Effects of spermatozoa–oviductal cell coincubation time and oviductal cell age on spermatozoa–oviduct interactions
Ahmed Aldarmahi A , Sarah Elliott A , Jean Russell B and Alireza Fazeli A CA Academic Unit of Reproductive and Developmental Medicine, University of Sheffield, Level 4, The Jessop Wing, Tree Root Walk, Sheffield S10 2SF, UK.
B Corporate and Computing Services, University of Sheffield, Sheffield S3 7RF, UK.
C Corresponding author. Email: a.fazeli@sheffield.ac.uk
Reproduction, Fertility and Development 26(2) 358-365 https://doi.org/10.1071/RD12222
Submitted: 10 July 2012 Accepted: 30 January 2013 Published: 4 April 2013
Abstract
The oviduct plays a crucial role in sperm storage, maintenance of sperm viability and sperm transport to the site of fertilisation. The aim of the present study was to investigate the effects of oviductal cell culture passage number, oviductal cell age and spermatozoa–oviduct coincubation times on gene expression in oviductal cells. Immortalised oviductal epithelial cells (OPEC) obtained from two different cell passages (36 and 57) were subcultured three times with and without spermatozoa for 24 h (control group). In a second study, OPEC were cocultured with spermatozoa for different time intervals (0, 4, 12 and 24 h). Expression of adrenomedullin (ADM), heat shock 70 kDa protein 8 (HSPA8) and prostaglandin E synthase (PGES) in OPEC was measured by quantitative polymerase chain reaction. The expression of ADM and HSPA8 was decreased significantly in OPEC cells from Passage 57, particularly in the later subculture group. These effects on HSPA8, but not ADM, expression in OPEC were further altered after coculture with spermatozoa for 24 h. We also demonstrated that spermatozoa–oviduct coculture for 12 and 24 h resulted in significantly higher expression of ADM, HSPA8 and PGES in OPEC. Overall, the data suggest that the OPEC lose some of their properties as a result of oviductal cell aging and that there are spermatozoa–oviduct interactions leading to increased oviductal cell gene expression.
Additional keywords: immortalised epithelial cells, in vitro culture, real-time polymerase chain reaction, spermatozoa.
Introduction
The oviduct plays a crucial role in sperm storage, maintenance of sperm viability and sperm transport to the site of fertilisation (Hunter 1981; Hunter and Nichol 1983; Menezo and Guerin 1997). In mammalian species, spermatozoa are transported through the female reproductive tract to reach the isthmic region of the oviduct, where they bind to the ciliated epithelial cells. In vivo, spermatozoa–oviductal epithelial cell binding prolongs sperm survival, stabilises the acrosome, induces sperm capacitation and creates a sperm reservoir (Scott 2000; Hunter and Birkhead 2002; Rodriguez-Martinez 2007).
Similarly, several oviductal epithelial cell culture systems have been shown to maintain the viability of spermatozoa and induce capacitation (Kervancioglu et al. 1994; Morales et al. 1996). In vitro cocultures from different species, including human, have been successfully established and characterised (Bongso et al. 1989; Thibodeaux et al. 1992; Hombach-Klonisch et al. 2006). These culture systems have been used to obtain valuable information on gene and protein expression (Umezu et al. 2003). They have also been used to gain a better understanding of the maternal interaction with gametes and embryos (Lee et al. 2002; Fazeli et al. 2004; Georgiou et al. 2007; Kodithuwakku et al. 2007; Ulbrich et al. 2010). Sperm–oviduct epithelial cell in vitro systems have been used in various species to study the physiology and molecular events of spermatozoa–oviduct interaction. Primary cell cultures can be used to study spermatozoa–oviductal interactions, but the primary cell cultures have a limited lifespan and tend to undergo cellular dedifferentiation in culture (Mulholland et al. 1988).
In contrast with in vivo models, in vitro models are more simple and easier to define. Hence, they provide useful means of understanding complex molecular interactions taking place in different biological systems. However, there are still several shortcomings in using in vitro cell culture. Specific features and functions of the oviductal epithelium are lost during in vitro culture (Bongso et al. 1989). Some studies report that the level of mRNA expression is altered with increasing culture passage number and presume that dedifferentiation of differentiated cells takes place in vitro after several cell culture passages (Neumann et al. 2010). In a previous study, we demonstrated that expression of adrenomedullin (ADM), heat shock 70 kDa protein 8 (HSPA8) and prostaglandin E synthase (PGES) in an immortalised oviductal epithelial cell line decreased as the number of oviductal culture passages increased (Aldarmahi et al. 2012). However, it was not clear whether the variation between different cell passages was affected by the handling of cells on different experimental days and/or altered in response to coculture with spermatozoa. Therefore, the aim of the present experiment study was to identify whether the decline in oviductal cell ADM and HSPA8 gene expression after increased passage of cell cultures is related to the age of the cells used or to differences in the handling of cells from one day to another. We also investigated the effects of different spermatozoa–oviductal cell incubation times on ADM, HSPA8 and PGES gene expression in oviductal epithelial cells (OPEC) in our defined in vitro spermatozoa–oviductal cell interaction system.
