants (Simpson 1945). Molecular and morphological evidence also groups them with the hyraxes in a clade named Paenungulata (Gheerbrant et al. 2005; Kellogg et al. 2007). The placentation of the African elephant has been studied in some detail. It is of the zonary, endotheliochorial type and the trophoblast of the interhaemal region is cellular not syncytial (Amoroso and Perry 1964; Perry 1974; Allen et al. 2003; Wooding et al. 2005). In contrast, Sirenia is a high-level clade that is poorly represented in placental research, although the placenta is known to be zonary in manatees and the dugong (Turner 1889; Wislocki 1935). The only detailed study of placentation in a sirenian, namely the West Indian manatee, concluded that the interhaemal region was haemochorial and contained syncytiotrophoblast (Wislocki 1935).
The present study focuses on the placenta of the Amazonian manatee Trichechus inunguis. Our hypothesis was that renewed study of the manatee placenta using a broader range of techniques, including immunohistochemistry and transmission electron microscopy (TEM), would reveal a closer resemblance to that of the elephant than previously had been thought (Wislocki 1935). We were also interested in re-examining the placenta for columnar trophoblast in regions regarded by Wislocki (1935) as haemophagous in function. In addition, by using scanning electron microscopy (SEM) of vessel casts, we aimed to give a more detailed description of the fetal vasculature. Finally, we wanted to confirm that the allantoic sac was partitioned into four chambers. This detail is important because it is shared by members of all the orders included within the Afrotheria (Stanhope et al. 1998) and is one of very few synapomorphies in support of the molecular evidence for this superordinal clade (Carter et al. 2006a; Mess and Carter 2006; Seiffert 2007).
The Amazonian manatee is a freshwater species that is restricted to the Amazon Basin (Husar 1978; Cantanhede et al. 2005). It is hunted for food by the indigenous peoples and is classified as vulnerable by the International Union for Conservation of Nature and Natural Resources (2007). It is therefore desirable to study the reproductive biology of this species. A captive breeding program has been established at the Robin Best Centre for Aquatic Mammals of the National Institute of Amazonian Research (INPA) at Manaus, Brazil, where the first conception and birth of the species in captivity was recorded in 1998. Subsequently, there have been four more deliveries here, as well as at the Centre of Protection and Research of Aquatic Mammals (CPPMA) in Balbina. In addition to its relevance to placentation and phylogenetics, the present study is a contribution to understanding reproduction in a vulnerable member of an order where one of five species became extinct in recent times.
Materials and methods
Manatees were kept in pools in mixed groups of adult males and females until pregnancy was detected. Then, the males were removed and the pregnancy monitored. Labour was detected in all cases except one (a stillborn calf) in the clear water pools at INPA, but not at CPPMA, where the water is always turbid. After birth, we waited for the placenta to be expelled. It was then retrieved with a leaf skimmer net at the end of a long pole. Birthweights are given in Table 1. As an example, the first placenta (mother and calf Boo-Erê; see Table 1) was expelled 40–47 h after birth. It weighed 935 g immediately after recovery from the pool but, 4 h later, after it had been allowed to drain, it weighed 700 g. This placenta was approximately 23 cm in diameter and 1–1.5 cm thick.
We had access to all five placentae delivered at INPA and three additional placentae from CPPMA, which included a premature stillbirth (Table 1). Three of the placentae had been frozen and were useful only for gross anatomy. Three had been fixed in 10% formalin and were used for histology. Two were fixed in formaldehyde and glutaraldehyde for semi-thin sectioning and transmission electron microscopy (TEM). Part of one placenta was used to make injection casts for SEM.
Pieces of tissue were excised and processed for histology by standard methods and embedded in paraffin (Paraplast; Oxford Labware, St Louis, MO, USA) or glycol methacrylate (Historesin; Leica, Wetzlar, Germany). The paraffin-embedded tissue was sectioned at 5 μm and stained with haematoxylin and eosin (HE), Masson’s triple stain and periodic acid-Schiff (PAS) or used for immunohistochemistry.
