Follicular dynamics and gene expression in granulosa cells, corpora lutea and oocytes from gilts of breeds with low and high ovulation rates
P. V. Silva A , S. E. F. Guimarães A E , J. D. Guimarães B , C. S. Nascimento A , P. S. Lopes A , J. B. Siqueira A , L. S. Amorim B , F. Fonseca e Silva C and G. R. Foxcroft DA Departamento de Zootecnia, Universidade Federal de Vicosa, Vicosa 36570-000, MG, Brasil.
B Departamento de Veterinária, Universidade Federal de Vicosa, Vicosa 36570-000, MG, Brasil.
C Departamento de Estatística, Universidade Federal de Vicosa, Vicosa 36570-000, MG, Brasil.
D Swine Reproduction-Development Program, Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton T6G 2P5, AB, Canada.
E Corresponding author. Email: sfacioni@ufv.br
Reproduction, Fertility and Development 26(2) 316-327 https://doi.org/10.1071/RD12257
Submitted: 7 August 2012 Accepted: 12 January 2013 Published: 7 March 2013
Abstract
Follicular dynamics and the expression of candidate genes using real-time polymerase chain reaction (PCR) were compared during the oestrous cycle of pig breeds with high (commercial line; n = 24) and low (local Brazilian Piau; n = 21) ovulation rates and prolificacy. Gilts were killed on Days 0, 4, 10 and 18 of the oestrous cycle and visible ovarian follicles were classified by follicular diameter. Recovered cumulus–oocyte complexes were classified as normal or atretic and frozen in liquid nitrogen until RNA extraction. Low ovulation rates and/or prolificacy in Piau gilts was associated with a different pattern of follicle development, with lower numbers of small follicles on Day 18, fewer large follicles on Days 0 and 18 (P ≤ 0.05) and a higher proportion of atretic follicles on Days 0 and 18 (P ≤ 0.05). Compared with commercial line gilts, less-prolific Piau gilts exhibited higher expression of apoptotic genes during luteolysis (CASP3 and FASL; P ≤ 0.05), decreased expression of TGFBR2 and BAX mRNA in the corpus luteum (P ≤ 0.05), higher expression of apoptotic genes (FAS, BCL2 and CASP8; P ≤ 0.05) in granulosa cells and a greater abundance (P ≤ 0.05) of genes controlling oocyte-secreted factors (GDF9, BMP15 and BMP6), suggesting underlying mechanisms controlling differences in follicular development, ovulation rate and inherent prolificacy in this pig breed.
Additional keywords: growth factors, litter size, ovulation.
Introduction
As in sheep, differences in ovulation rate in different breeds of pigs are presumably linked to differences in intraovarian regulatory mechanisms and overall prolificacy. For example, according to Manabe et al. (2004), the higher ovulation rate in the Meishan sow is related to differences in both follicular recruitment and atresia. The same group reported that a lower ovulation rate and a smaller number of pigs born in local Hungarian Mangalica sows compared with other European breeds was also related to differences in follicle recruitment and atresia (Rátky et al. 2005). It has been postulated that follicles of Meishan sows provide a ‘better’ environment for oocyte maturation than follicles of Large-White hybrid sows, which may also contribute to the prolificacy of Meishan sows (Bazer et al. 1988a; Haley and Lee 1993; Hunter and Picton 1995). Similarly, different patterns of follicle and oocyte development may exist between prolific commercial breeds and the less prolific Brazilian Piau breed, and comparative studies using these breeds may contribute to the identification of genes related to ovulation rate, oocyte quality and consequently embryonic development and survival in the pig. Preliminary studies comparing different traits in contemporary commercial sows with sows from the less-prolific Brazilian Piau breed (Silva et al. 2011) have revealed differences in ovulation rate (15.5 ± 1.9 vs 11.1 ± 2.4, respectively; P ≤ 0.05), the number of total pigs born (TB; 14.3 ± 3.7 vs 9.3 ± 2.7, respectively; P ≤ 0.05) and pigs born alive (BA; 12.7 ± 3.1, 7.9 ± 2.6, respectively; P ≤ 0.05). Because the Piau breed has never been subjected to intensive genetic selection, it may carry allelic variants that are no longer found in highly selected and more prolific commercial lines.
The growth and development of ovarian follicles requires a series of coordinated events that lead to follicular somatic cell differentiation, oocyte maturation and ovulation (Bonnet et al. 2008). In farm species, the number of ovulatory follicles is regulated mainly by the pituitary gonadotrophins FSH and LH (Hunter et al. 2004). However, it is well established that the ovulatory process is also influenced by endocrine and paracrine pathways that involve local growth factors secreted from granulosa cells (GC) and the growing oocytes (Manabe et al. 2004). Most recent studies have focused on the role of bone morphogenetic proteins (BMPs), which belong to the transforming growth factor (TGF)-β family, in the control of follicle development in mammals (Glister et al. 2004; Feary et al. 2007; Paradis et al. 2009). The BMPs modulate a wide range of cellular functions, such as proliferation and differentiation, steroidogenesis, metabolism and apoptosis (Shimasaki et al. 2004; Juengel and McNatty 2005; Gilchrist et al. 2008; Gilchrist and Ritter 2011), and can also affect the quality of the oocyte and consequently embryonic development and survival (Hunter et al. 2004; Hunter and Paradis 2009). Bidirectional communication between oocyte and somatic cells is also considered to be essential for follicular and theca cell development, as well as for oocyte maturation (Singh et al. 1993; Gilchrist et al. 2004). However, once having initiated development, most follicles become atretic before ovulating due to apoptosis of GC, irrespective of the stage of follicular development (Tilly 1996; Guthrie 2005). Therefore, the fate of follicles during follicular development is determined by the balance between pro-apoptotic and survival molecules. The molecules and the processes in which they are involved may be summarised as follows: (1) atresia: B/linfoma-2 cell family members (Bcl2), tumour necrosis factor (TNF) and caspases; (2) follicle selection: Bcl2, Bax, FSH, inhibin, Fas ligand (FASL) and caspases; and (3) luteolysis: Fas/FasL, caspase 3 (CASP3), Bax and BMP ligands and receptors (Hussein 2005). However, the precise role of these proteins and genes in regulating follicle selection and apoptosis in the pig has not been completely elucidated (Inoue et al. 2011). Because ovulation rate and oocyte quality are important determinants of reproductive efficiency, understanding the regulation of follicular growth leading to ovulation is crucial (Webb et al. 2007).
Because there are no gene expression data available to explain the lower prolificacy of the Brazilian Piau breed, the aims of the present study were to: (1) investigate and compare the length of the oestrous cycle and the dynamics of follicle growth between the less prolific Piau and commercial line sows; and (2) elucidate the expression pattern of candidate genes during the oestrous cycle using quantitative real-time polymerase chain reaction (PCR) in both genetic lines as a means of better understanding the mechanisms controlling ovarian follicular development in the pig.
Materials and methods
Animals
This experiment was conducted with approval from the Universidade Federal de Viçosa Animal Care Committee (Protocol #34/2010). The 24 commercial line (Landrace × Large-White × Pietrain) and 21 Brazilian Piau breed gilts used in the study were obtained from the pig farm at the Universidade Federal de Viçosa (Viçosa, Brazil). Oestrous behaviour was evaluated during the first to seventh consecutive oestrous periods. The onset of oestrus was checked once a day using a mature boar and was designated as Day 0. Groups of gilts were then killed by electrical stunning on Days 0, 4, 10 and 18 of the oestrous cycle (n = 6 per day of the oestrous cycle for commercial gilts; n = 5 gilts per day of the oestrous cycle for the Piau breed, except for Day 18 when n = 6). The selected time frame covers the initial phase of follicle development (Days 0–4), which is characterised as being gonadotrophin independent and during which initial recruitment and growth of primordial follicles and preantral follicle relies mostly on local ovarian factors (Foxcroft et al. 1994). Days 10–18 of the cycle correspond to the final selection phase when the preovulatory follicle population has been established (Grant et al. 1989; Hunter and Wiesak 1990). The gilts were fed to appetite with a standard diet twice daily and had free access to water.
