Effects of systemic progesterone during the early luteal phase on the availabilities of amino acids and glucose in the bovine uterine lumen
Michael P. Mullen A B F , Fuller W. Bazer C , Guoyao Wu C , Mervyn H. Parr A B , Alexander C. O. Evans D E , Mark A. Crowe B E and Michael G. Diskin AA Animal and Bioscience Research Department, Animal and Grassland Research and Innovation Centre, Teagasc, Mellows Campus, Athenry, Co. Galway, Ireland.
B UCD School of Veterinary Medicine, University College Dublin, Belfield, Dublin 4, Ireland.
C Department of Animal Science, Texas A&M University, College Station, TX 77843-2471, USA.
D UCD School of Agriculture and Food Science, University College Dublin, Belfield, Dublin 4, Ireland.
E UCD Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland.
F Corresponding author. Email: michael.mullen@teagasc.ie
Reproduction, Fertility and Development 26(2) 282-292 https://doi.org/10.1071/RD12319
Submitted: 5 October 2012 Accepted: 18 December 2012 Published: 4 February 2013
Abstract
The uterine histotroph provides essential nutrition to the developing conceptus during the preimplantation period of pregnancy. The objective of the present study was to examine the effects of cycle stage and progesterone (P4) concentrations in the blood on the recoverable quantities of amino acids and glucose in the histotroph during the preimplantaion period of conceptus development. Following oestrus, dairy heifers were assigned to low, control or high P4 groups (n = 6 heifers per treatment and time point). The uterine horn ipsilateral to the corpus luteum was flushed on either Day 7 or Day 13. The present study quantified 24 amino acids and glucose in the uterine flushings using HPLC and fluorometry, respectively. Heifers in the low P4 group had lower plasma concentrations of P4 throughout the cycle, whereas heifers in the high group had higher plasma concentrations of P4 between Days 3 and 7 compared with the control group (P < 0.05). Total recoverable neutral (Ser, Gln, Gly, Thr, Cit, β-Ala, Tau, Ala, Tyr, Trp, Met, Val, Phe, Ile, Leu, Pro and Cys), acidic (Glu) and basic (His, Arg, Orn and Lys) amino acids were greater (P < 0.05) on Day 13 than on Day 7. There was no significant difference in the amount of Asp or Asn between Day 7 and Day 13. The amount of amino acids recovered on Day 7 was similar across treatment groups. On Day 13, the amount of Asn, His and Thr was lower (P < 0.05) in the low P4 heifers compared with the controls and/or high P4 heifers. Quantities of glucose were not altered by cycle stage or P4 treatment. In conclusion, the stage of oestrous cycle and P4 play important roles in modulating amino acids in the histotroph, a potentially critical factor for early embryonic and/or conceptus survival.
Additional keywords: embryo loss, fertility, histotroph.
Introduction
The ability of the developing embryo to produce sufficient quantities of interferon-τ (IFNT) during the preimplantation phase is essential for maternal recognition of pregnancy, maintenance of a functional corpus luteum (CL), and subsequent maintenance of pregnancy in domestic ruminants, and must occur by Day 16 in cattle (Betteridge et al. 1980; Northey and French 1980; Spencer et al. 2007). Embryonic development, and therefore production of IFNT, during this critical preimplantation period is reliant on maternal provision (via secretion from endometrial glands) of a complex milieu of growth factors, enzymes, cytokines, transport proteins, vitamins, minerals, amino acids, glucose and other nutrients, collectively termed histotroph (Bazer et al. 1987; Gray et al. 2001; Gray et al. 2002; Gray et al. 2006; Spencer et al. 2007).
The central role that progesterone (P4) plays in the establishment and maintenance of pregnancy is well established (Bazer et al. 2008). Several studies have reported an association between the P4 concentrations and embryonic survival (Starbuck et al. 2001; Diskin and Sreenan 2005; Stronge et al. 2005; McNeill et al. 2006; Parr et al. 2012). Indeed, changes in systemic concentrations of P4 during the early luteal phase modulates embryonic development (Carter et al. 2008; Clemente et al. 2009; Forde et al. 2011a). The mechanisms by which systemic P4 affects embryonic development are proposed to be indirect via effects on the uterine endometrium (Clemente et al. 2009) that alter the timing and expression of endometrial genes (McNeill et al. 2006; Simmons et al. 2009; Satterfield et al. 2010; Forde et al. 2011a; Mullen et al. 2012b) and ultimately the composition of histotroph (Spencer et al. 2007; Forde et al. 2009; Forde et al. 2011a; Mullen et al. 2012b). The importance of the histotroph to survival of the conceptus (i.e. the embryo and its associated extraembryonic membranes) was clearly demonstrated in sheep when embryos transferred into uteri lacking endometrial glands for the secretion of histotroph failed to elongate (Gray et al. 2001, 2002).
During early embryonic development, amino acids and glucose serve important biological functions as major energy sources (Tiffin et al. 1991; Rieger et al. 1992; Partridge and Leese 1996; Lane and Gardner 1997; Kim et al. 2011a), as cell signalling molecules (Kimball and Jefferson 2004; Wu 2009) and substrates for protein synthesis (Alexiou and Leese 1992). In addition, they can act as intracellular osmolytes (Dawson et al. 1998; Tartia et al. 2009), regulators of pH (Baltz 1993; Edwards et al. 1998), antioxidants (Nasr-Esfahani et al. 1992), chelators (Van Winkle et al. 1990), substrates for synthesis of biologically important nitrogenous compounds (Kim et al. 2011a) and regulators of cell proliferation and differentiation (Gao et al. 2009b; Kim et al. 2011b).
Despite the requirement of histotroph by the early developing embryo and the significantly increased early embryonic mortality in dairy cattle over recent decades (Diskin et al. 2006; Diskin and Morris 2008), studies examining its composition and the effects of systemic P4 in the bovine are limited. Recent studies with cattle examined the effects of cycle stage on amino acid and energy substrates in oviducal fluids on Days 0, 2, 3, 4 and 6 and uterine fluids on Days 6, 8 and 14 of the oestrous cycle (Hugentobler et al. 2007, 2008). In addition, the same group examined the effects of P4 infused on Day 1–4 on ions, amino acids and energy substrates in oviducal and uterine fluids collected on Days 3 and 6, respectively (Hugentobler et al. 2010). These studies revealed novel information on the temporal and P4-induced effects on concentrations of amino acids and glucose in the bovine uterine lumen. However, there are no reports of the effects of systemic P4 during the early luteal phase on amino acids or glucose in the uterine lumen at later stages of the preimplantation period, coincident with critical stages of conceptus development in cattle. The hypothesis was that the availability of amino acids and glucose in the bovine uterus would change in accordance with the concentration of systemic P4 and stage of cycle. Therefore, the objective of the present study was to examine the effects of altered systemic concentrations of P4 during the early luteal phase on amino acid and glucose availability on Days 7 and 13 of the oestrous cycle in the bovine uterine lumen.