Tissues from two placentae were immersion-fixed in 2.5% phosphate-buffered glutaraldehyde and 2% formaldehyde, pH 7.4, then rinsed in phosphate-buffered saline (PBS), pH 7.4 (three times for 10 min each time). After fixation, the material was keep in 2% phosphate-buffered osmium tetroxide, pH 7.4, for 2 h. Tissues were then washed in phosphate buffer (three times for 10 min each time) and immersed in 3% uranyl acetate solution overnight. After again being washed in buffer (three times for 10 min each time), tissues were dehydrated in alcohol and immersed in propylene oxide for 10 min. Tissues were then immersed in a 1 : 1 mixture of propylene oxide and araldite (Polysciences, Warrington, PA, USA) for 1 h, in araldite for 12 h and in fresh araldite for 2 h. Finally, tissues were embedded in araldite and baked in an oven at 60°C for 3 days to complete polymerisation. Then, blocks were cut at 1 μm on an automatic ultramicrotome (Ultracut R; Leica Microsystems, Nussloch, Germany) and stained with either a 1% solution of toluidine blue or azure B to identify areas of interest.
Vascular casts
To study the fetal microvasculature, branches of the umbilical arteries and veins were injected with Mercox CL-2R (Okenshoji, Tokyo, Japan) under manual control, as described by Hodde et al. (1977). The procedures were similar to those we and others have applied to a range of placental types (Leiser et al. 1997; Miglino et al. 2004). Tissues were digested by immersion of the preparation in several changes of 10% NaOH solution at 50–60°C. Casts were rinsed thoroughly in distilled water and dried in an oven at 37°C. They were then placed in 20% gelatin and frozen. For SEM, parts of the casts were sectioned while still frozen. Sections were rinsed in distilled water to remove the gelatin, dried and mounted on stubs with conductive carbon cement (Neubauer, Münster, Germany). Sections were then coated with gold using a sputter coater (Model K550; Emitech Products, Houston, TX, USA) and examined in an SEM (Model 435 VP; Leo Electron Microscopy, Cambridge, UK).
Immunohistochemistry
Immunohistochemistry was performed for vimentin, to identify mesenchymal cells and stromal decidua, and α-smooth muscle actin, to identify vessel walls. Sections were rehydrated in an ethanol series and, in the course of this, they were submitted to blockade of endogenous peroxidase with 3% hydrogen peroxide (v/v) in ethanol for 20 min. Sections were then placed in 0.1 m citrate buffer, pH 6.0, and submitted to microwave irradiation at 700 MHz for 15 min. Sections were equilibrated in 0.1 m PBS, pH 7.4, and non-specific binding was blocked using Dako Protein Block (DakoCytomation, Carpinteria, CA, USA) for 20 min.
Tissues were incubated with primary antibodies overnight at 4°C in a humid chamber. Mouse monoclonal anti-human primary antibodies were used to detect vimentin (1 : 200; V9, sc-6260; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and α-smooth muscle actin (1 : 300; Clone 1A4; DakoCytomation). Slices were then rinsed in PBS and incubated with the biotinylated secondary antibody for 45 min, followed by streptavidin–horseradish peroxidase for 45 min (LSAB+ System-HRP; DakoCytomation). After rinsing in PBS, binding was visualised using aminoethyl carbazole (AEC Substrate Kit; Zymed Laboratories, San Francisico, CA, USA) as the chromagen. Sections were counterstained with haematoxylin and mounted in Faramount (DakoCytomation). Negative controls were performed using PBS instead of the primary antibody solution.