Tissue collection
Ovaries were collected immediately after the gilts had been killed and were transported on ice to the laboratory within 20 min. Before processing the ovaries, the number of CL was recorded in order to estimate ovulation rate. All visible ovarian follicles were classified on the basis of follicular diameter as small (≤3 mm), medium (3–6 mm) or large (≥6 mm), and individual follicles were aspirated using a 21-gauge needle attached to a 1-mL disposable syringe. Recovered cumulus–oocyte complexes (COCs) were classified on the basis of morphological criteria under a stereomicroscope as: (1) surrounded by intact layers of cumulus cells; (2) having a partial cumulus cell layer; (3) naked oocytes; (4) having expanded cumulus cells; or (5) degenerated oocytes. Because of the limited amount of material available, oocytes of the same size and classified morphologically as Group 1, 2 or 3 were pooled within sow and classified as relatively normal or healthy; similarly, oocytes classified morphologically as Group 4 or 5 were pooled within sow and classified as relatively degenerated or atretic oocytes. Oocytes classified as normal exhibited homogeneous cytoplasmic pigmentation and unexpanded zona pellucida, regardless of whether they were surrounded by cumulus cells; in contrast, atretic oocytes had an abnormal shape, lacked cytoplasmic pigmentation and did not have an intact zona pellucida. After oocyte isolation, the follicular fluid was centrifuged at 6000g for 5 min at 4°C to collect GC that were classified as either normal or atretic depending on the COCs from which they were harvested. The aspirated oocytes were denuded from the attached cumulus cells by repeated pipetting in 1× phosphate-buffered saline (PBS) with 0.1% (w/v) polyvinyl alcohol (PVA) and frozen immediately in liquid nitrogen until RNA extraction. The CL from each sow were dissected and treated with RNAlater (Ambion, Austin, TX, USA) and stored at –20°C for 48 h. After freezing, the CL of each sow were thawed and ground in liquid nitrogen, and the ground tissue was stored at –70°C until RNA extraction.
Isolation of RNA and cDNA synthesis
Immediately after classification of COCs, GC from follicles with the same classification from each animal were washed twice in 1× PBS by centrifugation at 5000g for 6 min at 4°C, stored in RLT Buffer provided in the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and frozen at –20°C until RNA extraction. The GC samples were thawed at 36°C for 5 min and extracted according the manufacturer’s instructions. RNA was isolated from luteal tissue using 30 mg ground tissue using the RNeasy Mini Kit (Qiagen). Similarly, oocytes were thawed on ice and total RNA was isolated using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. All samples were DNase treated using on-column DNase digestion with the RNase free DNase Set (Qiagen) and RNA was eluted in 30 µL for GC and CL, and in 10 µL for oocytes. Samples were quantified using a NanoVue Plus spectrophotometer (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) and RNA integrity was verified using an Agilent 2100 Bioanalyser Nano Kit (Agilent Technologies, Palo Alto, CA, USA). Oocyte, GC and CL total RNA was reverse transcribed using a ProtoScript M-MuLV First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA, USA) according to manufacturer’s instructions with 5 µM of oligo(dT). The cDNA was synthesised using 1 µg GC and CL total RNA and 6 µL oocyte total RNA. The cDNA was then stored at –20°C until analysis by quantitative real-time PCR.
Quantitative real-time PCR
Quantitative real-time PCR was performed using SyBr Green GoTaq qPCR Master Mix (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The primer oligonucleotides used for the reactions were designed using PrimerQuest software (Integrated DNA Technologies, Coralville, IA, USA) from swine sequences available in GenBank (htpp://www.ncbi.nlm.nih.gov, accessed 3 November 2010). In the present study, GAPDH was used as a reference gene against which all gene expression was normalised. The list of primer sequences and expected PCR product lengths are given in Table 1. Reactions were performed in duplicate in 96-well optical reaction plates sealed with optical adhesive film using 12.5 µL of 2× SyBr Green GoTaq qPCR Master Mix (Promega). Prior to quantification by quantitative real-time PCR, the amplification efficiency and optimal primer concentration was determined for each gene using serial dilution of cDNA from each cell type. The PCR efficiencies for all primers pairs were obtained using the formula E = 10–1/slope, where E is efficiency and slope is the gradient of the dilution series in the linear phase. Samples were amplified separately using an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with the following amplification program: 40 cycles of 30 s of melting at 95°C followed by 30 s of annealing and extension at 60°C. After the 40 amplification cycles, all samples were subjected to a melting curve analysis, in which they were heated in increments of 1°C per 30 s from 60°C to 94°C to validate the absence of non-specific products.
Statistical analysis
The Chi-squared test of independence was used to evaluate the distribution of follicles within different follicle size classes and the sample size deviation was corrected using the Fisher’s exact test. Statistical tests were performed using PROC FREQ in the SAS statistical package (SAS Institute, Cary, NC, USA), with a significance level of 5%. Confidence intervals for the independent proportions were then constructed by the PROP.TEST in the R program (R Development Core Team 2008). The number of CL and data on the interoestrous interval were analysed using SAS PROC Univariate to verify differences in ovulation rate and oestrous cycle length between genetic groups.
The expression for each gene was calculated using the ΔCt method (target gene Ct – GAPDH gene Ct) for all individual samples, where Ct is the PCR cycle number at which the fluorescence generated within a reaction crosses an arbitrary threshold. Differences in gene expression were estimated using the 2–ΔCt method of Livak and Schmittgen (2001). For statistical analysis, six follicle classifications were used: (1) large normal; (2) large atretic; (3) normal medium; (4) medium atretic; (5) small normal; and (6) small atretic. These were compared between genetic group × day of oestrous cycle. However, because follicle classifications varied by genetic line and day of cycle, not all comparisons were possible. The comparisons were made using the SAS GLM PROCEDURE and defined as (commercial – Piau) for GC and oocytes. For the CL, comparisons were made using the same model with balanced samples, regardless of follicle classification. Unless indicated otherwise, data are given as the mean ± s.e.m.
Results
Dynamics of follicle development
Compared with commercial line gilts, the less-prolific Piau gilts tended to have a shorter oestrous cycle (19.4 ± 1.7 vs 20.0 ± 1.0 days, respectively; P = 0.068) and a different pattern of follicle development, as well as a lower ovulation rate (15.5 ± 1.9 vs 11.1 ± 2.4, respectively; P ≤ 0.05).