Materials and methods
Experimental design and collection of uterine flushings
All experimental procedures involving animals were licensed by the Department of Health and Children, Ireland, in compliance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EC and were sanctioned by the University College Dublin Animal Research Ethics Committee.
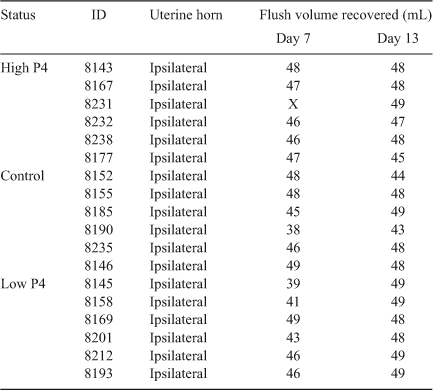
The heifers used in the present study were part of a larger cohort from which uterine flushings were collected, as described previously (Mullen et al. 2012b). Briefly, 40 Holstein–Friesian (HF) heifers were subjected to repeated prostaglandin (PG) F2α-induced synchronisation of oestrus and assigned to one of three groups representing high P4, control and low P4 (n = 10, 18 and 12 respectively). To generate groups divergent for systemic P4, intramuscular injections of PGF2α on Days 3, 3.5 and 4 and insertion of progesterone releasing intravaginal device (PRID) on Days 3–6 were performed to induce low and high systemic concentrations of P4, respectively. Heifers that were unresponsive to treatment or underwent complete CL regression in the low P4 group were excluded, as described previously (Beltman et al. 2009). Heifers that received a PRID to increase plasma concentrations of P4 were chosen as those with the greatest deviation from the mean to avoid an overlap in P4 profiles. The uterine horns of heifers were flushed on each of two separate cycles to obtain a uterine flush sample on both Day 7 and Day 13. There were six heifers per treatment group that yielded flushings on both days, with the exception of one heifer in the high P4 group that failed to yield a suitable uterine flushing on Day 7. Thus, the total numbers of samples on Days 7 and 13 were 17 and 18, respectively (Table 1).
|
Uterine flushings were recovered from the uterine horn ipsilateral to the CL by an experienced commercial operative (BoviGenetics, Cavan, Ireland), and involved the careful introduction and removal of 50 mL of 100 mM Tris buffer (pH 7.2; Sigma Aldrich, Dublin, Ireland). All uterine flushings were immediately centrifuged on-site at 4000g for 30 min at 4°C. The supernatants were aliquoted and snap frozen in liquid nitrogen before being transported on dry ice for storage at –80°C.
Analysis of amino acids and glucose
The analysis of amino acids and glucose in uterine flushes was performed as described previously (Gao et al. 2009a). Briefly, uterine flushings (0.5 mL) were deproteinised with an equal volume of 1.5 M HClO4, followed by the addition of 0.25 mL of 2 M K2CO3. The extract was then analysed for glucose using a flourometric method involving hexokinase and glucose-6-phosphate dehydrogenase, as described previously (Wu 1995). Amino acids in the extract were determined by flourometric HPLC involving precolumn derivatisation with o-phthaldialdehyde, as described previously (Wu et al. 1997). Integration of chromatographic peaks was performed using Millenium-32 software (Waters, Milford, MA, USA).
Circulating P4 concentrations
Plasma concentrations of P4 were determined by radioimmunoassay (RIA) in a direct assay (Coat-A-Count; Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) without prior extraction, as described previously (Forde et al. 2011a). The intra- and inter-assay CV for samples containing mean concentrations of 0.43, 2.59 and 7.02 ng mL–1 P4 were 16.2% and 8.8%, 8.0% and 3.5%, and 6.7% and 2.9%, respectively. The minimum detectable concentration of P4 in the assay was 0.04 ng mL–1.
Statistical analysis
To account for variations in the volume of uterine flushing recovered (Table 1), total recovered quantities of each substrate were calculated (by multiplying the concentration by the volume of flushing obtained) and used for subsequent comparisons herein.
The effect of treatment on circulating P4 concentrations and on recovered quantities of amino acids and glucose were calculated using PROC MIXED in SAS version 9.1 (SAS Institute, Cary, NC, USA). Terms for day and treatment × day were included in the model. Heifer was fitted as a repeated-measures term and the covariance structure chosen was unstructured. The DIFF option along with LS means in PROC MIXED was used for multiple comparisons. P ≤ 0.05 was considered significant.
The relationship between circulating P4 concentrations averaged between Days 3–7, 8–10 and 11–13 and amino acid and glucose recovery were evaluated using regression analysis. Where a significant linear relationship was recorded, circulating P4 concentrations were fitted as a quadratic term and retained in the final model if statistically significant (P ≤ 0.05).
Results
Progesterone model
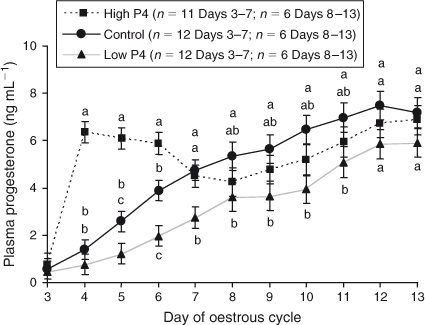
Plasma concentrations of P4 from Day 3 to 13 ranged between 0.76 and 6.87 ng mL–1, 0.59 and 7.49 ng mL–1 and 0.45 and 5.92 ng mL–1 for the high, control and low P4 groups, respectively (Fig. 1). Plasma concentrations of P4 were higher for heifers in the high P4 group from Day 3 to Day 7 and lower in the low P4 group from Day 3 to the day of collection of uterine flushings compared with levels in the control group (area under the curve (AUC) P < 0.05).
|
Volume of uterine flushing recovered
The mean (± s.d.) volume of uterine flushing recovered on Day 7 and 13 was 45.4 ± 3.3 and 47.6 ± 1.8 mL, respectively (Table 1).
Effects of P4 and cycle stage on recoverable amounts of glucose and amino acids in uterine flushings
Total recoverable glucose in uterine flushings was similar across both Day 7 and Day 13, as well as across P4 treatment groups (P > 0.05; Table 2).