Results
Umbilical cord
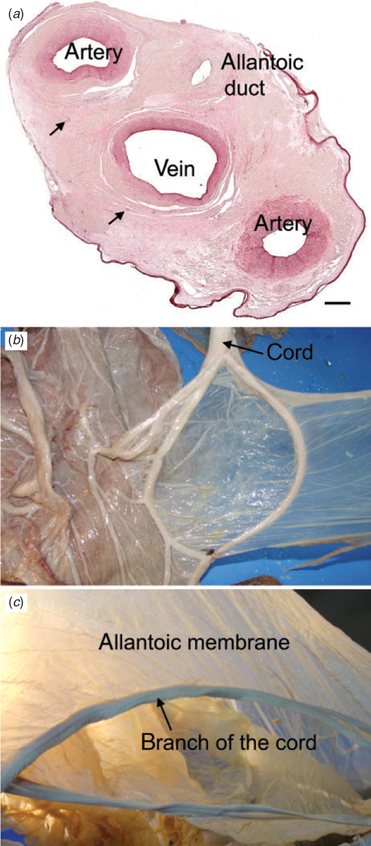
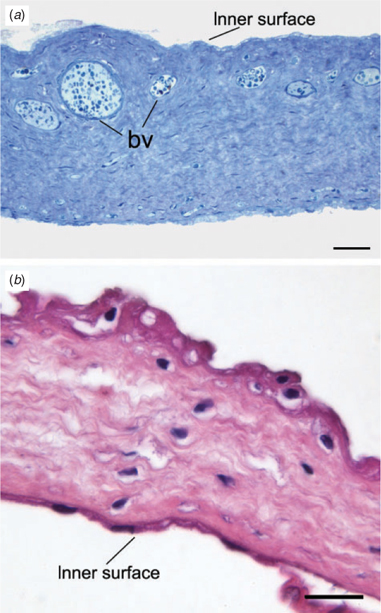
The umbilical cord included two arteries, a vein and the allantoic duct (Fig. 1a). It branched twice, sending four sets of vessels to the placenta (Fig. 1b). The true cord was relatively short compared with the length of the branches. The surface of the cord was covered by amniotic epithelium. In addition to the vasa vasorum of the umbilical arteries and vein, small blood vessels occurred in the stroma.
|
Fetal membranes
The allantois was well supplied with blood vessels (Fig. 2a). It was lined on both surfaces by simple squamous epithelium; on the outer surface, this was extremely attenuated. There were several folds of allantoic membrane. Although the structure was disrupted during parturition, it was possible to reconstruct a four-lobed allantoic sac. The four main branches of the cord subtended the folds of membrane that partitioned the sac into these four lobes (Fig. 1c). The amnion (Fig. 2b) was avascular, but was thicker and tougher than the allantois. The inner surface was lined by simple squamous epithelium. The outer surface was covered by stratified squamous epithelium with regions of squamous metaplasia; there were also some round cells.
|
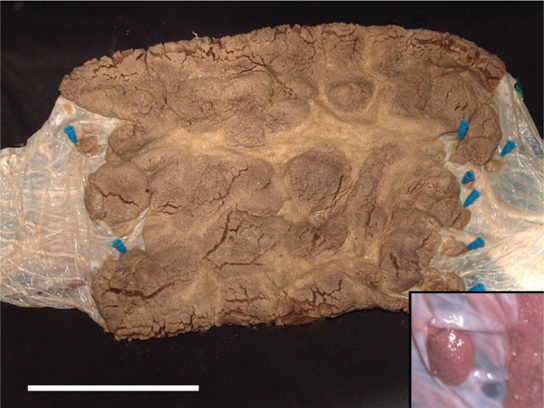
Gross anatomy of the placenta
The gestational sac was shaped like a croissant with the annular placental ring at its centre. Seen from the maternal surface, there were thicker lobes separated by thinner, interlobar areas (Fig. 3). The margins of the placenta were irregular and isolated lobes also occurred (Fig. 3, inset). Filamentous cords extended from the lobes of the delivered placenta. Some were branched. Their length exceeded that of the villi, suggesting they may have provided additional anchorage to the uterine wall.