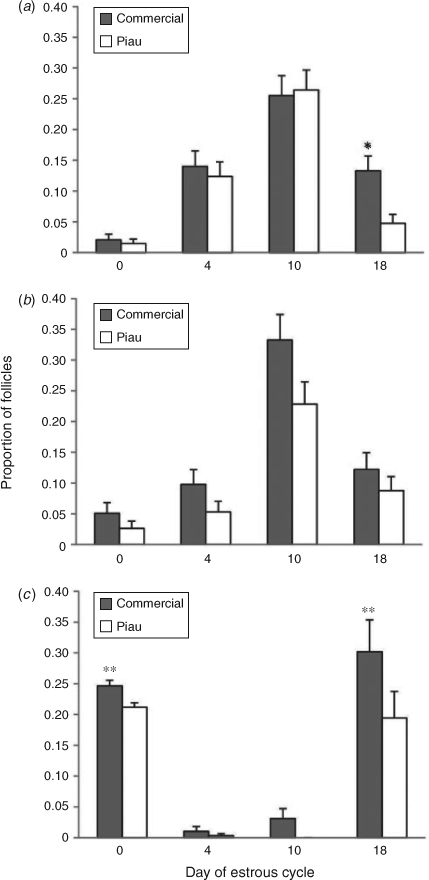
The preovulatory population of follicles for both Piau and commercial gilts reached 6–10 mm in diameter. The distribution of both small (P ≤ 0.05) and large (P ≤ 0.01) ovarian follicles during the oestrous cycle differed between commercial and Piau gilts throughout the oestrous cycle (see Table S1 available as Supplementary Material to this paper). The confidence interval for the difference between proportions allowed the identification of days of the oestrous cycle on which the follicular number from Piau and commercial line gilts differed significantly, as illustrated in Fig. 1. The pattern of follicle development in the Piau breed was characterised by a lower number of small follicles on Day 18 and large follicles on Day 0, reflecting a lower ovulation rate compared with the commercial line gilts.
|
The proportion of normal and atretic follicles during the oestrous cycle for all size follicles in commercial and Piau gilts is shown in Fig. 2. The pattern of atresia seems to differ between breeds, although the growth of large follicles on Days 18 and 0 in both breeds is marked by a significant decrease in the number of small and medium-sized follicles. In addition to the decline in follicle numbers, the incidence of estimated atresia in the total follicle population increased from 17.3% before selection (Day 10) to 50% before oestrous (Day 18) in the Piau breed, compared with a decrease in atresia from 59.3% to 25.7% in the commercial line gilts.

|
Expression of mRNA in the CL
Few differences between the genetic lines were observed for mRNA expression of apoptosis-related genes in the CL during the oestrous cycle (Fig. 3). The abundance of BCL2 mRNA, a major anti-apoptotic molecule, was similar in commercial and Piau gilts on Day 0, whereas the mRNA abundance of the pro-apoptotic factor BAX was higher in commercial line gilts on Days 4 and 18 (P ≤ 0.05 and P ≤ 0.01, respectively). Expression of one of the components of the Fas system, namely FASL, was higher in the Piau breed on Day 18 (P ≤ 0.01). However, no differences in expression were observed for FAS and the cascade initiator caspase-8 (CASP8). In contrast, the abundance of CASP3 mRNA, one of the downstream components of CASP8, was higher in the Piau breed on Days 0 and 4 (P ≤ 0.05 and P ≤ 0.01, respectively). Similar results were observed for TGFBR2, with higher expression in commercial line gilts on Days 0 and 4 (P ≤ 0.0001 and P ≤ 0.05, respectively) than in the Piau breed.
Expression of mRNA in GC
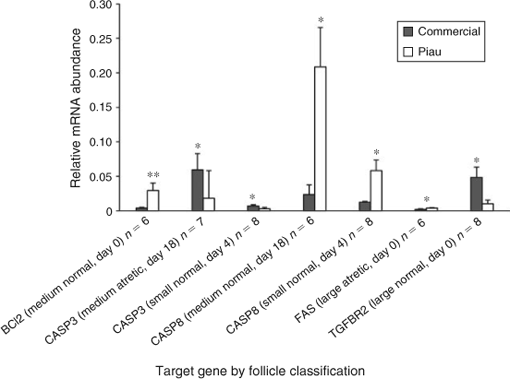
Figure 4 shows mRNA expression in the commercial and Piau breeds of components of the death receptor apoptotic pathway, with the exception of FASL, which could not be detected in the GC with the current primer set, and TGFBR2. In large normal follicles, the abundance of TGFBR2 mRNA was higher in commercial line gilts on Day 0; however, no significant difference was observed in large atretic follicles. Expression of FAS mRNA expression in large atretic follicles was higher in Piau than commercial gilts (P ≤ 0.05) on Day 0, whereas no significant difference was observed in large normal follicles. On Day 0, medium normal follicles exhibited higher BCL2 mRNA expression in GC from the Piau breed (P ≤ 0.01), whereas CASP8 mRNA expression was higher in the Piau breed for the medium normal follicle category on Day 18 of the oestrous cycle. Expression of CASP3 mRNA was higher in commercial line gilts for medium atretic follicles on Day 18. In small normal follicles on Day 4, CASP3 mRNA expression was higher in commercial line gilts, whereas CASP8 mRNA expression was higher in the Piau breed. Additional comparisons between follicle size and health status were limited by the number of experimental units on specific days of the oestrous cycle.
|
Oocyte mRNA expression profiles
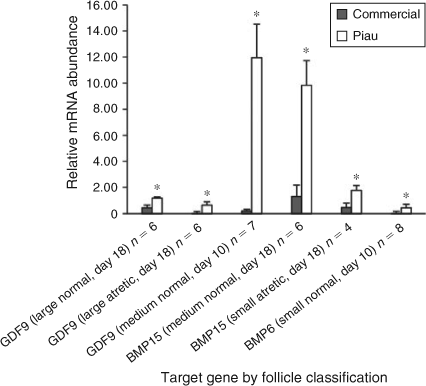
Figure 5 shows the contrast of oocyte gene expression for growth differentiation factor 9 (GDF9), BMP15 and BMP6 between breeds according to follicle size on a particular day of the oestrous cycle. Interestingly, for all genes analysed, negative values were obtained, indicating that the expression of these genes was higher in the Piau oocytes than in oocytes recovered from commercial line gilts. The relative expression of GDF9 in oocytes from large normal and large atretic follicles on Day 18, and in oocytes from medium normal follicles on Day 10, was higher (P ≤ 0.05) in the Piau breed, whereas no differences in GDF9 expression were observed for medium atretic follicles. For small follicles, no differences in GDF9 mRNA abundance were observed in either normal or atretic follicles on the days studied. Oocyte BMP15 mRNA expression was higher on Day 18 in the Piau breed in medium normal and small atretic follicles (P ≤ 0.05). Only one contrast was significant for oocyte BMP6 mRNA abundance, which was higher in the Piau breed for small normal follicles on Day 10 (P ≤ 0.05).
|
Discussion
The key ovarian phenotypic traits of the less-prolific Piau breed were a lower ovulation rate and a tendency for a shorter oestrous cycle than the more prolific commercial line gilts. A shorter oestrous cycle has also been observed in the hyperprolific Meishan breed compared with less-prolific breeds (Bazer et al. 1988b). It has been postulated that the longer behavioural oestrus in the Meishan breed would result in the recovery of follicles at the onset of oestrus at a relatively earlier time relative to ovulation than in other breeds, and Meishan sows appear to maintain a larger number of follicles in the proliferating pool during the follicular phase, thus contributing to their high prolificacy (Bazer et al. 1988b). Although also showing a tendency for a shorter oestrous cycle, as observed in Meishan, the Piau breed represents the other extreme of prolificacy. Despite the lack of information about the precise time course of events during the oestrous cycle in the Piau breed, the findings of the present study indicate a decrease in the number of small follicles (≤3 mm diameter) in the early follicular phase (Day 18), as illustrated in Fig. 1a. This reduction in the number of small follicles, together with a higher rate of atresia on Day 18 (see Fig. 2), suggests that a block to follicle replacement in the proliferating pool, the opposite situation to that in Meishan sows, may be a primary determinant of low prolificacy in the Piau breed.