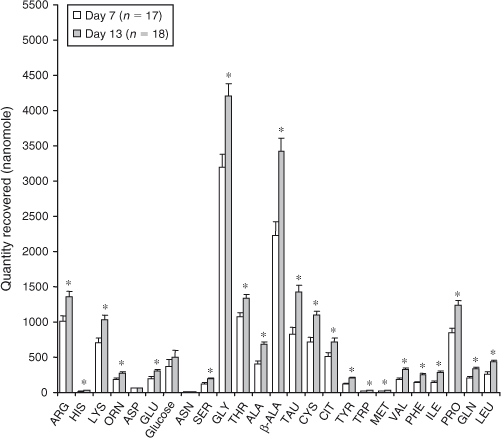
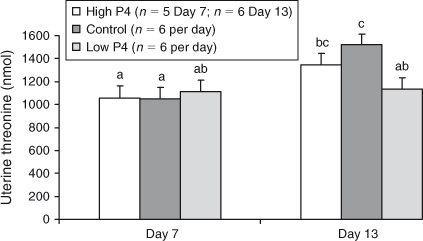
Total recoverable amounts of Arg, His, Lys, Orn, Glu, Ser, Gly, Thr, Ala, β-Ala, Tau, Cys, Cit, Tyr, Trp, Met, Val, Phe, Ile, Pro, Gln and Leu were higher on Day 13 than on Day 7 (day effect P < 0.01; Fig. 2; Table 2). Total recoverable amounts of Asp and Asn were not altered by cycle stage (day effect P > 0.05; Table 2).
|
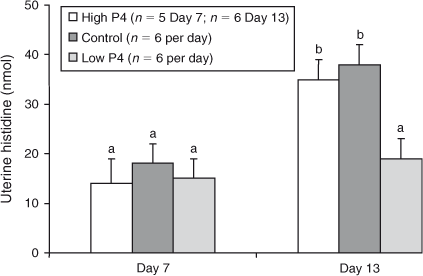
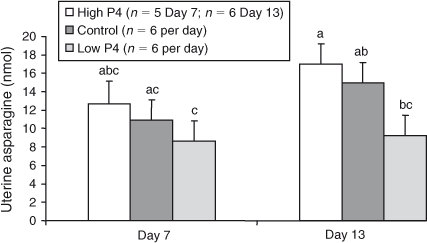
The effects of P4 treatment on total recoverable amounts of any of the amino acids on either Day 7 or Day 13 were not significant (P > 0.05), with the exceptions of His, Asn and Thr, for which heifers with lower plasma concentrations of P4 yielded lower amounts of His, Asn, and Thr on Day 13 than did heifers in the control or high P4 groups (P < 0.02; Figs 3–5).
|
|
|
To illustrate the effect of day, and in the absence of significant treatment by day interactions, the mean quantities of amino acids recovered on each day across P4 groups are shown in Fig. 2.
Relationship between circulating P4 concentrations during the early luteal phase and amino acids and glucose amounts in uterine flushings
There were no significant relationships (P > 0.05) between mean P4 plasma concentrations on Days 3–7, 8–10 or 11–13 and the amount of either amino acids or glucose in uterine flushings collected on Day 7. However, the amount of recoverable Ser on Day 7 tended to have a positive linear relationship with mean plasma P4 concentrations averaged between Days 3 and 7 (P < 0.1).
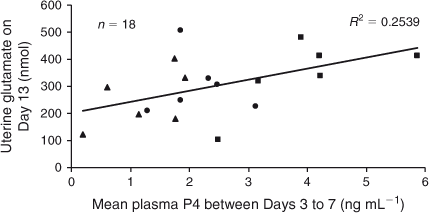
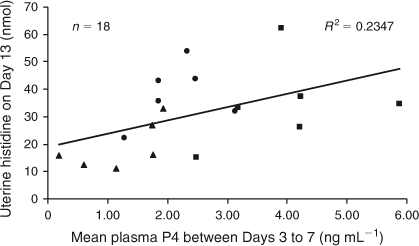
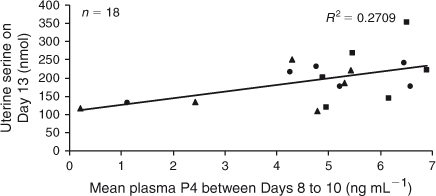
On Day 13, mean plasma P4 concentrations between Days 3 and 7 had a positive linear relationship with Glu and His recovery (P < 0.05; Figs 6, 7), as well as a tendency for greater Asn and Tau recovery (P ≤ 0.08) in uterine flushings. Plasma concentrations of P4 averaged from Day 8 to Day 10 had a positive linear relationship with Ser and a tendency for higher Met recovery in uterine flushings on Day 13 (P < 0.03 and P < 0.1, respectively; Fig. 8). Although there was no significant relationship between mean P4 concentrations from Day 11 to Day 13 and amino acid recovery, there was a trend for a positive linear relationship with Ser in uterine flushings collected on Day 13 (P < 0.1).
|
|
|
Discussion
The main findings of the present study were that: (1) the most abundant amino acids in bovine histotroph were Gly and β-Ala, for which there was an approximate 250-fold difference compared with the least abundant amino acid, Asn; (2) the maternal provision of 23 amino acids increased in uterine histotroph between Days 7 and 13, which coincides with the increasing requirements of rapidly developing conceptuses for nutrients; (3) low circulating P4 concentrations at different stages of the early luteal phase modulated the availability of amino acids His, Thr and Asn on Day 13 in uterine histotroph, an important period in conceptus development during the preimplantation period; and (4) the availability of glucose in the uterine fluid during this developmental period was not significantly altered by P4 or cycle stage.
Within the first 2 weeks after oestrus, available evidence in cattle suggests that the uterine environment is largely independent of the developing embryo and/or conceptus, but then undergoes significant temporal modulation and assumes a pregnant state until there is either recognition of pregnancy by approximately Day 16 or luteolysis occurs in advance of a return to oestrus (Spencer et al. 2007; Forde et al. 2011b, 2012; Mullen et al. 2012a). During these first 2 weeks of pregnancy the embryo undergoes rapid growth and development, increasing over 160-fold in protein content between Day 7 and Day 13 (Grealy et al. 1996). Therefore, given the importance of histotroph to conceptus survival, analysis of the uterine environment in high-fertility dairy heifers and its response to systemic P4 during this critical time period was expected to yield additional information regarding factors required for successful establishment of pregnancy in the bovine.