Materials and methods
Sperm preparation
Boar semen was obtained from an AI company (JSR Genetic, Thorpe Willoughby, UK). After collection, ejaculates were filtered through gauze and subsequently diluted 1 : 9 (v/v) in Beltsville thawing solution (Pursel and Johnson 1975). The viability of the samples was assessed and determined upon arrival in the laboratory.
On the day of the experiment, boar semen was washed through a two-step gradient of 70% and 30% iso-osmotic Percoll (GE Healthcare, Amersham, UK). To prepare the iso-osmotic Percoll, 9 mL Percoll was mixed with 1 mL of 10× HEPES; to prepare 70% Percoll, 7 mL iso-osmotic Percoll was mixed with 3 mL of 1× HEPES; finally, to prepare 30% Percoll, 3 mL iso-osmotic Percoll was mixed with 7 mL of 1× HEPES. For gradient separation, 2 mL of the 70% (v/v) Percoll was added to a 15-mL conical tube and then overlaid with 2 mL of 30% (v/v) Percoll (Harrison 1976). The diluted semen (4 mL) was then placed on top of the Percoll. The gradient tube was centrifuged at 200g for 15 min, followed by another 15 min at 1000g at room temperature. The supernatant was discarded carefully and the pellet was resuspend in 2 mL modified Tyrode's solution (Parrish et al. 1988) and centrifuged at 1000g for 15 min at room temperature. The supernatant was then removed and the pellet was resuspend in 4 mL Tyrode’s albumin lactate pyruvate (TALP) medium, pH 7.2 (Fazeli et al. 1999). The concentration of washed spermatozoa (1 × 106 spermatozoa mL–1) was adjusted by using an Improved Neubauer counting chamber (CAMLAB, Cambridge, UK). The entire procedure was undertaken using aseptic techniques at room temperature and the washed spermatozoa were used immediately.
The viability of the samples was assessed and determined using ethidium homodimer and Calcein-AM (Viability/Cytotoxicity kit; Molecular Probes, Eugene, OR, USA). The dyes were added to 100 µL semen aliquots (5 × 106 mL–1) to final concentrations of 0.08 µM ethidium homodimer and 0.4 µM Calcein-AM. Samples were mixed and incubated for 30 min at 39°C in 5% v/v CO2. An aliquot (10 µL) of each semen sample was placed on a slide and evaluated under a fluorescence microscope (CKX41; Olympus, Southend-on-Sea, UK) using a dual rhodamine–fluorescein isothiocyanate (FITC) filter. Three slides were prepared for each sample and a minimum of 200 spermatozoa was evaluated and observed at ×40 magnification. Green fluorescent spermatozoa were classified as live, whereas red fluorescent spermatozoa were classified as dead.
Oviducal epithelial cell culture
Porcine telomerase reverse transcriptase (TERT)-immortalised OPEC were provided by Dr Sabine Hombach-Klonisch (University of Manitoba, Winnipeg, Canada; Hombach-Klonisch et al. 2006). Originally, the porcine oviductal cells were collected from gilts at the pro-oestrous stage of the sexual cycle between 210 and 215 days of age weighing 115–120 kg (Hombach-Klonisch et al. 2006). The TERT-OPEC were cultured with Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F-12 (DME/F12; Sigma, Poole, UK) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 200 mM l-glutamine, 160 ng mL–1 human insulin (Invitrogen, Paisley, UK), 1 nM β-oestradiol (Sigma) and 1% antibiotic antimycotic solution containing 1% (v/v) penicillin G, streptomycin and 0.5% (v/v) amphotericin B (Sigma). Cells were cultured at 37°C under 5% CO2 and 95% humidity. Cells were detached with 2 mL trypsin–EDTA (Sigma), containing 0.5 mg mL–1 trypsin and 0.2 mg mL–1 EDTA, for 5–7 min at 37°C.
The TERT-OPEC were initially cultured in T-75 flasks (Greiner, Frickenhausen, Germany) and then subcultured into six-well plates (Greiner) under the same conditions. Only plates with confluent cells (>80% confluency) were used in the experiments. The cellular integrity of the cells was assessed using the Trypan blue (Sigma) viability test. Cells were frozen and thawed in order to culture different cell passages on the same day under the same conditions using our in-house freezing and thawing protocol. Briefly, the freezing medium was made up of Ham’s F-12 (Sigma) supplemented with 10% dimethylsulfoxide (DMSO; Sigma) and 20% heat-inactivated FBS (Invitrogen). No antibiotics were added. The freezing medium was kept in an ice-water bath. The OPEC were harvested from the T-75 flasks using a standard protocol (washing with Ca2+/Mg2+-free buffer). A 1-mL aliquot of each cell suspension was centrifuged at 300g for 5 min at 4°C to pellet the cells. The supernatant was discarded and the pellet was resuspended in freezing medium. Aliquots of the cell suspension were transferred into cryovials and kept in an ice-water bath (0–1°C) for 15–20 min. The cryovials were transferred into freezing containers (Nalgene, Rochester, NY, USA) and stored at –80°C overnight before being transferred to liquid nitrogen. For thawing, the cryovials were defrosted quickly in warm water and seeded into prepared flasks with Ham’s F-12 medium. The flasks were cultured at 37°C under 5% CO2 and 95% humidity until they reached confluence.