The observation of fewer small follicles and the higher rate of atresia in large follicles limit the number of follicles available for selection and subsequent ovulation in the less-prolific Piau compared with commercial line gilts on Day 18 of the oestrous cycle and is consistent with reports that sows with a higher ovulation rate have a larger number of follicles in the proliferating pool (Clark et al. 1973) and maintain a higher number of follicles during the follicular phase than do non-prolific breeds (Miller et al. 1998). However, Piau gilts also had a lower incidence of atresia in medium- and small-sized follicles on Days 4 and 10. This pool of follicles begins to grow after ovulation and will constitute the pool of follicles in the recruitment phase, which occurs on Days 14 and 16 of the oestrous cycle in pigs (Foxcroft and Hunter 1985). Collectively, these observations corroborate the suggestion that ovulation rate is a complex trait related to differences in both follicular recruitment and atresia (Manabe et al. 2004).
The follicular dynamics between breeds were further corroborated by gene expression analysis using quantitative real-time PCR. Genes of the TGF-β superfamily, such as those for BMPs, GDF9 and their receptors, are known modulators of mammalian folliculogenesis and were selected for investigation. The expression of BMP6, BMP15 and GDF9 transcripts, as well as that of their cell receptors, has recently been reported in porcine oocytes from preovulatory follicles (Paradis et al. 2009; Sun et al. 2010). However, less information is available about the mechanisms underlying their physiological role in follicular development between species, or differences in their expression in divergent breeds of pigs. Therefore, a comparative study using the less-prolific Piau breed and more prolific contemporary commercial line gilts provided the opportunity to identify genes related to ovulation rate and oocyte quality, and consequently embryonic development and survival, in these two breeds.
Interestingly, in the present study, the significant contrasts between two breeds identified higher expression of oocyte-secreted factors in the Piau breed than in the commercial line. Because oocyte-secreted factors act as key regulators of oocyte maturation and folliculogenesis (McNatty et al. 2004; Juengel and McNatty 2005; Gilchrist et al. 2008), they may be related to the differences in ovulation rate between breeds. The local Piau breed exhibited higher GDF9 mRNA abundance in large normal and large atretic follicles on Day 18, as well as in medium normal follicles on Day 10. Expression of BMP15 mRNA was detected in the oocyte, consistent with previous studies in the pig (Quinn et al. 2004; Zhu et al. 2008; Paradis et al. 2009), with higher expression in medium-sized healthy follicles and small-sized atretic follicles in the Piau breed on Day 18. Working with in vitro oocytes, Li et al. (2008) reported that these two genes and their encoded proteins were differentially expressed during the maturation process, especially during cumulus cell expansion. Therefore, these results suggest that the lower prolificacy of the Piau breed may be related to differences in the expression of both oocyte-secreted factors, resulting in changes in the timing of cumulus cell expansion and potentially also affecting GC steroidogenesis. Furthermore, it is possible that the differential expression of GDF9 and BMP15 verified in the present study is due to relative differences in the stage of follicular maturation, because previous studies reported that the Meishan preovulatory follicle is in a more advanced state of maturation than that of the Large-White (Hunter et al. 1993; Faillace and Hunter 1994; Xu et al. 1998).
Expression of BMP6 mRNA was detected in porcine oocytes in the present study, corroborating previous findings (Zhu et al. 2008; Paradis et al. 2009). The differential expression of BMP6 mRNA from Piau oocytes may play an important role in FSH-dependent follicle development and in the regulation of luteinisation, again affecting the difference in ovulation rate between breeds. It has been suggested in rats that BMP6 mRNA derived from GC is lost during selection of the dominant follicle and that BMP6 mRNA is strongly expressed in GC during atresia (Erickson and Shimasaki 2003). Moreover, investigations into the mechanism of action found that BMP6, unlike BMP15 and GDF9, does not have proliferative properties on rat GC and is able to suppress FSH-induced progesterone production (Otsuka et al. 2001). Therefore, the results of the present study suggest breed differences in BMP6, BMP15 and GDF9 mRNA expression, and indicate that a larger number of follicles can escape from atresia during early folliculogenesis in the Piau breed, resulting in the lower incidence of atresia on Days 4 and 10 compared with commercial line gilts. These results initially seem contradictory, because the Piau breed is the less prolific. However, oocyte-secreted factors may be involved in the recruitment process, leading to differences in oocyte quality (Gilchrist et al. 2008) and the number of tertiary and atretic follicles between lines, as suggested by Manabe et al. (2004). Also associated with their lower prolificacy, differences in oocyte-expressed factors may be contributing to the ability of the Piau to use smaller follicles for selection and ovulation, as described for the Meishan breed (Miller et al. 1998).
Once the pool of follicles initiates its growth, atresia and ovulation are the only possible fates (Knox 2005). Atresia limits the number of oocytes capable of fertilisation and embryonic development, and is physiologically important for the elimination of degenerated oocytes (Guthrie et al. 1995; Guthrie 2005). It is not clear whether this process differs between pig breeds. In the present study, mRNA expression of candidate genes involved in apoptotic pathways in GC and luteal cells differed between breeds during the oestrous cycle. The Fas–FasL system has been reported in many species as the major mechanisms regulating GC (Matsuda-Minehata et al. 2008) and luteal cell (Juengel et al. 1993; Rueda et al. 1997; Sakamaki et al. 1997) apoptosis. In the present study, FAS mRNA was more abundant in GC from healthy than atretic follicles during the oestrous cycle, consistent with previous findings in several species (Porter et al. 2001; Dharma et al. 2003; Inoue et al. 2006). However, in contrast, Inoue et al. (2006) reported that FAS mRNA expression increased in GC from atretic compared with healthy follicles.
We also detected FAS and FASL mRNA expression in pig luteal tissue, as reported in murine and human studies (Kondo et al. 1996; Sakamaki et al. 1997). Interestingly, in the present study no differences were observed for the FAS transcript in CL between breeds, whereas FAS mRNA expression in GC was significantly higher in large atretic follicles from Piau gilts than from commercial line sows on Day 0. According to Manabe et al. (2004), differences in the initiation of GC apoptosis between species indicate local mechanisms of regulation, mainly the apoptotic stimuli induction mechanism. In this context, the present study indicates that apoptosis signalling may be differently activated in atretic follicles between distinct breeds. Therefore, it can be suggested that the follicle in the Piau breed provides a different environment for follicle apoptosis than that in commercial line follicles, which may contribute to the lower prolificacy of the Piau breed. Many reports have shown that apoptosis occurs during luteolysis and it has also been established that apoptosis plays a role in CL regression at the end of the oestrous cycle (Rueda et al. 1997). In the present study the amount of transcript that encodes the membrane protein Fas in the CL was similar between breeds, whereas expression of FASL mRNA was higher in Piau CL, which may result in a more effective apoptosis signalling towards luteolysis in this breed.
Several genes belonging to the bcl2 family, including both anti- and pro-apoptotic family members, are known to be expressed in luteal cells of various species (Rodger et al. 1995; Rueda et al. 1997; Goodman et al. 1998). The Bcl2 : Bax expression ratio within a cell is related to its potential to become apoptotic, and it has been proposed that Bax homodimers promote cell death, whereas Bcl2 homodimers function as repressors of cell death (Oltval et al. 1993). The findings of the present study indicate that BCL2 mRNA abundance in the CL did not differ between breeds during the oestrous cycle. However, the mRNA abundance of the pro-apoptotic factor BAX was higher in commercial line gilts on Days 4 and 18, resulting in a higher ratio of BAX : BCL2 mRNA compared with that in the Piau breed, a change consistent with bax-mediated apoptosis (Rueda et al. 1997). This indicates a possible occurrence of an increased ratio of apoptosis in the CL of commercial line compared with Piau sows. Similar higher expression of BAX mRNA in the regressing CL was reported during luteolysis in cattle and humans (Rueda et al. 1997; Sugino et al. 2000; Pretheeban et al. 2010). Although in the present study the expression of BCL2 mRNA was similar between breeds on Day 10, previous studies in humans demonstrated higher expression of BCL2 mRNA in the mid-luteal phase CL (Sugino et al. 2000). Together, these findings suggest that the BAX gene may be important in the regulation of the CL lifespan by controlling the rate of apoptosis and that it potentially underlies differences in oestrous cycle length and cyclicity between breeds. However, it is important to emphasise that these events may also be regulated at the post-translational level and modulated by interactions with other molecules, such as p53 tumour suppressor protein (Miyashita et al. 1994). In addition, the abundance of transcripts is not always associated with differences in protein secretion (Griffin et al. 2002) and studies at the proteomic level are required.