|
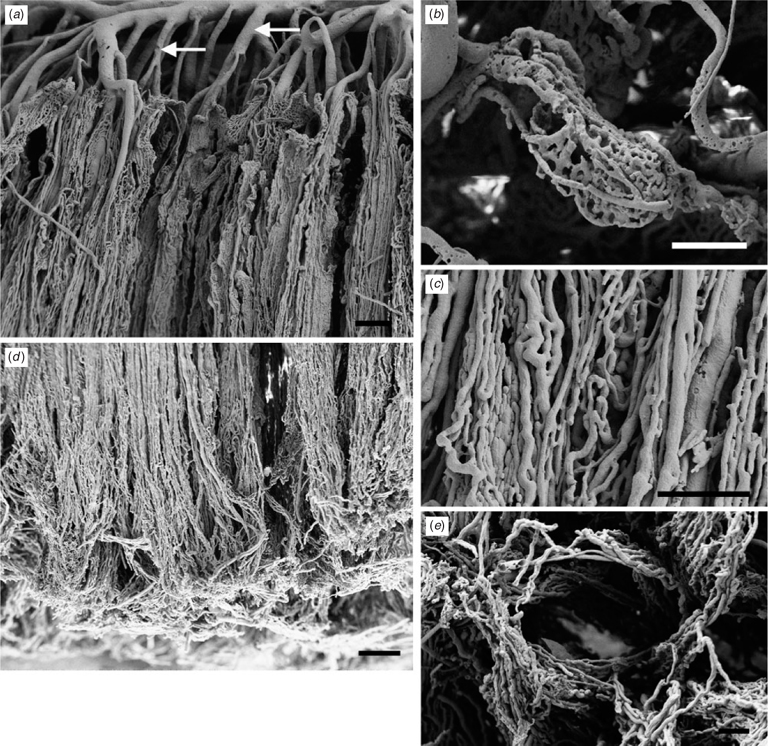
Fetal vasculature
The fetal vasculature was demonstrated by examining vessel casts with SEM. Ramification of the vessels occurred in a region between the chorionic plate and the labyrinth (Fig. 4a). A few of these vessels supplied small baskets of vessels aligned parallel to the chorionic plate (Fig. 4b). Some of the major branches maintained their calibre until reaching the maternal surface of the labyrinth. It is likely that these were arteries and gave rise to the dense capillary network. The shorter branches nearer the chorionic plate would then be veins draining the capillaries. The capillaries ran in parallel through the entire thickness of the labyrinth. They anastomosed with one another and exhibited sinusoidal dilatations (Fig. 4c). Towards the maternal surface of the delivered placenta, the capillaries outlined spaces that, before delivery, probably had been occupied by uterine trabeculae (Fig. 4d, e).