RNA extraction and purification, and cDNA synthesis
Total RNA was extracted using TRI Reagent (Sigma) according to the manufacturer’s instructions. To remove any potential genomic DNA contamination from the samples, the extracted RNA was treated with DNase I (DNA-free; Ambion, Huntingdon, UK) according to the manufacturer’s instructions. Subsequently, the quantity and quality of the RNA were determined using a NanoDrop ND-1000 Spectrophotometer (Labtech International, Ringmer, UK) at wavelengths of 260 and 280 nm. The extracted RNA was only used when the ratio of absorption at 260 and 280 nm (Abs260/Abs280) was between 1.8 and 2.02. The quality of the extracted RNA was determined using a BioAnalyzer (Agilent, West Lothian, UK). First-strand cDNA was synthesised using 0.3 µg µL–1 oligo(dT)12–18 primers (Metabion, Martinsried, Germany) and 1–5 µg total RNA. Reverse transcription (RT) was performed using a SuperScript II reverse transcriptase system (200 U µL–1; Invitrogen) according to the manufacturer’s instructions. Escherichia coli RNase H (3 units; Ambion) was added to remove any contaminating RNA. A negative control (no reverse transcriptase added) was included for all samples. The cDNA samples were stored at –20°C until further analysis.
Primer design
Forward and reserve primers for the selected genes were designed to span introns or to bridge an exon–exon junction (exon boundaries). All primers were tested for specificity using National Center for Biotechnology Information (NCBI) blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed 1 January 2011) and for primer–dimer and secondary structure using the DNA analysis tools on the integrated DNA technology website (http://eu.idtdna.com/scitools/scitools.aspx, accessed 1 January 2011). Primers for β-actin (BACT), ADM, HSPA8 and PGES were designed from the DNA sequence of Sus scrofa species, as described previously (Aldarmahi et al. 2012). The efficiencies of the reference and targets genes were assessed and determined. The primers were purchased from Integrated DNA Technology (Leuven, Belgium).
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qPCR) was performed in triplicate for each biological replicate using SYBR Green Jump Start Taq Ready mix (Sigma). The Master mix contained 1 µL each primer (200 nmol), 1 µL cDNA, 10 µL SYBR Green and 7 µL RNase-Free Water in a total volume of 20 µL in each well of a 96-well plate (Greiner). The amplification conditions were 95°C for 30 s for DNA denaturation, followed by 55°C for 1 min to anneal the primers and 72°C for 1 min to extend the primers. These conditions were repeated for 40 cycles. The final extension was at 72°C for 3 min. One positive control (1 µL each cDNA sample) and two negative controls (no template control and no RT control) were run for each experiment. Primer efficiency and melting curves were also determined. All qPCR runs were performed on the Stratagene 3005x (Agilent, Santa Clara, CA, USA). Cycle threshold (CT) values were normalised against threshold values for the reference BACT gene. To assess the stability of the reference BACT gene during the experiments, a panel of β-actin CT values from qPCR were compared. The BACT gene is stably expressed in oviductal epithelial cells and has been used in different investigations as a single reference gene for normalisation of qPCR data (Lee et al. 2001; Ebers et al. 2009; Shin et al. 2010). We also complied with minimum information for publication of quantitative real-time polymerase chain reaction experiment (MIQE) guidelines to ensure reliable and reproducible data (Bustin et al. 2009).
Data and statistical analysis
The qPCR data were analysed using the comparative CT method. Briefly, the difference in cycle threshold (ΔCT) was determined as the difference between the number of cycles required for amplification of the test and reference genes. The relative RNA expression levels for all samples were then calculated using the 2(–ΔΔCT) method (Winer et al. 1999). Then, ΔΔCT was determined by finding the difference between the groups (Livak and Schmittgen 2001). All RNA expression data were imported into Microsoft Office Excel 2007 (Microsoft UK, Readings, UK) and analysed using STATISTICA 7.0 (StatSoft, Inc., Tulsa, OK, USA). Two-way analysis of variance (ANOVA) was used to compare the effects of passage and day factors on changes in oviductal epithelial cell gene expression. Paired t-tests were used in the second experiment to compare OPEC–spermatozoa to the control OPEC group. Results are expressed as the mean ± s.e.m. P < 0.05 was considered significant.