In the present study, Gly and β-Ala were the most abundant amino acids in the uterine fluid, followed by Thr, Arg, Tau and Pro. The identification of Gly as a highly abundant amino acid in female reproductive tract fluids is consistent with previous observations in cattle (Fahning et al. 1967; Tiffin et al. 1991; Moore and Bondioli 1993; Steeves and Gardner 1999; Elhassan et al. 2001; Hugentobler et al. 2007), sheep (Hill et al. 1997; Moses et al. 1997; Gao et al. 2009b), horses (Engle et al. 1984) and rabbits (Miller and Schultz 1987). The relatively high abundance of Gly in the uterine lumen across species suggests that it has an important role in embryo and/or conceptus development, and this is further supported by its abundance in the fetuses of swine throughout pregnancy (Wu et al. 1999). Indeed, the addition of Gly to the culture medium enhances the development of porcine and bovine embryos in vitro (Moore and Bondioli 1993; Lee and Fukui 1996; Takahashi and Kanagawa 1998; Mito et al. 2012). Although the mechanisms are not well defined, Gly may serve several functions during embryo and/or conceptus development, including acting as a precursor to protein or nucleic acid synthesis, regulation of intracellular pH and osmoregulation (Edwards et al. 1998; Steeves et al. 2003; Tartia et al. 2009; Moravek et al. 2012). The developing embryo is particularly susceptible to changes in intracellular pH (FitzHarris and Baltz 2009) and increased osmolarity (Dawson et al. 1998; Hammer and Baltz 2003). The abundance (and temporal increase) of effective osmoregulators such as Gly (Dawson et al. 1998; Moravek et al. 2012), β-Ala (Dawson et al. 1998; Hammer and Baltz 2003), Pro (Dawson et al. 1998) and Tau (Menezo and Guerin 1997) in uterine flushings in the present study, coupled with the significant production of Gly by the embryo in vitro (Lee and Fukui 1996), suggests that these amino acids play an important role in osmotic regulation during the preimplantation period in the bovine. In addition, the organic osmoregulatory effects of Gly and β-Ala are additive during murine embryo development, indicating a common mode of action (Dawson et al. 1998).
A significant effect of day of cycle was evident in the present study, with total recoverable quantities of 22 amino acids being higher, and two (Asn and Asp) being similar, on Day 13 compared with Day 7. Day 7 and Day 13 of pregnancy were selected because they represent days for two critical developmental events for rapidly developing bovine embryos as they reach the blastocyst stage and then initiate elongation as a conceptus, respectively (Spencer et al. 2007). These results indicate an increasing requirement for a large proportion of the amino acids examined by the developing conceptus during the initiation of elongation. To the best of our knowledge only two other studies (Fahning et al. 1967; Hugentobler et al. 2007) have examined the effects of cycle stage on amino acid availability in bovine uterine fluid. However, the present study, which includes the analysis of amino acids Orn, β-Ala, Cys, Cit and Pro, represents the most comprehensive information to date with regard to the identification of all protein amino acids and major non-protein amino acids in bovine histotroph.
Hugentobler et al. (2007) reported a temporal increase in the concentrations of eight amino acids (Ser, Thr, Tyr, Met, Val, Ile and Leu) in uterine fluid from either Days 6 or 8 and Day 14 in cyclic beef heifers, which is consistent with our findings. However, in contrast with the results of the present study, Hugentobler et al. (2007) reported a temporal decrease in the concentrations of Gly, Tau, His and Phe and no significant differences in the concentrations of Asp, Glu, Asn, Gln, Arg, Ala, Trp or Lys between Days 6, 8 and 14. Although we also found no temporal effect on uterine Asp or Asn, we did observe a temporal increase in the recovery of Gly, Tau, His, Phe, Glu, Gln, Arg, Ala, Trp and Lys as the cycle progressed. The reasons for these differences in results are not clear as both studies used comparable methods for amino acid analysis; however, there were differences in the methods used for sample collection. Hugentobler et al. (2007) collected neat uterine fluid in vivo using a moderate period (3 h) of anaesthesia in comparison to the collection of uterine flushings. Whether the use of general anaesthesia, as pointed out by Hugentobler et al. (2007), affects the uterine environment remains unknown. However, a uterine flushing approach can result in alterations in the concentration of analytes detected, therefore normalisation according to recovery volume is required and results expressed as total quantities recovered. It should also be noted that examination of any secretome introduces the risks of the introduction of intracellular material due to cell damage that, despite one’s best efforts, cannot be ruled out as a source of variation among studies.
Of particular interest in the present study was the temporal increase and relative abundance of Arg, Lys, His and Cit in uterine flushings. Arg, Lys and His (all basic amino acids that share the same transport systems) are essential amino acids that cannot be synthesised by blastocysts, but are required during in vitro culture for murine blastocyst development and expansion, suggesting an important role during conceptus development and the establishment of pregnancy (for a review, see Bazer et al. 2012). Subsequent studies revealed that Arg or Leu are central to murine blastocyst expansion and further development, initiating signalling pathways (including mammalian target of rapamycin), increasing mRNA expression and production of nitric oxide and polyamines essential for conceptus development and successful pregnancy (Bazer et al. 2012). Other studies have demonstrated the importance of Arg during pregnancy. For example, intravenous administration of l-Arg or l-Cit (a precursor for the synthesis of Arg) enhanced Arg availability in the ovine fetus, which enhanced fetal growth and survival in multiple birth pregnancies and prevented fetal growth restriction in malnourished ewes (Lassala et al. 2009, 2010, 2011). Furthermore, in humans, Arg deficiency in preterm infants results in hyperammonaemia and cardiovascular, pulmonary, neurological and intestinal dysfunction (Becker et al. 2000; Wu et al. 2004).
Other temporally modulated amino acids of interest include Orn and Cys. Orn is an intermediary in the synthesis of polyamines, derived from the conversion of Arg to Orn by arginase followed by conversion of Orn to polyamines by ornithine decarboxlyase (ODC1), which is essential for embryogenesis and placental development (Fozard et al. 1980; Bazer et al. 2012). Cys, a member of the functional amino acids (defined as regulators of key metabolic pathways benefiting survival, growth, development, reproduction and health; Wu et al. 2009), has been shown to improve the development of porcine blastocysts in vitro (Choe et al. 2010).
The models used herein to modulate systemic concentrations of P4 were described previously by Beltman et al. (2009) and Carter et al. (2008). They were designed primarily to generate divergent P4 profiles primarily between Days 3 and 7 of the oestrous cycle, as reported in several studies in beef cattle, to examine the effects of P4 on endometrial gene expression and subsequent embryo and/or conceptus development (Carter et al. 2008, 2010; Forde et al. 2011a). This approach addresses the difficulty associated with determining the effects of systemic P4 on the composition of uterine fluid due to the variation in endogenous concentrations of P4 among animals on the same day and among cycles (Hugentobler et al. 2010; Parr et al. 2012). This model results in significant divergence in concentrations of systemic P4 during Days 3–7 and, to a lesser extent, between Days 8 and 13 after oestrus; in beef heifers, its use has clearly demonstrated the effects of a high and low P4 environment during the early luteal phase on the timing of endometrial gene expression and resultant advancement or retardation of conceptus development (Carter et al. 2008; Forde et al. 2009, 2011a).