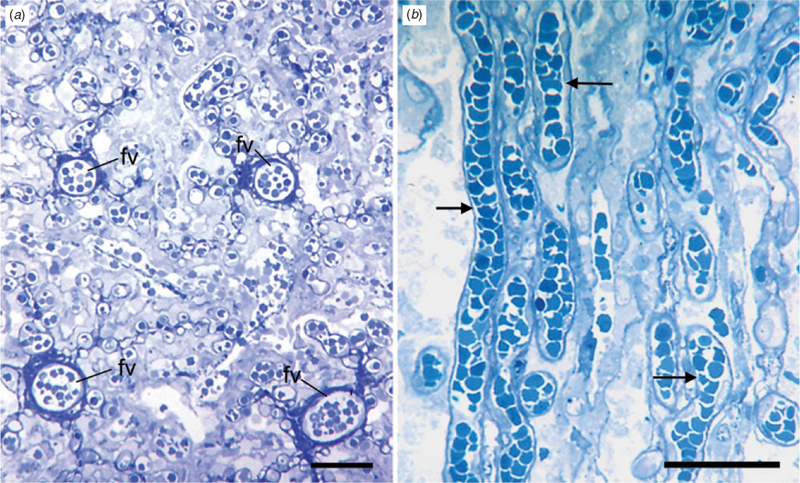
Histology
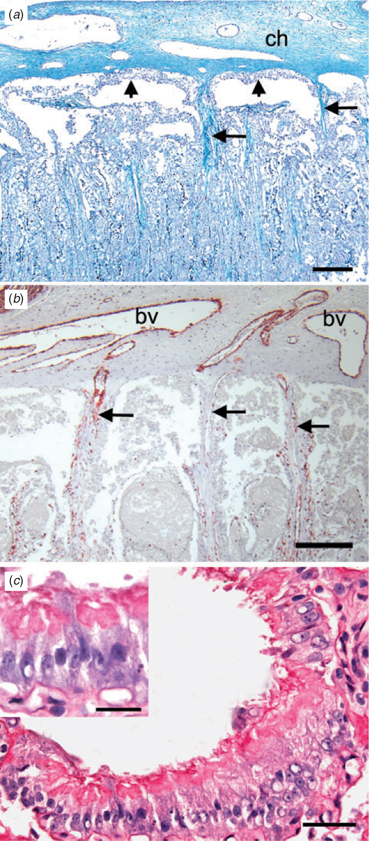
The major branches of the umbilical vessels ran within the connective tissue of the chorionic plate and parallel to its surface (Fig. 5a). Connective tissue septae descended from the outer surface of the plate and carried blood vessels to the labyrinth (Fig. 5b). There were spaces between the surface of the chorion and the labyrinth. These spaces were lined by columnar trophoblast and, as discussed below, corresponded to the haemophagous areas mentioned by Wislocki (1935). We were able to identify additional spaces near the inner surface of the labyrinth that were lined by columnar trophoblast (Fig. 5c). Within the labyrinth, maternal and fetal capillaries ran parallel to one another, separated by cellular trophoblast and some fetal connective tissue. The way in which fetal vessels were distributed was best seen in sections taken in the same plane as the chorionic plate. The larger vessels entered with ample connective tissue and, from them, the smaller vessels radiated out (Fig. 6a). In sections through the labyrinth, it could be seen that the maternal vessels pursued a rather straight course (Fig. 6b), in contrast with what is usually seen in endotheliochorial placentae.
|
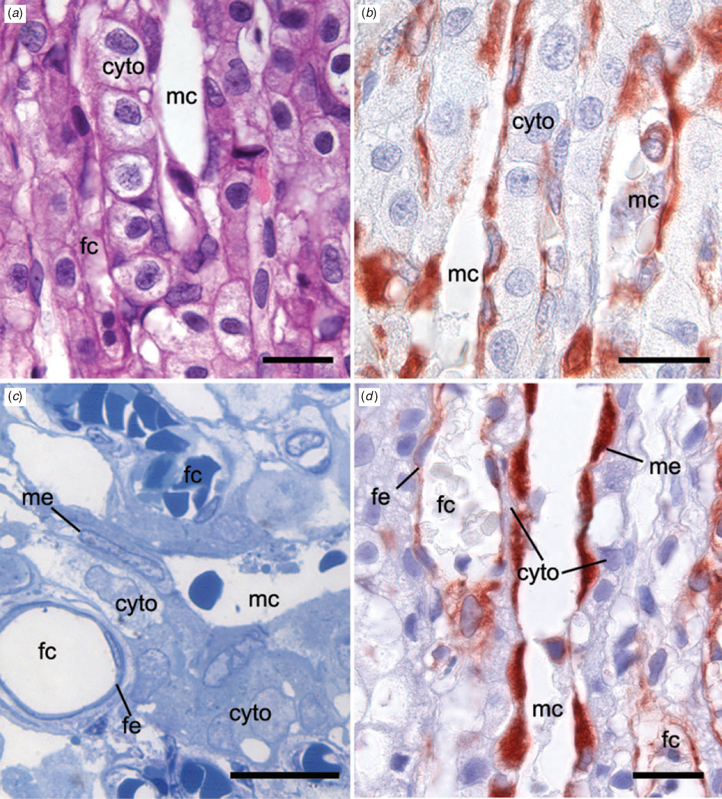
Maternal and fetal capillaries were separated by cytotrophoblasts (Fig. 7a, b) and no evidence of syncytiotrophoblast was found. The endothelia of maternal and fetal vessels were immunopositive for vimentin (Fig. 7b, d) and the cells lining maternal capillaries were larger than normal. The interhaemal barrier comprised fetal capillary endothelium, a single layer of cytotrophoblast and maternal capillary endothelium (Fig. 7c). Fetal mesenchymal cells were found, but did not form part of the interhaemal barrier. In places, attenuation of the cytotrophoblast made the interhaemal distance very short (Fig. 7d).