Experimental design
Effects of cell age and cell handling on the OPEC response to coculture with spermatozoa
Two cell cultures were prepared at Passages 36 and 57. Each passage was subcultured on consecutive days to produce three subcultures (P1, P2 and P3). These cell cultures were frozen until the day of the experiment. On each experimental day, one passage from each culture was thawed and used in the studies. These subcultures were: Passages 36P1 and 57P1 used on Day 1 of experiments; 36P2 and 57P2 used on Day 2; and 36P3 and 57P3 used on Day 3. Cells were cultured in six-well plates with spermatozoa (1 × 106 spermatozoa mL–1) in a total volume of 1 mL at 37°C under 5% CO2 and 95% humidity for 24 h. The control consisted of OPEC alone without spermatozoa. After coincubation, RNA was extracted from the OPEC for the synthesis of cDNA. Expression of ADM and HSPA8 mRNA was determined by qPCR. There were three biological replicates that were each measured three times (n = 9).
Effects of spermatozoa–OPEC coincubation time on OPEC gene expression
The OPEC were cultured in six-well plates and then cocultured with spermatozoa (1 × 106 spermatozoa mL–1) at 37°C under 5% CO2 and 95% humidity for different times (0, 4, 12 and 24 h). At each time point, two groups were cultured; one group with spermatozoa and the other group alone, without spermatozoa, as a control. The experiment was repeated three times for each time point and each biological replicate was measured three times (n = 9). At all time points, OPEC expression of ADM, HSPA8 and PGES was determined by qPCR after RNA extraction and cDNA synthesis.
Results
Expression of the reference gene BACT did not differ significantly between experimental treatments or cell passages and was an appropriate reference gene for normalisation. The qPCR efficiency value for the reference gene BACT was 99.2%, whereas those for the genes ADM, HSPA8 and PGES were 100.1%, 91.6% and 96.5%, respectively.
Effects of cell age and cell handling on the OPEC response to coculture with spermatozoa
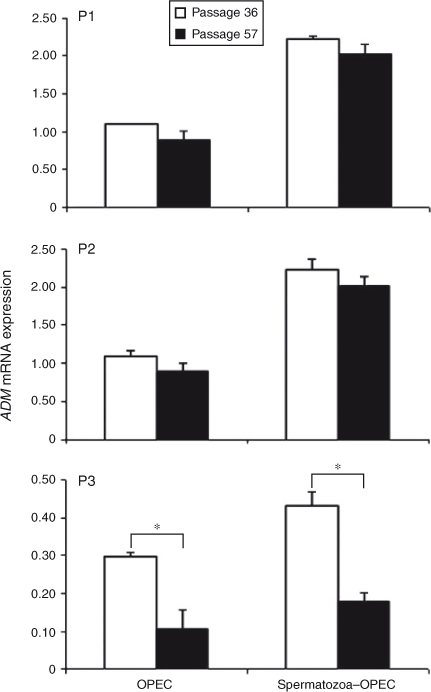
There was no effect of cell passage on the expression of ADM in subcultures P1 and P2 in the absence or presence of spermatozoa, but it was significantly decreased in subculture (P3) of Passage 57 compared with Passage 36 (Fig. 1). The presence of spermatozoa with OPEC increased ADM expression in Passages 36 and 57 in subcultures P1 and P2, but only slightly increased ADM expression in Passage 36 cells at P3 (Fig. 1).
|
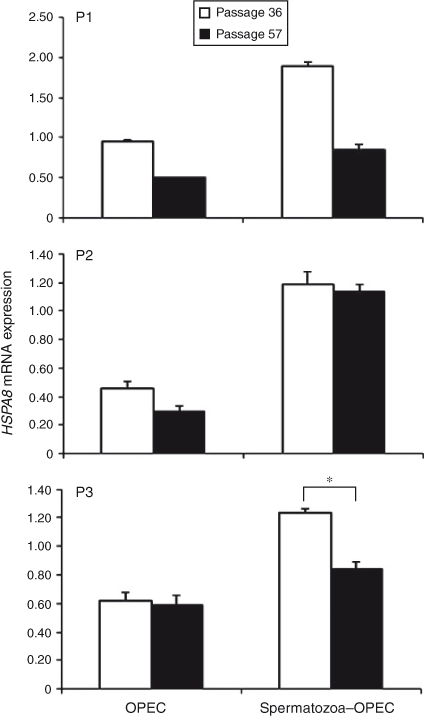
In contrast, HSPA8 gene expression was significantly lower in cells obtained from Passage 57 compared with Passage 36 in all subcultures (P1, P2 and P3), but the effects of coculture with spermatozoa differed between the subculture groups (Fig. 2). At P1 of Passage 57, HSPA8 expression was decreased in OPEC cultured with and without spermatozoa compared with Passage 36. However, in P2 of Passage 57, only OPEC cultured without spermatozoa exhibited lower HSPA8 expression, whereas at P3 of Passage 57 only OPEC cocultured with spermatozoa exhibited decreased HSPA8 expression. In general, the presence of spermatozoa with OPEC increased HSPA8 expression in both Passages 36 and 57, although the effect was most evident in Passage 36 cells.