Many findings indicate that Bcl2 family proteins modulate the apoptosis of GC in mammals, in which BCL2 overexpression is related to reduced follicular atresia and increased litter size (Hsu et al. 1996; Choi et al. 2004). In the present study BAX mRNA abundance in GC was similar between breeds during the oestrous cycle, whereas BCL2 mRNA abundance in medium-sized healthy follicles was higher in the Piau breed compared with commercial line gilts on Day 0, again suggesting decreased apoptosis in Piau GC during early follicular development. CASP3 has been reported as an essential molecule for the apoptosis of GC (Manabe et al. 2004; Matsuda-Minehata et al. 2008). Many studies in GC have described changes in caspase-3 protein expression and activity associated with the progression of atresia in ovarian follicles (Boone and Tsang 1998; Berardinelli et al. 2004). In the present study, CASP3 mRNA abundance was significantly higher in GC from medium-sized atretic and small-sized healthy follicles on Day 4 in commercial line gilts than in the Piau breed. However, little other information is available about CASP3 mRNA expression patterns in different breeds of pigs during the oestrous cycle. During the early luteal phase in the pig, a new group of medium-sized follicles starts to growth between Days 3 and 8 (Guthrie et al. 1995). Although the functional lifespan of these follicles is unknown, it is likely that within a day or two after ovulation the medium-sized growing follicles will be eventually deleted by atresia, as described in cattle (Sirois and Fortune 1988). Therefore, the data on CASP3 mRNA expression in medium- and small-sized follicles in commercial line gilts suggest that this breed may be more susceptible to atresia than the Piau breed on Day 4, driving the different pattern of follicular development observed between breeds. These findings also agree well with the higher incidence of atresia (23.1% vs 14.5%) on Day 4 in commercial line gilts compared with the Piau gilts in the total follicle population (Fig. 2).
It is also known that apoptosis occurs during luteolysis in the ovary, and it is recognised that controlled cell death is important to physical removal of the CL from the ovary at the end of the oestrous cycle (Rueda et al. 1997). Previous studies reported CASP3 expression in the CL during luteolysis induced by prostaglandin in the rat (Boone and Tsang 1998) and in sheep (Rueda et al. 1999) CL. Moreover, abundant expression of CASP3 in the human CL was reported and considered to be important for luteal regression (Krajewska et al. 1997). However, little is known regarding the expression and role of caspases in the pig CL. In the present study, CASP3 mRNA was expressed in luteal cells throughout the oestrous cycle and its abundance was higher in the Piau breed on Days 0 and 4. Apoptotic cell death during luteolysis is important to maintain oestrous cyclicity (Manabe et al. 2004). However, premature disruption of normal CL function could result in a reduction of reproductive efficiency due to irregular oestrous cycles and loss of pregnancy (Rueda et al. 1997). In this context, the results of the present study indicate that apoptosis mediated by CASP3 may be differently regulated between the breeds studied and may reflect differences in the length of the oestrous cycle and the time of luteal regression. Recent findings have addressed roles for TGF-β in the follicle maturation and luteinisation processes in the pig (Paradis et al. 2009; Sriperumbudur et al. 2010). In the present study, TGFBR2 mRNA expression was decreased in Piau CL on Days 0 and 4 compared with the commercial line, indicating that the regression process may differ between breeds. In addition, TGFBR2 mRNA expression in the present study was higher in GC from large follicles of commercial line gilts on Day 0 and may mediate the differences in follicular dynamics leading to differences in ovulation rate and oocyte quality in gilts from the different breeds. This finding is further supported by Sriperumbudur et al. (2010), who suggested that TGFBR2 may have roles in mediating the luteinisation process in postovulatory porcine follicles.
Conclusions
In conclusion, the findings of the present study indicate differences in the characteristics of the oestrous cycle and follicular development in the less-prolific Piau gilt than in commercial line gilts. The pattern of follicular growth in Piau gilts is characterised by a small population of preovulatory follicles, combined with a higher incidence of atresia in the preovulatory stage of the cycle compared with commercial line gilts. The gene expression profiles observed were consistent with the different follicular dynamics between the breeds, with the relative expression of BCL2 and BAX genes influencing important decisions between proliferation and atresia in CL and GC of gilts of the different breeds. Because transcriptional profiling identifies only one level of cellular regulation, the differences in mRNA abundance observed in the present study may not necessarily correspond to differences in secreted proteins and do not provide information about possible protein modifications, activity or location (Zeng et al. 2004). However, the expression data suggest that further proteomic studies could be of value. In addition, from a functional perspective, techniques like RNA interference (RNAi) could be used in future experiments to downregulate the expression of specific genes of interest in in vitro culture systems and thus investigate the potential role of those genes in mediating cumulus cell expansion and to confirm their pro-apoptotic activity in granulosa cells during atresia. Apoptosis in the CL, mediated by differences in FASL and CASP3 and by decreased expression of TGFBR2 mRNA in Piau compared with commercial line CL on Days 0 and 4 of oestrous cycle may also reflect the tendency for a shorter oestrous cycle and faster luteal regression in the Piau breed. Finally, the higher expression of oocyte-secreted factors (GDF9, BMP15 and BMP6) in Piau oocytes may play a role in inhibiting the luteinisation process, as well as affect follicle development and induce a lower ovulation rate that is a key component of the reduced prolificacy of this breed.
Together, our results support the hypothesis that differential expression of genes and/or gene pathways controlling follicle growth mediates the different pattern of follicle development observed between the breeds studied. This may affect not only ovulation rate, but also oocyte and embryo quality.
Acknowledgements
This research was supported by Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Instituto Nacional de Ciência e Tecnologia de Ciência Animal (INCT)–Ciência Animal.
References
Bazer, F. W., Thatcher, W. W., Martinat-Botte, F., and Terqui, M. (1988a). Conceptus development in Large White and prolific Chinese Meishan pigs. J. Reprod. Fertil. 84, 37–42.| Conceptus development in Large White and prolific Chinese Meishan pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2Fjs12muw%3D%3D&md5=e7004bb367d66ddb1a385e3815b68cb5CAS | 3184056PubMed |
Bazer, F. W., Thatcher, W. W., Martinat-Botte, F., and Terqui, M. (1988b). Sexual maturation and morphological development of the reproductive tract in large white and prolific Chinese Meishan pigs. J. Reprod. Fertil. 83, 723–728.
| Sexual maturation and morphological development of the reproductive tract in large white and prolific Chinese Meishan pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1czhs1ynsw%3D%3D&md5=ece0e1c15f576a131305ddb6f86b1900CAS | 3411562PubMed |
Berardinelli, ., Russo, V., Martelli, A., Nardinocchi, D., Di Giacinto, O., Barboni, B., and Mattioli, M. (2004). Colocalization of DNA fragmentation and caspase-3 activation during atresia in pig antral follicles. Anat. Histol. Embryol. 33, 23–27.