Despite the effects of systemic P4 on endometrial gene expression in beef cattle (Carter et al. 2008, 2010; Forde et al. 2009, 2011a) and amino acid content in uterine flushings from sheep and pigs (Satterfield et al. 2010; Bazer et al. 2012), we found only subtle effects on uterine amino acid composition in Holstein–Friesian heifers. The high P4 environment had no effect on amino acid or glucose recovery on either Day 7 or Day 13, whereas the low P4 environment modulated the availability of three amino acids His, Thr and Asn on Day 13, with reduced recovery in low P4 heifers compared with their high P4 or control counterparts. This would suggest that the curvilinear relationship observed in several studies between systemic concentrations of P4 during the early luteal phase and embryo survival rates in beef and dairy cattle (Diskin and Sreenan 2005; Stronge et al. 2005; McNeill et al. 2006), most recently demonstrated by Parr et al. (2012), is, at least in the case of high P4, not contributed to by modulation of uterine amino acid or glucose availability (before implantation). However, the reduced uterine availability of His, Thr and Asn observed in the low P4 heifers may contribute to the retardation of conceptus elongation on Day 14 (following embryo transfer on Day 7) observed in a similar low P4 environment (generated using the model herein) by Forde et al. (2011a) and the suboptimal pregnancy rates associated with low P4 in the aforementioned studies (Diskin and Sreenan 2005; Stronge et al. 2005; McNeill et al. 2006; Parr et al. 2012). Recoverable levels of Asn and Thr in uterine flushings are modulated by P4 in ovine and porcine uteri (Bazer et al. 2012), but this is the first report of an effect of P4 on the availability of His in uterine fluid. His, a basic amino acid, is a precursor for histamine synthesis via histidine decarboxylase that is essential for preimplantation embryo development in mice (Dey and Johnson 1980; Hudgins et al. 1982) and it has an important role in tissues undergoing rapid growth (Russell and Snyder 1968; Dzodzœ and Rosengren 1971). Threonine is a limiting factor for fetal growth in rats (Metcoff et al. 1981), whereas evidence supporting an important role for Asn is limited. Asn added to culture medium along with Asp, Glu, Arg and Ile has embryotrophic effects (Lee et al. 2004).
However, it should be noted that because the effects in the present study were only significant in the low P4 environment, it cannot be rule out, despite the short half-life of PGF2α of 8 min (Kindahl et al. 1976) and endogenous production throughout the oestrous cycle (Murakami et al. 2001), that PGF2α administration did not contribute directly or indirectly to the effects on the uterine environment.
In cattle, only two studies by Hugentobler et al. (2007, 2010) have related systemic progesterone concentrations to uterine amino acid composition by comparing: (1) endogenous variation of systemic P4 throughout the oestrous cycle with uterine constituents on Days 6, 8 and 14; and (2) the effects of intravenous administration of P4 on Days 1–4 on oviducal and uterine amino acids on Days 3 and 6, respectively. Hugentobler et al. (2007, 2010) found no effect of endogenous systemic concentrations of P4 on uterine amino acid composition (possibly due to a lack of sufficient variation), but did identify a positive association between administered P4 and uterine fluid content of glucose and Val on Day 6. This suggests a temporal delay in the uterine response to systemic P4, in agreement with previous observations by our group on the uterine abundance of retinol-binding protein (RBP; Mullen et al. 2012b), wherein systemic P4 during Days 3–7, while showing weak relationships with histotroph constituents on Day 7, more effectively modulated the uterine environment later in the cycle, between Day 7 and Day 13. Interestingly, in the present study, linear regression analysis of mean plasma P4 concentrations between Days 3–7, 8–10 and 11–13 identified positive linear relationships between P4 during the early luteal phase (Days 3–7) and Ser (an immediate precursor of glycine) availability on Day 7 and P4 during the mid-luteal phase (Days 8–10 and 11–13) and Ser availability on Day 13. These results indicate that: (1) P4 alters the timing of nutrient availability in a specific manner (supporting a stage- or function-specific role of amino acids on embryonic development; Lee et al. 2004); and (2) systemic P4 after Day 7 also modulates the composition of the histotroph during the preimplantation period of pregnancy in cattle.
Glucose is an essential source of energy to the developing embryo (Petters et al. 1990) and can be used to form not only glycogen, but also nucleic acids, proteins and lipids. However, oocytes and early embryos up to the 8-cell stage have a preference for pyruvate before switching to glucose at the blastocyst stage (Leese 1991; Boland et al. 2001). In fact, high concentrations of glucose during the initial cleavage stage are inhibitory to embryonic development in the bovine (Takahashi and First 1992), porcine (Flood and Wiebold 1988), murine (Chatot et al. 1989), hamster (Schini and Bavister 1988) and human (Conaghan et al. 1993) due to the Crabtree effect, resulting in accelerated glycolysis (Schini and Bavister 1988). Although the bovine embryo does not require glucose until Day 3 or 4 in vitro (Kim et al. 1993), it is an essential nutrient from the early morula stage onwards (Rieger et al. 1992), which corresponds to approximately Day 6. Our findings that the cycle stage, although numerically higher on Day 13 than on Day 7, did not significantly affect uterine glucose availability are consistent with the results of previous studies with cyclic beef heifers (Hugentobler et al. 2008) in which glucose availability was examined on Days 6, 8 and 14. In addition, in the sheep, uterine glucose recovery was not affected by the day of the oestrous cycle, but increased significantly between Days 10 and 16 of pregnancy (Gao et al. 2009b). In contrast with the present study, systemic P4 affected several components of the histotroph in ewes, including recoverable glucose, Asp, Asn, Ser, Ala, Gln, β-Ala, Cit, Arg and Lys on Day 9 and Lys and Arg on Day 12, which may reflect differences in hormonal regulation of the histotroph between species.
In conclusion, the results of the present study add novel information on the maternal environment during early embryonic development in the bovine and affirm previous observations for a central role of cycle stage in concert with the P4 environment in modulating histotroph composition and, possibly, an important role in the nutrition of developing embryos before maternal recognition of pregnancy. In addition, these results may assist in the optimisation of culture media for in vitro studies of embryonic development.