|
Effects of spermatozoa–OPEC coincubation time on OPEC gene expression
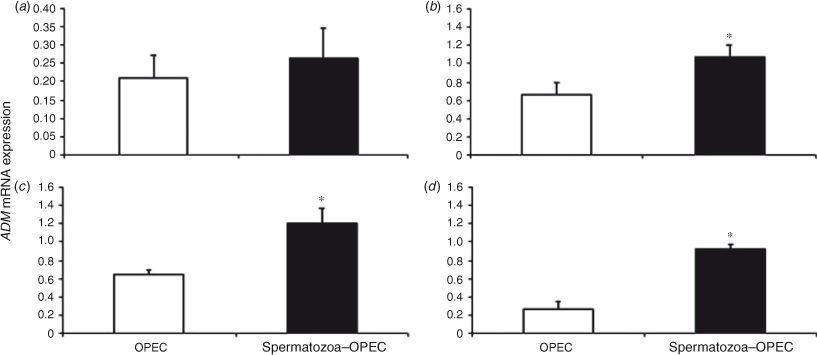
Over the course of time, ADM expression did not change significantly in OPEC cultured with or without spermatozoa. However, direct comparison between the two treatment groups revealed higher ADM in OPEC cocultured with spermatozoa for 4, 12 and 24 h (Fig. 3). Similarly, there was no significant change in HSPA8 or PGES expression in OPEC over time, but direct comparisons between groups demonstrated higher gene expression in OPEC cocultured with spermatozoa for 12 and 24 h (Figs 4, 5).
Discussion
The first aim of the present study was to determine whether the decline in ADM and HSPA8 gene expression in OPEC after increased cell culture passage is related to the age of the cells or to differences in the handling of cells from one day to another. We designed the experiments to expose OPEC from different passages to spermatozoa in one experimental day. Therefore, if a decrease in gene expression was observed between cells with increasing passage number, then it could not be the result of the different handling of cells on different experimental days. We showed that OPEC from Passage 57 and subculture P3 were more vulnerable to changes in gene expression compared with Passage 36 cells. In some instances, particularly in the case of HSPA8 expression, there were differential effects of coculture with spermatozoa. These findings suggest that the OPEC may lose some of their properties as a result of oviductal cell aging. Neumann et al. (2010) also reported that the level of mRNA expression was altered with increasing culture passage number in primary rheumatoid arthritis synovial fibroblasts. Early passages (<3rd passage) were compared with later passages (>7th passage) to evaluate changes in gene expression as a consequence of cell culture. Gene expression changed by over 10% between the early and later passages (Neumann et al. 2010). In addition, Lin et al. (2007) reported dedifferentiation of chondrocyte primary cells during culture passage and significantly lower relative gene expression in a chondrocyte monolayer culture compared with cartilage tissue.
Changes in cell morphology, responses to stimuli, growth rates, gene expression profiles and protein production are frequent with increased cell passaging and aging (Chang-Liu and Woloschak 1997; Wenger et al. 2004). There were significant differences in cell differentiation of low- versus high-passage human colorectal adenocarcinoma (Caco-2) cells, and alkaline phosphatase activity was also reduced in high-passage Caco-2 cells (Yu et al. 1997). Immortalised cells serve as an alternative cell source to investigate functional spermatozoa–oviductal interactions and have the advantage of providing a constant source of a particular cell type compared with primary cell culture. For this reason we opted to use the immortalised OPEC line. However, we found that cell aging may also affect these cells despite the fact that they are immortalised. This point should be taken into consideration when experiments are designed and conducted over a longer period of time.
We also investigated the effects of different spermatozoa–oviductal cell incubation times on ADM, HSPA8 and PGES gene expression in OPEC in our defined in vitro spermatozoa–oviductal cell interaction system. Changes in ADM, HSPA8 and PGES gene expression were observed after 12 and 24 h incubation of OPEC with spermatozoa. Previous work demonstrated that direct contact of spermatozoa with oviductal cells was essential for the upregulation of ADM expression in oviductal tissue and in OE-E6/E7 cells cocultured with spermatozoa (Li et al. 2010). These data suggest that the spermatozoa–oviduct interaction initiates a specific transduction cascade to alter gene expression in OPEC. However, it is unclear whether the induction of gene expression in OPEC results from spermatozoa binding to these cells. It has been proposed that spermatozoa–oviduct binding is mediated by lectin-like secretory proteins from the male genital tract, which become associated with the sperm surface during ejaculation (Topfer-Petersen 1999; Suarez 2002). In pigs, complex mannose structures are thought to be involved in the carbohydrate-based initial spermatozoa–oviduct binding mechanism (Green et al. 2001; Wagner et al. 2002). In the present experiments we demonstrated that the presence of spermatozoa in coculture with oviductal cells alters oviductal cell gene expression. However, it is important to note that we measured gene expression in many oviductal cells in culture irrespective of the individual oviductal cell interaction with spermatozoa. It is unclear whether changes in gene expression occur as a result of spermatozoa contact with a limited number of oviductal cells or most of them.