| Colocalization of DNA fragmentation and caspase-3 activation during atresia in pig antral follicles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7jsFemsg%3D%3D&md5=11ebef4743a5b718ccf14cee9a3ee2b8CAS | 15027958PubMed |
Bonnet, A., Lê, C. K., Sancristobal, M., Benne, F., Robert-Granié, C., Law-So, G., Fabre, S., Besse, ., De Billy, E., Quesnel, H., Hatey, F., and Tosser-Klopp, G. (2008). In vivo gene expression in granulosa cells during pig terminal follicular development. Reproduction 136, 211–224.
| In vivo gene expression in granulosa cells during pig terminal follicular development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVGqtbbL&md5=b2ab68bcb81380acd61b08ddf21c7bbdCAS | 18456903PubMed |
Boone, D. L., and Tsang, B. K. (1998). Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biol. Reprod. 58, 1533–1539.
| Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjsFShur8%3D&md5=cc98698edc5ff73722d7e82711eab9e6CAS | 9623616PubMed |
Choi, D., Hwang, S., Lee, E., Yoon, S., Yoon, B. K., and Bae, D. (2004). Expression of mitochondria-dependent apoptosis genes (p53, Bax, and Bcl-2) in rat granulosa cells during follicular development. J. Soc. Gynecol. Invest. 11, 311–317.
| Expression of mitochondria-dependent apoptosis genes (p53, Bax, and Bcl-2) in rat granulosa cells during follicular development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlt1Wmsbk%3D&md5=64515a9f2764f9b8f81f033820634d73CAS |
Clark, J. R., Eday, T. N., First, N. L., Chapman, A. B., and Casida, L. E. (1973). The effects of four genetic groups and two feed levels of feeding on ovulation rate and follicular development in pubertal gilts. J. Anim. Sci. 36, 1164–1169.
| 1:STN:280:DyaE3s3gtlWlug%3D%3D&md5=05df9ff86f9e27f5cc425223a867e262CAS | 4736529PubMed |
Dharma, S. J., Kelkar, R. L., and Nandedkar, T. D. (2003). Fas and Fas ligand protein and mRNA in normal and atretic mouse ovarian follicles. Reproduction 126, 783–789.
| Fas and Fas ligand protein and mRNA in normal and atretic mouse ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFWktQ%3D%3D&md5=ff74af478c7cf938f69cdd1deb234de7CAS | 14748697PubMed |
Erickson, G. F., and Shimasaki, S. (2003). The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod. Biol. Endocrinol. 1, 1–20.
Faillace, L. S., and Hunter, M. G. (1994). Follicle development and oocyte maturation during the immediate preovulatory period in Meishan and White hybrid gilts. J. Reprod. Fertil. 101, 571–576.
| Follicle development and oocyte maturation during the immediate preovulatory period in Meishan and White hybrid gilts.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FlsFCjtg%3D%3D&md5=a463d69627d69c60815e628d15ed1b60CAS | 7966010PubMed |
Feary, E. S., Juengel, J. L., Smith, ., French, M. C., O’connell, A. R., Lawrence, S. B., Galloway, S. M., Davis, G. H., and Mcnatty, K. . (2007). Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the Woodlands FecX2W mutation. Biol. Reprod. 77, 990–998.
| Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the Woodlands FecX2W mutation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVSgtLbP&md5=64e0364f7d305bf29928ff760151324bCAS | 17715428PubMed |
Foxcroft, G. R., and Hunter, M. G. (1985). Basic physiology of follicular maturation in the pig. J. Reprod. Fertil. Suppl. 33, 1–19.
| 1:STN:280:DyaL287hsFWhtg%3D%3D&md5=e80ea50c6a78df3644ee50b30fbbf718CAS | 3003359PubMed |
Foxcroft, G. R., Cosgrove, J., Ding, J., Hofacker, S., and Wiesak, T. (1994). Reproductive function: current concepts. In ‘Principles of Pig Science’. (Eds D. J. A. Cole, J. Wiseman and M. Varley.) pp. 225–252. (Nottingham University Press: Nottingham, UK.)
Gilchrist, R. B., and Ritter, L. J. (2011). Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction 142, 647–657.
| Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFOhsbrM&md5=f29f971d5f33315248b71a659000f9d8CAS | 21896635PubMed |
Gilchrist, R. B., Ritter, L. J., and Armstrong, D. T. (2004). Oocyte–somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 82–83, 431–446.
| Oocyte–somatic cell interactions during follicle development in mammals.Crossref | GoogleScholarGoogle Scholar | 15271471PubMed |
Gilchrist, R. B., Lane, M., and Thompson, J. G. (2008). Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 14, 159–177.
| Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVKmurY%3D&md5=97f7a19cad1bb4c5a191a9fe4e6ab0c5CAS | 18175787PubMed |
Glister, C., Kemp, C. F., and Knight, . G. (2004). Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4,-6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 127, 239–254.
| Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4,-6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitVWrtL8%3D&md5=6ac97304298d9dccd17780b70c091d74CAS | 15056790PubMed |
Goodman, S. B., Kugu, K., Chen, S. H., Preutthipan, S., Tilly, K. I., Tilly, J. L., and Dharmarajan, A. M. (1998). Estradiol-mediated suppression of apoptosis in the rabbit corpus luteum is associated with a shift in expression of bcl-2 family members favoring cellular survival. Biol. Reprod. 59, 820–827.
| Estradiol-mediated suppression of apoptosis in the rabbit corpus luteum is associated with a shift in expression of bcl-2 family members favoring cellular survival.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmsVGrsb4%3D&md5=c39c1f8a086e0b573be7017781c6f281CAS | 9746731PubMed |
Grant, S. A., Hunter, M. G., and Foxcroft, G. R. (1989). Morphological and biochemical characteristics during ovarian follicular development in the pig. J. Reprod. Fertil. 86, 171–183.
| Morphological and biochemical characteristics during ovarian follicular development in the pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktFOqs7g%3D&md5=f99fbaeb091fde93e0a11ef7232b6093CAS | 2754637PubMed |
Griffin, T. J., Gygi, S. ., Ideker, T., Rist, B., Eng, J., Hood, L., and Aebersold, R. (2002). Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol. Cell. Proteomics 1, 323–333.
| Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktFSmtLo%3D&md5=72d36e3c77d94ccd47acd06c8dc5977fCAS | 12096114PubMed |
Guthrie, H. D. (2005). The follicular phase in pigs: follicle populations, circulating hormones, follicle factors and oocytes. J. Anim. Sci. 83, 79–89.
Guthrie, H. D., Grimes, R. W., Cooper, B. S., and Hammond, J. M. (1995). Follicular atresia in pigs: measurement and physiology. J. Anim. Sci. 73, 2834–2844.
| 1:CAS:528:DyaK2MXotFajsro%3D&md5=e65d44c2706317968fad50dcacea5f0dCAS | 8582874PubMed |
Haley, C. S., and Lee, G. J. (1993). Genetic basis of prolificacy in Meishan pigs. J. Reprod. Fertil. Suppl. 48, 247–259.
| 1:STN:280:DyaK2c7psVCksg%3D%3D&md5=9fd9eac66cbcb82490d94579fd8ff937CAS | 8145208PubMed |
Hsu, S. Y., Lai, R. J., Finegold, M., and Hsueh, A. J. (1996). Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 137, 4837–4843.
| Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xms1GjtLY%3D&md5=b9526135be2006515571e13a00afed23CAS | 8895354PubMed |
Hunter, M. G., and Paradis, F. (2009). Intra-follicular regulatory mechanisms in the porcine ovary. Soc. Reprod. Fertil. Suppl. 66, 149–164.
| 1:CAS:528:DC%2BC3cXjslKjtLg%3D&md5=6031526c68b037434a482eb1a6471c42CAS | 19848278PubMed |
Hunter, M. G., and Picton, H. M. (1995). Effect of hCG administration at the onset of oestrus on early embryo survival and development in Meishan gilts. Anim. Reprod. Sci. 38, 231–238.