Acknowledgements
This study was funded by the Science Foundation Ireland (grant no. 07/SRC/B1156). The authors thank P. Joyce, G. Burke and W. Connolly for animal care and A. Glynn for help with the P4 analyses.
References
Alexiou, M., and Leese, H. J. (1992). Purine utilisation, de novo synthesis and degradation in mouse preimplantation embryos. Development 114, 185–192.| 1:CAS:528:DyaK38XhvFWls7c%3D&md5=44210d4b937cf9c0a9cc7657932ef354CAS | 1576959PubMed |
Baltz, JM (1993). Intracellular pH regulation in the early embryo. Bioessays 15, 523–530.
| Intracellular pH regulation in the early embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhtVaku78%3D&md5=d9d0e0be186d7187f38a7ca2e01397c1CAS | 8135765PubMed |
Bazer, F. W., Vallet, J. L., Ashworth, C. J., Anthony, R. V., and Roberts, R. M. (1987). The role of ovine conceptus secretory proteins in the establishment of pregnancy. Adv. Exp. Med. Biol. 230, 221–235.
| The role of ovine conceptus secretory proteins in the establishment of pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXit12ju7o%3D&md5=565cc97a8f976760222221c3966c1fe8CAS | 3454121PubMed |
Bazer, F. W., Burghardt, R. C., Johnson, G. A., Spencer, T. E., and Wu, G. (2008). Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod. Biol. 8, 179–211.
| Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways.Crossref | GoogleScholarGoogle Scholar | 19092983PubMed |
Bazer, F. W., Kim, J., Ka, H., Johnson, G. A., Wu, G., and Song, G. (2012). Select nutrients in the uterine lumen of sheep and pigs affect conceptus development. J. Reprod. Dev. 58, 180–188.
| Select nutrients in the uterine lumen of sheep and pigs affect conceptus development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVKju7k%3D&md5=4b2279d48a8540dbb5381ff94ae9d519CAS | 22738901PubMed |
Becker, R. M., Wu, G., Galanko, J. A., Chen, W., Maynor, A. R., Bose, C. L., and Rhoads, J. M. (2000). Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatr. 137, 785–793.
| Reduced serum amino acid concentrations in infants with necrotizing enterocolitis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlCisg%3D%3D&md5=7cd49827bf0734a7c63459b2e05afc78CAS | 11113834PubMed |
Beltman, M. E., Roche, J. F., Lonergan, P., Forde, N., and Crowe, M. A. (2009). Evaluation of models to induce low progesterone during the early luteal phase in cattle. Theriogenology 72, 986–992.
| Evaluation of models to induce low progesterone during the early luteal phase in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFGlsrrP&md5=df82d073fb359e6ca7dc763dfec2b657CAS | 19716596PubMed |
Betteridge, K. J., Eaglesome, M. D., Randall, G. C., and Mitchell, D. (1980). Collection, description and transfer of embryos from cattle 10–16 days after oestrus. J. Reprod. Fertil. 59, 205–216.
| Collection, description and transfer of embryos from cattle 10–16 days after oestrus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c3ltFGhsQ%3D%3D&md5=0c5a84da1a770fb19b86502e8466460cCAS | 7401037PubMed |
Boland, M. P., Lonergan, P., and O’Callaghan, D. (2001). Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology 55, 1323–1340.
| Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjsFSis70%3D&md5=d54ab782fb7aab78204f273ef2c1b158CAS | 11327687PubMed |
Carter, F., Forde, N., Duffy, P., Wade, M., Fair, T., Crowe, M. A., Evans, A. C. O., Kenny, D. A., Roche, J. F., and Lonergan, P. (2008). Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod. Fertil. Dev. 20, 368–375.
| Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtVKksLg%3D&md5=64ca51d0d9b619dd0572e72474aeae4aCAS | 18402756PubMed |
Carter, F., Rings, F., Mamo, S., Holker, M., Kuzmany, A., Besenfelder, U., Havlicek, V., Mehta, J. P., Tesfaye, D., Schellander, K., and Lonergan, P. (2010). Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol. Reprod. 83, 707–719.
| Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGlsrfF&md5=ebe7ce372fc067fe585e777f0f15cc0bCAS | 20631399PubMed |
Chatot, C. L., Ziomek, C. A., Bavister, B. D., Lewis, J. L., and Torres, I. (1989). An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 86, 679–688.
| An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1Mzkt1emtA%3D%3D&md5=9ac09b097fe004a33cabb71e8cd7924fCAS | 2760894PubMed |
Clemente, M., de La Fuente, J., Fair, T., Al Naib, A., Gutierrez-Adan, A., Roche, J. F., Rizos, D., and Lonergan, P. (2009). Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138, 507–517.
| Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOgtrvL&md5=6038faeaab282ac53cbf3ad10c7005d7CAS | 19556439PubMed |
Choe, C., Shin, Y. W., Kim, E. J., Cho, S. R., Kim, H. J., Choi, S. H., Han, M. H., Han, J., Son, D. S., and Kang, D. (2010). Synergistic effects of glutathione and β-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J. Reprod. Dev. 56, 575–582.
| Synergistic effects of glutathione and β-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhslOhsro%3D&md5=c6b18b1caba4f34f589dafe82e53b0deCAS | 20657156PubMed |
Conaghan, J., Handyside, A. H., Winston, R. M., and Leese, H. J. (1993). Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J. Reprod. Fertil. 99, 87–95.
| Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlsFCg&md5=437e9566fb8ea4a8bf3d0d99cf52519cCAS | 8283458PubMed |
Dawson, K. M., Collins, J. L., and Baltz, J. M. (1998). Osmolarity-dependent glycine accumulation indicates a role for glycine as an organic osmolyte in early preimplantation mouse embryos. Biol. Reprod. 59, 225–232.
| Osmolarity-dependent glycine accumulation indicates a role for glycine as an organic osmolyte in early preimplantation mouse embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltFGnsb0%3D&md5=2d90ac2d98ee9445fc848cbcdbf92e1cCAS | 9687289PubMed |
Dey, S. K., and Johnson, D. C. (1980). Histamine formation by mouse preimplantation embryos. J. Reprod. Fertil. 60, 457–460.
| Histamine formation by mouse preimplantation embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXisVyr&md5=541b4ce3b390fa332357b87b73822007CAS | 7431350PubMed |
Diskin, M. G., and Morris, D. G. (2008). Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 43, 260–267.
| Embryonic and early foetal losses in cattle and other ruminants.Crossref | GoogleScholarGoogle Scholar | 18638133PubMed |
Diskin, M. G., and Sreenan, J. M. (2005). Repeatability of embryo survival in beef heifers. J. Anim. Sci. 83, 38.