In conclusion, our data suggest that the OPEC lose some of their properties as a result of oviductal cell aging, despite being immortalised epithelial cells. This has important implications for the study of spermatozoa–oviduct interactions in cell culture systems. In addition, we showed that there are spermatozoa–oviduct interactions leading to increased oviductal cell gene expression, although the effect of a single spermatozoon on an individual OPEC remains to be determined.
References
Aldarmahi, A., Elliott, S., Russell, J., Klonisch, T., Hombach-Klonisch, S., and Fazeli, A. (2012). Characterisation of an in vitro system to study maternal communication with spermatozoa. Reprod. Fertil. Dev. 24, 988–998.| Characterisation of an in vitro system to study maternal communication with spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Grs7vP&md5=9fc7300090dbcec41fed3abc29981f1bCAS | 22935160PubMed |
Bongso, A., Ng, S. C., Sathananthan, H., Ng, P. L., Rauff, M., and Ratnam, S. S. (1989). Establishment of human ampullary cell cultures. Hum. Reprod. 4, 486–494.
| 1:STN:280:DyaK3c%2FgvFansw%3D%3D&md5=ce4b814380e314cf65444afbef91dd5fCAS | 2794010PubMed |
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M. W., Shipley, G. L., Vandesompele, J., and Wittwer, C. T. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622.
| The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktVWqs7g%3D&md5=540b071d42e66eb1d74d96cb642fb4e1CAS | 19246619PubMed |
Chang-Liu, C. M., and Woloschak, G. E. (1997). Effect of passage number on cellular response to DNA-damaging agents: cell survival and gene expression. Cancer Lett. 113, 77–86.
| Effect of passage number on cellular response to DNA-damaging agents: cell survival and gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXht1Khsb8%3D&md5=d9c986ff58dfeede5a779f299ff09f48CAS | 9065805PubMed |
Ebers, K. L., Zhang, C. Y., Zhang, M. Z., Bailey, R. H., and Zhang, S. (2009). Transcriptional profiling avian beta-defensins in chicken oviduct epithelial cells before and after infection with Salmonella enterica serovar Enteritidis. BMC Microbiol. 9, 153.
| Transcriptional profiling avian beta-defensins in chicken oviduct epithelial cells before and after infection with Salmonella enterica serovar Enteritidis.Crossref | GoogleScholarGoogle Scholar | 19642979PubMed |
Fazeli, A., Duncan, A. E., Watson, P. F., and Holt, W. V. (1999). Sperm–oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol. Reprod. 60, 879–886.
| Sperm–oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVGru7k%3D&md5=15a0627e8744591789e1523310c67240CAS | 10084961PubMed |
Fazeli, A., Affara, N. A., Hubank, M., and Holt, W. V. (2004). Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 71, 60–65.
| Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFKktLY%3D&md5=b8610d8dfad0899ffa343262cbc678a3CAS | 14973272PubMed |
Georgiou, A. S., Snijders, A. P., Sostaric, E., Aflatoonian, R., Vazquez, J. L., Vazquez, J. M., Roca, J., Martinez, E. A., Wright, P. C., and Fazeli, A. (2007). Modulation of the oviductal environment by gametes. J. Proteome Res. 6, 4656–4666.
| Modulation of the oviductal environment by gametes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWmu73O&md5=8fa3c4314ecb940b90356ab83af6ff42CAS | 18004800PubMed |
Green, C. E., Bredl, J., Holt, W. V., Watson, P. F., and Fazeli, A. (2001). Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro. Reproduction 122, 305–315.
| Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFWjtb0%3D&md5=b67ad09c348fb1e135e379d2952fe029CAS | 11467982PubMed |
Harrison, R. A. (1976). A highly efficient method for washing mammalian spermatozoa. J. Reprod. Fertil. 48, 347–353.
| A highly efficient method for washing mammalian spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s%2FltlKksA%3D%3D&md5=8b2790421e803d06a4f23f37dd7e89afCAS | 994106PubMed |
Hombach-Klonisch, S., Pocar, P., Kauffold, J., and Klonisch, T. (2006). Dioxin exerts anti-estrogenic actions in a novel dioxin-responsive telomerase-immortalized epithelial cell line of the porcine oviduct (TERT-OPEC). Toxicol. Sci. 90, 519–528.
| Dioxin exerts anti-estrogenic actions in a novel dioxin-responsive telomerase-immortalized epithelial cell line of the porcine oviduct (TERT-OPEC).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xit1Ons7k%3D&md5=38a3f5b391f79eecabd7f66cd7402699CAS | 16431846PubMed |
Hunter, F. M., and Birkhead, T. R. (2002). Sperm viability and sperm competition in insects. Curr. Biol. 12, 121–123.