| Effect of hCG administration at the onset of oestrus on early embryo survival and development in Meishan gilts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlslCnt7k%3D&md5=a8abae26cd2ce809993e1c0e869a5f75CAS |
Hunter, M. G., and Wiesak, T. (1990). Evidence for and implications of follicular heterogeneity in pigs. J. Reprod. Fertil. Suppl. 40, 163–177.
| 1:STN:280:DyaK3c3osF2gsw%3D%3D&md5=3d5fba7f75b070005ebfa00694b6f978CAS | 2192035PubMed |
Hunter, M. G., Biggs, C., Foxcroft, G. R., McNeilly, A. S., and Tilton, J. E. (1993). Comparisons of endocrinology and behavioural events during the periovulatory period in Meishan and Large–White hybrid gilts. J. Reprod. Fertil. 97, 475–480.
| Comparisons of endocrinology and behavioural events during the periovulatory period in Meishan and Large–White hybrid gilts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkt1SrsLg%3D&md5=f4144abd1044179812d4340e76fd2b54CAS | 8501718PubMed |
Hunter, M. G., Robinson, R. S., Mann, G. E., and Webb, R. (2004). Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim. Reprod. Sci. 82–83, 461–477.
| Endocrine and paracrine control of follicular development and ovulation rate in farm species.Crossref | GoogleScholarGoogle Scholar | 15271473PubMed |
Hussein, M. R. (2005). Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update 11, 162–178.
| 15705959PubMed |
Inoue, N., Maeda, A., Matsuda-Minehata, F., Fukuta, K., and Manabe, N. (2006). Expression and localization of Fas ligand and Fas during atresia in porcine ovarian follicles. J. Reprod. Dev. 52, 723–730.
| Expression and localization of Fas ligand and Fas during atresia in porcine ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2ltbo%3D&md5=d59ee4c1c73e488920988d67b9d30729CAS | 16926525PubMed |
Inoue, N., Matsuda, F., Goto, Y., and Manabe, N. (2011). Role of cell-death ligand–receptor system of granulosa cells in selective follicular atresia in porcine ovary. J. Reprod. Dev. 57, 169–175.
| Role of cell-death ligand–receptor system of granulosa cells in selective follicular atresia in porcine ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsFKmtb4%3D&md5=7026704746e2ff7791a7fa3c69440f32CAS | 21551974PubMed |
Juengel, J. L., and McNatty, K. . (2005). The role of proteins of the transforming growth factor-β superfamily in the intraovarian regulation of follicular development. Hum. Reprod. 11, 144–161.
Juengel, J. L., Garverick, H. A., Johnson, A. L., Youngquist, R. S., and Smith, M. F. (1993). Apoptosis during luteal regression in cattle. Endocrinology 132, 249–254.
| Apoptosis during luteal regression in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhsVSmsbs%3D&md5=c24eb4934502e33ebdb3e5c831875acbCAS | 8419126PubMed |
Knox, R. V. (2005). Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig. Domest. Anim. Endocrinol. 29, 385–397.
| Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvFKnsLw%3D&md5=cde420e8245e6cc45e5bbd5f088091e8CAS | 15998504PubMed |
Kondo, H., Maruo, T., Peng, X., and Mochizuki, M. (1996). Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia. J. Clin. Endocrinol. Metab. 81, 2702–2710.
| Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XktFaku7c%3D&md5=a2b106dfda8cb0e27e12318c12a4adbcCAS | 8675599PubMed |
Krajewska, M., Wang, H. G., Krajewski, S., Zapata, J. M., Shabaik, A., Gascoyne, R., and Reed, J. C. (1997). Immunohistochemical analysis of in vivo patterns of expression of CPP32 (caspase-3), a cell death protease. Cancer Res. 57, 1605–1613.
| 1:CAS:528:DyaK2sXis12lsb8%3D&md5=999b8565080a2749b836d73e22f3b121CAS | 9108467PubMed |
Li, H. K., Kuo, T. Y., Yang, H. S., Chen, L. R., Li, S. S.-L., and Huang, H. W. (2008). Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim. Reprod. Sci. 103, 312–322.
| Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVSmtb7O&md5=40e930914a58b7ee3aba33cbde3acc8eCAS | 17222994PubMed |
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCt method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCt method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFelt7s%3D&md5=9cad4ff7133d540452bb50aaaa4ededfCAS | 11846609PubMed |
Manabe, N., Goto, Y., Matsuda-Minehata, F., Inoue, N., Maeda, A., Sakamaki, K., and Miyano, T. (2004). Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 50, 493–514.
| Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia.Crossref | GoogleScholarGoogle Scholar | 15514456PubMed |
Matsuda-Minehata, F., Maeda, A., Cheng, Y., Sai, T., Gonda, H., Goto, Y., and Manabe, N. (2008). Regulation of granulosa cell apoptosis by death ligand–receptor signaling. Anim. Sci. 79, 1–10.
| 1:CAS:528:DC%2BD1cXjt1Wlt70%3D&md5=be4605a49ddb8ba1bd182eb47d9833deCAS |
McNatty, K. ., Moore, L. G., Hudson, N. L., Quirke, L. D., Lawrence, S. B., Reader, K., Hanrahan, J. ., Smith, ., Groome, N. ., Laitinen, M., Ritvos, O., and Luengel, J. L. (2004). The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction 128, 379–386.
| The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptlGjs7Y%3D&md5=74488dcb6179ea22f9ffe440dc1b1b3aCAS | 15454632PubMed |
Miller, A. T., Picton, H. M., Craigon, J., and Hunter, M. G. (1998). Follicle dynamics and aromatase activity in high-ovulating Meishan sows and in Large–White hybrid contemporaries. Biol. Reprod. 58, 1372–1378.
| Follicle dynamics and aromatase activity in high-ovulating Meishan sows and in Large–White hybrid contemporaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjsFShtL4%3D&md5=4044138738b590cef5b229839882a07dCAS | 9623595PubMed |
Miyashita, T., Krajewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Liebermann, D., Hoffman, A. B., and Reed, J. C. (1994). Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9, 1799–1805.
| 1:CAS:528:DyaK2cXkslCqurc%3D&md5=3d1fd197ee197503c9088b80c45fc132CAS | 8183579PubMed |
Oltval, Z. N., Milliman, C. L., and Korsmeyer, S. J. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619.
| Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death.Crossref | GoogleScholarGoogle Scholar |
Otsuka, F., Moore, R. K., and Shimasaki, S. (2001). Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J. Biol. Chem. 276, 32 889–32 895.
| Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvFCmtLw%3D&md5=fa5acb1bfc9e3fe0da380dc252e3274eCAS |
Paradis, F., Novak, S., Murdoch, G. K., Dyck, M. K., Dixon, W. T., and Foxcroft, G. R. (2009). Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 138, 115–129.
| Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFemsbk%3D&md5=03ff34c38d398c14e3c3a9f947c9b6e2CAS | 19359354PubMed |
Porter, D. A., Harman, R. M., Cowan, R. G., and Quirk, S. M. (2001). Relationship of Fas ligand expression and atresia during bovine follicle development. Reproduction 121, 561–566.
| Relationship of Fas ligand expression and atresia during bovine follicle development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXivFKgsLo%3D&md5=0f7201f89f4caa644c182b5058be4ce0CAS | 11277875PubMed |
Pretheeban, T., Balendran, A., Gordon, M. B., and Rajamahendran, R. (2010). mRNA of luteal genes associated with progesterone synthesis, maintenance, and apoptosis in dairy heifers and lactating dairy cows. Anim. Reprod. Sci. 121, 218–224.
| mRNA of luteal genes associated with progesterone synthesis, maintenance, and apoptosis in dairy heifers and lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsrrF&md5=a7850a1e7eb4efd3b2ba7a4e122272e2CAS | 20599333PubMed |
Quinn, R. L., Shuttleworth, G., and Hunter, M. G. (2004). Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary. J. Anat. 205, 15–23.
| Immunohistochemical localization of the bone morphogenetic protein receptors in the porcine ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntlOqsrs%3D&md5=23ce7664c69b02c12938f6483bb9c746CAS | 15255958PubMed |
R Development Core Team (2008). ‘R: A Language and Environment for Statistical Computing’. (R Foundation for Statistical Computing: Vienna.) Available from http://www.R-project.org [Verified 8 August 2012].