Ultrastructure
At the ultrastructural level, there was loss of cellular integrity, particularly by the trophoblast and maternal endothelial cells. We attribute this to peripartum hypoxia rather than immersion in fresh water because cells just beneath the chorionic plate were among those affected. It was, however, possible to evaluate the tissue layers comprising the interhaemal barrier and confirm the impression gained from the histology. In addition, we observed that an irregular interstitial lamina lay under the endothelium of the maternal capillaries. The fetal capillaries had a normal basal lamina (data not shown).
Discussion
We have shown that the placenta of the Amazonian manatee is zonary and endotheliochorial. The trophoblast of the interhaemal area is cellular not syncytial. This is at odds with Wislocki’s (1935) interpretation of the manatee placenta as haemochorial with syncytiotrophoblast. Interestingly, Mossman (1987; p. 267) mooted the possibility that the placenta was endotheliochorial, although it is not clear whether he examined the original slides. It is conceivable that Wislocki was misled by the rather straight course of the vessels in the labyrinth because, in other endotheliochorial placentae, the blood vessels tend to pursue a more tortuous course. We had recourse to tools that were not available to Wislocki (1935). First, we used immunohistochemistry to demonstrate that both fetal and maternal vessels were lined by intact endothelium. Second, we applied TEM to confirm this observation and reveal the irregular interstitial lamina supporting the maternal endothelium. This is an interesting feature that the manatee placenta shares with other endotheliochorial placentae. There was thinning of the trophoblast cells to reduce the interhaemal distance, as shown in the elephant by TEM (Wooding et al. 2005). Our interpretation is confirmed by H. Soma (pers. comm.), who has examined delivered placentae from the West Indian manatee, the species studied by Wislocki (1935), as well as the dugong (see Carter et al. 2006b). Therefore, placentation in Sirenia must be regarded as endotheliochorial.
Placentation in the manatee resembles that in the African elephant (Amoroso and Perry 1964; Perry 1974; Allen et al. 2003; Wooding et al. 2005; Allen 2006). This is in agreement with our hypothesis, which was based on morphological, molecular and other evidence that Sirenia and Proboscidea are closely related (Gheerbrant et al. 2005; Kellogg et al. 2007). According to a recent cladistic analysis (Mess and Carter 2006), the earliest common ancestor of eutherian mammals had an endotheliochorial placenta, so this shared characteristic is plesiomorphic. Of greater interest is the presence of cytotrophoblasts in the interhaemal area, a characteristic that is shared with the rock hyrax (Oduor-Okelo et al. 1983). The common ancestor of Eutheria and Afrotheria had syncytiotrophoblast in the interhaemal region (Mess and Carter 2006); thus, the presence of cytotrophoblast is an apomorphic characteristic of Paenungulata.