| Sperm viability and sperm competition in insects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtVKru7s%3D&md5=882dc078a99e7274dabba3eff5dfbd9bCAS | 11818062PubMed |
Hunter, R. H. (1981). Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J. Reprod. Fertil. 63, 109–117.
| Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL38%2FhtVeqtA%3D%3D&md5=6f133cf739c239d42cd2cc02949c54c8CAS | 6895091PubMed |
Hunter, R. H., and Nichol, R. (1983). Transport of spermatozoa in the sheep oviduct: preovulatory sequestering of cells in the caudal isthmus. J. Exp. Zool. 228, 121–128.
| Transport of spermatozoa in the sheep oviduct: preovulatory sequestering of cells in the caudal isthmus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c7htlCjtw%3D%3D&md5=ef8131a544de494cdff00f956427160bCAS | 6663251PubMed |
Kervancioglu, M. E., Djahanbakhch, O., and Aitken, R. J. (1994). Epithelial cell coculture and the induction of sperm capacitation. Fertil. Steril. 61, 1103–1108.
| 1:STN:280:DyaK2c3lt1Cgsg%3D%3D&md5=b6d55fd5e5ad8973cb5f353a1f7a805cCAS | 8194625PubMed |
Kodithuwakku, S. P., Miyamoto, A., and Wijayagunawardane, M. P. (2007). Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reproduction 133, 1087–1094.
| Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVGnu7Y%3D&md5=e06579204d199c8da22f3acb4c9b14d5CAS | 17636163PubMed |
Lee, K. F., Chow, J. F., Xu, J. S., Chan, S. T., Ip, S. M., and Yeung, W. S. (2001). A comparative study of gene expression in murine embryos developed in vivo, cultured in vitro, and cocultured with human oviductal cells using messenger ribonucleic acid differential display. Biol. Reprod. 64, 910–917.
| A comparative study of gene expression in murine embryos developed in vivo, cultured in vitro, and cocultured with human oviductal cells using messenger ribonucleic acid differential display.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsVKjtrY%3D&md5=49b3d26e976ac803776bd74249afa514CAS | 11207208PubMed |
Lee, K. F., Yao, Y. Q., Kwok, K. L., Xu, J. S., and Yeung, W. S. (2002). Early developing embryos affect the gene expression patterns in the mouse oviduct. Biochem. Biophys. Res. Commun. 292, 564–570.
| Early developing embryos affect the gene expression patterns in the mouse oviduct.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitFeitL8%3D&md5=877afebe0234d6047570b25b0db26e7eCAS | 11906198PubMed |
Li, H. W., Liao, S. B., Chiu, P. C., Tam, W. W., Ho, J. C., Ng, E. H., Ho, P. C., Yeung, W. S., Tang, F., and O, W. S. (2010). Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J. Clin. Endocrinol. Metab. 95, E18–E25.
| Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium.Crossref | GoogleScholarGoogle Scholar | 20534761PubMed |
Lin, C. Y., Strom, A., Li Kong, S., Kietz, S., Thomsen, J. S., Tee, J. B., Vega, V. B., Miller, L. D., Smeds, J., Bergh, J., Gustafsson, J. A., and Liu, E. T. (2007). Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 9, R25.
| Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer.Crossref | GoogleScholarGoogle Scholar | 17428314PubMed |
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFelt7s%3D&md5=9cad4ff7133d540452bb50aaaa4ededfCAS | 11846609PubMed |
Menezo, Y., and Guerin, P. (1997). The mammalian oviduct: biochemistry and physiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 73, 99–104.
| The mammalian oviduct: biochemistry and physiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslemtLg%3D&md5=9c604c0ed7f94eee95d5246b043bf06eCAS | 9175697PubMed |
Morales, P., Palma, V., Salgado, A. M., and Villalon, M. (1996). Sperm interaction with human oviductal cells in vitro. Hum. Reprod. 11, 1504–1509.
| Sperm interaction with human oviductal cells in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zisVKqtw%3D%3D&md5=ea069517b51fada8ec65303fd7988966CAS | 8671493PubMed |
Mulholland, J., Winterhager, E., and Beier, H. M. (1988). Changes in proteins synthesized by rabbit endometrial epithelial cells following primary culture. Cell Tissue Res. 252, 123–132.
| Changes in proteins synthesized by rabbit endometrial epithelial cells following primary culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXhs1Gnsro%3D&md5=12bc1da4d67902c05c5e4545bc383d56CAS | 3378256PubMed |
Neumann, E., Riepl, B., Knedla, A., Lefevre, S., Tarner, I. H., Grifka, J., Steinmeyer, J., Scholmerich, J., Gay, S., and Muller-Ladner, U. (2010). Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res. Ther. 12, R83.
| Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts.Crossref | GoogleScholarGoogle Scholar | 20462438PubMed |
Parrish, J. J., Susko-Parrish, J., Winer, M. A., and First, N. L. (1988). Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180.
| Capacitation of bovine sperm by heparin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkslWit7g%3D&md5=b29a8be059be8768df05d3950d54d736CAS | 3408784PubMed |
Pursel, V. G., and Johnson, L. A. (1975). Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40, 99–102.
| 1:STN:280:DyaE2M%2FnslCjtw%3D%3D&md5=64a08be65961983b91263161a1d1a8deCAS | 1110222PubMed |
Rodriguez-Martinez, H. (2007). Role of the oviduct in sperm capacitation. Theriogenology 68, S138–S146.
| Role of the oviduct in sperm capacitation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotlaitLY%3D&md5=2d98f45c68d1b3d8a622bff48d0971aeCAS | 17452049PubMed |
Scott, M. A. (2000). A glimpse at sperm function in vivo: sperm transport and epithelial interaction in the female reproductive tract. Anim. Reprod. Sci. 60–61, 337–348.
| A glimpse at sperm function in vivo: sperm transport and epithelial interaction in the female reproductive tract.Crossref | GoogleScholarGoogle Scholar | 10844205PubMed |
Shin, S., Dimitri, C. A., Yoon, S. O., Dowdle, W., and Blenis, J. (2010). ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol. Cell 38, 114–127.
| ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvFynsbY%3D&md5=c8074ca22f7cdda47497228bfdb3a554CAS | 20385094PubMed |
Suarez, S. S. (2002). Formation of a reservoir of sperm in the oviduct. Reprod. Domest. Anim. 37, 140–143.
| Formation of a reservoir of sperm in the oviduct.Crossref | GoogleScholarGoogle Scholar | 12071887PubMed |
Thibodeaux, J. K., Myers, M. W., Goodeaux, L. L., Menezo, Y., Roussel, J. D., Broussard, J. R., and Godke, R. A. (1992). Evaluating an in vitro culture system of bovine uterine and oviduct epithelial cells for subsequent embryo co-culture. Reprod. Fertil. Dev. 4, 573–583.
| Evaluating an in vitro culture system of bovine uterine and oviduct epithelial cells for subsequent embryo co-culture.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3jsVyhsA%3D%3D&md5=098d548f21fef11209deeee92edbb205CAS | 1299832PubMed |
Topfer-Petersen, E. (1999). Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum. Reprod. Update 5, 314–329.
| Carbohydrate-based interactions on the route of a spermatozoon to fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvVKjtbY%3D&md5=c545061923e05ba331278eda573c4f8eCAS | 10465523PubMed |
Ulbrich, S. E., Zitta, K., Hiendleder, S., and Wolf, E. (2010). In vitro systems for intercepting early embryo–maternal cross-talk in the bovine oviduct. Theriogenology 73, 802–816.
| In vitro systems for intercepting early embryo–maternal cross-talk in the bovine oviduct.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivFGltr0%3D&md5=d9905d2d367e7a6fed98b01373224371CAS | 19963260PubMed |
Umezu, T., Hanazono, M., Aizawa, S., and Tomooka, Y. (2003). Characterization of newly established clonal oviductal cell lines and differential hormonal regulation of gene expression. In Vitro Cell. Dev. Biol. Anim. 39, 146–156.
| 1:CAS:528:DC%2BD3sXotlyksLc%3D&md5=6f930d43ec9cbb46b97c2045bc5c6935CAS | 14505432PubMed |
Wagner, A., Ekhlasi-Hundrieser, M., Hettel, C., Petrunkina, A., Waberski, D., Nimtz, M., and Topfer-Petersen, E. (2002). Carbohydrate-based interactions of oviductal sperm reservoir formation–studies in the pig. Mol. Reprod. Dev. 61, 249–257.
| Carbohydrate-based interactions of oviductal sperm reservoir formation–studies in the pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xlt1Giuw%3D%3D&md5=5048be4e228bc654532118ebe4eb71e3CAS | 11803561PubMed |
Wenger, S. L., Senft, J. R., Sargent, L. M., Bamezai, R., Bairwa, N., and Grant, S. G. (2004). Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci. Rep. 24, 631–639.
| Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpvFOksLk%3D&md5=a24a69d1c4b6a3c42736b5b198cebfb5CAS | 16158200PubMed |
Winer, J., Jung, C. K., Shackel, I., and Williams, P. M. (1999). Development and validation of real-time quantitative reverse transcriptase–polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 270, 41–49.
| Development and validation of real-time quantitative reverse transcriptase–polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtVersr0%3D&md5=d4f353b2c700cd9549ced42ae1d28bfdCAS | 10328763PubMed |
Yu, H., Cook, T. J., and Sinko, P. J. (1997). Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages. Pharm. Res. 14, 757–762.
| Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkt1yit7Y%3D&md5=a417f53ecdc2f0f151ed77f2e7cdbe31CAS | 9210193PubMed |