Rátky, J., Torner, H., Egerszegi, I., Schneider, F., Sarlos, ., Manabe, N., and Brüssow, K. . (2005). Ovarian activity and oocyte development during follicular development in pigs at different reproductive phases estimated by the repeated endoscopic method. J. Reprod. Dev. 51, 109–115.
| Ovarian activity and oocyte development during follicular development in pigs at different reproductive phases estimated by the repeated endoscopic method.Crossref | GoogleScholarGoogle Scholar | 15750302PubMed |
Rodger, F. E., Fraser, H. M., Duncan, W. C., and Illingworth, . J. (1995). Immunolocalization of Bcl-2 in the human corpus luteum. Hum. Reprod. 10, 1566–1570.
| Immunolocalization of Bcl-2 in the human corpus luteum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnt12hsr0%3D&md5=1d9e968f725c6ed41e3c04d860a3f0bcCAS | 7593540PubMed |
Rueda, B. R., Hamernik, D. L., Hoyer, P. B., and Tilly, J. L. (1997). Potential regulators of physiological cell death in the corpus luteum. In ‘Cell Death in Reproductive Physiology. Serono Symposia’. (Eds J. L. Tilly, J. Strauss and M. Tenniswood.) pp. 161–181. (Springer Verlag: New York.)
Rueda, B. R., Hendry, I. R., Tilly, J. L., and Hamernik, D. L. (1999). Accumulation of caspase-3 messenger ribonucleic acid and induction of caspase activity in the ovine corpus luteum following prostaglandin F2alpha treatment in vivo. Biol. Reprod. 60, 1087–1092.
| Accumulation of caspase-3 messenger ribonucleic acid and induction of caspase activity in the ovine corpus luteum following prostaglandin F2alpha treatment in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXislegt7g%3D&md5=b673f83c40a7b82475fbf7df18a39f73CAS | 10208968PubMed |
Sakamaki, K., Yoshida, H., Nishimura, Y., Nishikawa, S., Manabe, N., and Yonehara, S. (1997). Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol. Reprod. Dev. 47, 11–18.
| Involvement of Fas antigen in ovarian follicular atresia and luteolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXislGntbY%3D&md5=cda42e93e9d31457fde33feefc50f155CAS | 9110309PubMed |
Shimasaki, S., Moore, R. K., Otsuka, F., and Erickson, G. F. (2004). The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 25, 72–101.
| The bone morphogenetic protein system in mammalian reproduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvFOmt78%3D&md5=b7c87fb3816c2964af1184f02b21c27eCAS | 14769828PubMed |
Silva, P. V., Guimarães, S. E. F., Oliver, G., Patterson, J., Smit, M., Dixon, W. T., and Foxcroft, G. R. (2011). Comparison of gene expression between High and Low birth weight phenotype in pigs using EmbryoGENE microarray. PowerPoint presented at EmbryoGENE Annual General Meeting, 2011. Quebec City, Quebec, Canada. Available from http://embryogene.ca/kms/files/Priscila%20Silva[1].pdf [Verified 3 February 2013].
Singh, B., Barbe, G. J., and Armstrong, D. T. (1993). Factors influencing resumption of meiotic maturation and cumulus expansion of porcine oocyte-cumulus cell complexes in vitro. Mol. Reprod. Dev. 36, 113–119.
| Factors influencing resumption of meiotic maturation and cumulus expansion of porcine oocyte-cumulus cell complexes in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXht1Ggurs%3D&md5=b7a80090f212eef7f43250a59b752d7fCAS | 8398125PubMed |
Sirois, J., and Fortune, J. E. (1988). Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol. Reprod. 39, 308–317.
| Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXls1eksbc%3D&md5=266d40df70fde3bb282f12b0c886dd2cCAS | 3052602PubMed |
Sriperumbudur, R., Zorrilla, L., and Gadsby, J. E. (2010). Transforming growth factor-β (TGF-β) and its signaling components in peri-ovulatory pig follicles. Anim. Reprod. Sci. 120, 84–94.
| Transforming growth factor-β (TGF-β) and its signaling components in peri-ovulatory pig follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFWrt7w%3D&md5=d9f56e6c80fe39e9f813ed7c429346ecCAS | 20378284PubMed |
Sugino, N., Suzuki, T., Kashida, S., Karube, A., Takiguchi, S., and Kato, H. (2000). Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: regulation by human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 85, 4379–4386.
| Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: regulation by human chorionic gonadotropin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotlWmsLs%3D&md5=ee98833027dc4665876c01f5e444c803CAS | 11095483PubMed |
Sun, R. Z., Lei, L., Cheng, L., Jin, Z. F., Zu, S. J., Shan, Z. Y., Wang, Z. D., Zhang, J. X., and Liu, Z. H. (2010). Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles. J. Mol. Histol. 41, 325–332.
| Expression of GDF-9, BMP-15 and their receptors in mammalian ovary follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlyksb3P&md5=6e35fdd57e16ddcfe5ec2007c46b5282CAS | 20857181PubMed |
Tilly, J. L. (1996). Apoptosis and ovarian function. Rev. Reprod. 1, 162–172.
| Apoptosis and ovarian function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmtFWqurw%3D&md5=0edbf5b9ef872ca26d7cd8696f93ff39CAS | 9414454PubMed |
Webb, R., Garnsworthy, . C., Campbell, B. K., and Hunter, M. G. (2007). Intra-ovarian regulation of follicular development and oocyte competence in farm animals. Theriogenology 68, S22–S29.
| Intra-ovarian regulation of follicular development and oocyte competence in farm animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotlaitbs%3D&md5=c91c9cdd27476533966d2ca2d18110c1CAS | 17540442PubMed |
Xu, X., Faillace, L. S., Harding, R. T., Foxcroft, G. R., and Hunter, M. G. (1998). Evidence that Meishan and Large–White hybrid preovulatory follicles may differentially affect oocyte in vitro maturation and fertilization. Anim. Reprod. Sci. 51, 307–319.
| Evidence that Meishan and Large–White hybrid preovulatory follicles may differentially affect oocyte in vitro maturation and fertilization.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czltV2gsg%3D%3D&md5=c19ef4325d073b233f312e0fd3a7b3d9CAS | 9686313PubMed |
Zeng, F., Baldwin, D., and Schultz, R. M. (2004). Transcript profiling during preimplantation mouse development. Dev. Biol. 272, 483–496.
| Transcript profiling during preimplantation mouse development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmt1CjtrY%3D&md5=ae0d9273fc157863417369e7a827e367CAS | 15282163PubMed |
Zhu, G., Guo, B., Pan, D., Mu, Y., and Feng, S. (2008). Expression of bone morphogenetic proteins and receptors in porcine cumulus–oocyte complexes during in vitro maturation. Anim. Reprod. Sci. 104, 275–283.
| Expression of bone morphogenetic proteins and receptors in porcine cumulus–oocyte complexes during in vitro maturation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOmsb8%3D&md5=896606bd0fc445d99ca87e9fe2269932CAS | 17368971PubMed |