In the African elephant, there is a prominent haemophagous region along the margins of the placenta where maternal erythrocytes seep into spaces lined by columnar trophoblast and are ingested to provide iron for the fetus (Wooding et al. 2005). Wislocki (1935) described the spaces beneath the chorionic plate of the manatee placenta as haemophagous. He showed that maternal blood vessels communicated with both the capillaries of the labyrinth (which he called blood channels) and trophoblast-lined lacunae, where the blood was stagnant and taken up by the trophoblastic lining (Wislocki 1935, fig. 1). We can confirm the existence of lacunae lined by columnar trophoblast, but have not seen evidence of erythrocyte uptake in the term placenta. It needs to be borne in mind that columnar trophoblast is found in a wide range of placental types where it is heterophagous in nature, taking up uterine secretions, cell debris and the occasional erythrocyte (Enders and Carter 2006).
We could confirm two related features that were well described by Wislocki (1935), who examined a placenta in situ. The cord is short and divides twice to send four leashes of vessels to the placenta. Folds of membrane partition the allantoic sac, forming four chambers, of which two are slightly larger than the others. The four main branches of the cord subtend the folds of the allantoic membrane. The significance of these features is that a four-lobed allantoic sac is a shared derived character (synapomorphy) present in some members of all six orders of Afrotheria (Carter et al. 2006a; Mess and Carter 2006; Seiffert 2007). It is interesting that these features were recognised by early authors, but given different interpretations. Thus, Wislocki (1935) included them in the group of characteristics supporting a relationship between manatees, elephants and hyraxes, whereas Perry (1974) felt that such characteristics were of limited value in assessing phylogenetic affinities. Neither had the advantage of considering the trees established by molecular phylogenetics (Springer et al. 2005); in hindsight, Wislocki was the greater visionary.
Acknowledgements
These studies were supported by The Carlsberg Foundation, Denmark (A.M.C.), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP; M.A.M.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; M.A.M. and V.M.F.S.). The authors are very grateful to Dr Allen C. Enders for his valuable input on the interpretation of the fine structure of the placenta. The authors thank the team of INPA and CPPMA for their dedicated work with the captive manatees.
Allen, W. R. (2006). Ovulation, pregnancy, placentation and husbandry in the African elephant (Loxodonta africana). Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 821–834.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Hodde, K. C. , Miodonski, A. , Bakker, C. , and Veltman, W. (1977). Scanning electron microscopy of microcorrosion casts with special attention on arteriovenous differences and application to the rat’s cochlea. Scan. Electron Microsc. 2, 477–484.
Kellogg, M. E. , Burkett, S. , Dennis, T. R. , Stone, G. , Gray, B. A. , McGuire, P. M. , Zori, R. T. , and Stanyon, R. (2007). Chromosome painting in the manatee supports Afrotheria and Paenungulata. BMC Evol. Biol. 7, 6.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Oduor-Okelo, D. , Musewe, V. O. , and Gombe, S. (1983). Electron microscopic study of the chorioallantoic placenta of the rock hyrax (Heterohyrax brucei). J. Reprod. Fertil. 68, 311–316.
| PubMed |
Simpson, G. G. (1945). The principles of classification and a classification of mammals. Bull. Am. Mus. Nat. Hist. 85, 1–350.
Stanhope, M. J. , Waddell, V. G. , Madsen, O. , de Jong, W. , Hedges, S. B. , Cleven, G. C. , Kao, D. , and Springer, M. S. (1998). Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. Proc. Natl Acad. Sci. USA 95, 9967–9972.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Turner, W. (1889). On the placentation of Halicore dugong. Trans. R. Soc. Edinb. 35, 641–662.

Wislocki, G. B. (1935). The placentation of the manatee (Trichechus latorostris). Mem. Mus. Comp. Zool. Harvard Coll. 54, 158–178.

Wooding, F. B. P. , Stewart, F. , Mathias, S. , and Allen, W. R. (2005). Placentation in the African elephant, Loxodonta africana: III. Ultrastructural and functional features of the placenta. Placenta 26, 449–470.
| Crossref | GoogleScholarGoogle Scholar | PubMed |