Diskin, M. G., Murphy, J. J., and Sreenan, J. M. (2006). Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 96, 297–311.
| Embryo survival in dairy cows managed under pastoral conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nitFGgsg%3D%3D&md5=e067a53a9bf58dd81f82258a108eac2bCAS | 16963203PubMed |
Dzodzœ, Y. C., and Rosengren, E. (1971). Decarboxylases of histidine and ornithine in chick embryo. Br. J. Pharmacol. 41, 294–301.
| Decarboxylases of histidine and ornithine in chick embryo.Crossref | GoogleScholarGoogle Scholar | 5572279PubMed |
Edwards, L. J., Williams, D. A., and Gardner, D. K. (1998). Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum. Reprod. 13, 3441–3448.
| Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltFOksA%3D%3D&md5=4aa9eb03ff8657ab9c3165fe4e7c4ec1CAS | 9886531PubMed |
Elhassan, Y. M., Wu, G., Leanez, A. C., Tasca, R. J., Watson, A. J., and Westhusin, M. E. (2001). Amino acid concentrations in fluids from the bovine oviduct and uterus and in ksom-based culture media. Theriogenology 55, 1907–1918.
| Amino acid concentrations in fluids from the bovine oviduct and uterus and in ksom-based culture media.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvFarsbY%3D&md5=5e1e03e241469cfa43f1f814f03c51aeCAS | 11414495PubMed |
Engle, C. C., Foley, C. W., Plotka, E. D., and Witherspoon, D. M. (1984). Free amino-acids and protein concentrations in reproductive-tract fluids of the mare. Theriogenology 21, 919–930.
| Free amino-acids and protein concentrations in reproductive-tract fluids of the mare.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXltVyisrY%3D&md5=8014701aa4c68e7a3ac1dfb060025258CAS |
Fahning, M. L., Schultz, R. H., and Graham, E. F. (1967). The free amino acid content of uterine fluids and blood serum in the cow. J. Reprod. Fertil. 13, 229–236.
| The free amino acid content of uterine fluids and blood serum in the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXktlSgurc%3D&md5=b08442bbbdcc44d4afd0bcf7d32d78d0CAS | 6067172PubMed |
FitzHarris, G., and Baltz, J. M. (2009). Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction 138, 619–627.
| Regulation of intracellular pH during oocyte growth and maturation in mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlShur3K&md5=0e968358b2f29426f18675468d7e3063CAS | 19520797PubMed |
Flood, M. R., and Wiebold, J. L. (1988). Glucose metabolism by preimplantation pig embryos. J. Reprod. Fertil. 84, 7–12.
| Glucose metabolism by preimplantation pig embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlvVOqsrk%3D&md5=7eacace71a244693eff973d14802496dCAS | 3184061PubMed |
Forde, N., Carter, F., Fair, T., Crowe, M. A., Evans, A. C. O., Spencer, T. E., Bazer, F. W., McBride, R., Boland, M. P., O’Gaora, P., Lonergan, P., and Roche, J. F. (2009). Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 81, 784–794.
| Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhsLbM&md5=b886d18b83f7eafebec7bdb868eac690CAS | 19553605PubMed |
Forde, N., Beltman, M. E., Duffy, G. B., Duffy, P., Mehta, J. P., O’Gaora, P., Roche, J. F., Lonergan, P., and Crowe, M. A. (2011a). Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol. Reprod. 84, 266–278.
| Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVeltr4%3D&md5=61a2e2dc9f6a05ff10d8194c1307c89aCAS | 20881316PubMed |
Forde, N., Carter, F., Spencer, T. E., Bazer, F. W., Sandra, O., Mansouri-Attia, N., Okumu, L. A., McGettigan, P. A., Mehta, J. P., McBride, R., O’Gaora, P., Roche, J. F., and Lonergan, P. (2011b). Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol. Reprod. 85, 144–156.
| Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotFOlsL4%3D&md5=11f719f39ba17575fcafd885e45e10b9CAS | 21349821PubMed |
Forde, N., Duffy, G. B., McGettigan, P. A., Browne, J. A., Mehta, J. P., Kelly, A. K., Mansouri-Attia, N., Sandra, O., Loftus, B. J., Crowe, M. A., Fair, T., Roche, J. F., Lonergan, P., and Evans, A. C. O. (2012). Evidence for an early endometrial response to pregnancy in cattle: both dependent upon and independent of interferon tau. Physiol. Genomics 44, 799–810.
| Evidence for an early endometrial response to pregnancy in cattle: both dependent upon and independent of interferon tau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslamuw%3D%3D&md5=87cfdd385238dad92ffcbe059db49248CAS | 22759920PubMed |
Fozard, J. R., Part, M. L., Prakash, N. J., Grove, J., Schechter, P. J., Sjoerdsma, A., and Koch-Weser, J. (1980). l-Ornithine decarboxylase:an essential role in early mammalian embryogenesis. Science 208, 505–508.
| l-Ornithine decarboxylase:an essential role in early mammalian embryogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXktVCks7w%3D&md5=700ea8ffca9c59142cfb55c5f08ebe79CAS | 6768132PubMed |
Gao, H., Wu, G., Spencer, T. E., Johnson, G. A., and Bazer, F. W. (2009a). Select nutrients in the ovine uterine lumen. V. Nitric oxide synthase, GTP cyclohydrolase, and ornithine decarboxylase in ovine uteri and peri-implantation conceptuses. Biol. Reprod. 81, 67–76.
| Select nutrients in the ovine uterine lumen. V. Nitric oxide synthase, GTP cyclohydrolase, and ornithine decarboxylase in ovine uteri and peri-implantation conceptuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslaru7Y%3D&md5=40e93d2ae1ce3c46210c90cc683ce9a1CAS | 19246319PubMed |
Gao, H., Wu, G., Spencer, T. E., Johnson, G. A., Li, X., and Bazer, F. W. (2009b). Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol. Reprod. 80, 86–93.
| Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosFOg&md5=8337c6de3d512c8f25516a7ede66e455CAS | 18753605PubMed |
Gray, C. A., Taylor, K. M., Ramsey, W. S., Hill, J. R., Bazer, F. W., Bartol, F. F., and Spencer, T. E. (2001). Endometrial glands are required for preimplantation conceptus elongation and survival. Biol. Reprod. 64, 1608–1613.
| Endometrial glands are required for preimplantation conceptus elongation and survival.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvFGgsbs%3D&md5=afcd4b3289a59b337bce8d8b23ce07e3CAS | 11369585PubMed |
Gray, C. A., Burghardt, R. C., Johnson, G. A., Bazer, F. W., and Spencer, T. E. (2002). Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124, 289–300.
| Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVCitL8%3D&md5=191a453784160e002d35839abf6c8418CAS | 12141942PubMed |
Gray, C. A., Abbey, C. A., Beremand, P. D., Choi, Y., Farmer, J. L., Adelson, D. L., Thomas, T. L., Bazer, F. W., and Spencer, T. E. (2006). Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol. Reprod. 74, 383–394.
| Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1Kktg%3D%3D&md5=af768bc8ba1521028375de408424c550CAS | 16251498PubMed |
Grealy, M., Diskin, M. G., and Sreenan, J. M. (1996). Protein content of cattle oocytes and embryos from the two-cell to the elongated blastocyst stage at Day 16. J. Reprod. Fertil. 107, 229–233.
| Protein content of cattle oocytes and embryos from the two-cell to the elongated blastocyst stage at Day 16.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmsFGgtr0%3D&md5=23d42298e9a8117292eaeeb963b78140CAS | 8882289PubMed |
Hammer, M. A., and Baltz, J. M. (2003). Beta-alanine but not taurine can function as an organic osmolyte in preimplantation mouse embryos cultured from fertilized eggs. Mol. Reprod. Dev. 66, 153–161.
| Beta-alanine but not taurine can function as an organic osmolyte in preimplantation mouse embryos cultured from fertilized eggs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnt1Wntr8%3D&md5=7f9cb43e4291b3bd4ceedab61129b6fdCAS | 12950102PubMed |
Hill, J. L., Wade, M. G., Nancarrow, C. D., Kelleher, D. L., and Boland, M. P. (1997). Influence of ovine oviducal amino acid concentrations and an ovine oestrus-associated glycoprotein on development and viability of bovine embryos. Mol. Reprod. Dev. 47, 164–169.
| Influence of ovine oviducal amino acid concentrations and an ovine oestrus-associated glycoprotein on development and viability of bovine embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXivFWmtb8%3D&md5=29981c4633101a01876ee4333f78720aCAS | 9136117PubMed |
Hudgins, L., Mukerjee, S., and Dey, S. K. (1982). Preimplantation embryo development in the mouse: role of histidine decarboxylase. Gamete Res. 6, 121–125.
| Preimplantation embryo development in the mouse: role of histidine decarboxylase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xlsl2gur8%3D&md5=78ef34ab5b786345e1730bc967e54364CAS |
Hugentobler, S. A., Diskin, M. G., Leese, H. J., Humpherson, P. G., Watson, T., Sreenan, J. M., and Morris, D. G. (2007). Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol. Reprod. Dev. 74, 445–454.
| Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhvFCqtLg%3D&md5=1ff68bfd8ae5ea025824bd86e49376f0CAS | 16998855PubMed |
Hugentobler, S. A., Humpherson, P. G., Leese, H. J., Sreenan, J. M., and Morris, D. G. (2008). Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle. Mol. Reprod. Dev. 75, 496–503.
| Energy substrates in bovine oviduct and uterine fluid and blood plasma during the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhs1Crsrw%3D&md5=816a2c32d42da64c218660038e88c21fCAS | 17926343PubMed |
Hugentobler, S. A., Sreenan, J. M., Humpherson, P. G., Leese, H. J., Diskin, M. G., and Morris, D. G. (2010). Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod. Fertil. Dev. 22, 684–694.
| Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvF2isLY%3D&md5=3b613adb9726d0a177f46ff72b904e8aCAS | 20353728PubMed |
Kim, J. H., Funahashi, H., Niwa, K., and Okuda, K. (1993). Glucose requirement at different developmental stages of in vitro fertilized bovine embryos cultured in semi-defined medium. Theriogenology 39, 875–886.
| Glucose requirement at different developmental stages of in vitro fertilized bovine embryos cultured in semi-defined medium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktVGlsbw%3D&md5=5dca31cab68bba760646f266501a6782CAS | 16727260PubMed |
Kim, J., Burghardt, R. C., Wu, G., Johnson, G. A., Spencer, T. E., and Bazer, F. W. (2011a). Select nutrients in the ovine uterine lumen. IX. Differential effects of arginine, leucine, glutamine, and glucose on interferon tau, ornithine decarboxylase, and nitric oxide synthase in the ovine conceptus. Biol. Reprod. 84, 1139–1147.
| Select nutrients in the ovine uterine lumen. IX. Differential effects of arginine, leucine, glutamine, and glucose on interferon tau, ornithine decarboxylase, and nitric oxide synthase in the ovine conceptus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFemurw%3D&md5=c493acb0280b3ff2a79852856214f577CAS | 21293034PubMed |
Kim, J. Y., Burghardt, R. C., Wu, G., Johnson, G. A., Spencer, T. E., and Bazer, F. W. (2011b). Select nutrients in the ovine uterine lumen. VII. Effects of arginine, leucine, glutamine, and glucose on trophectoderm cell signaling, proliferation, and migration. Biol. Reprod. 84, 62–69.
| Select nutrients in the ovine uterine lumen. VII. Effects of arginine, leucine, glutamine, and glucose on trophectoderm cell signaling, proliferation, and migration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlvVegtbY%3D&md5=19e5c986da9f26e036a472e3dea653c3CAS | 20844282PubMed |
Kimball, S. R., and Jefferson, L. S. (2004). Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Curr. Opin. Clin. Nutr. Metab. Care 7, 39–44.
| Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjs12qsbw%3D&md5=6667699775ad230f659aa3410153a834CAS | 15090902PubMed |
Kindahl, H., Edqvist, L. E., Granstrom, E., and Bane, A. (1976). The release of prostaglandin F2alpha as reflected by 15-keto-13,14-dihydroprostaglandin F2alpha in the peripheral circulation during normal luteolysis in heifers. Prostaglandins 11, 871–878.
| The release of prostaglandin F2alpha as reflected by 15-keto-13,14-dihydroprostaglandin F2alpha in the peripheral circulation during normal luteolysis in heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28Xks1Snsb0%3D&md5=e0689652fee4a0e83fd8693874496e07CAS | 945590PubMed |
Lane, M., and Gardner, D. K. (1997). Differential regulation of mouse embryo development and viability by amino acids. J. Reprod. Fertil. 109, 153–164.
| Differential regulation of mouse embryo development and viability by amino acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhs1Gls78%3D&md5=73157417dce478ba8f92bb7b17cbdfbaCAS | 9068427PubMed |
Lassala, A., Bazer, F. W., Cudd, T. A., Li, P., Li, X., Satterfield, M. C., Spencer, T. E., and Wu, G. (2009). Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus. J. Nutr. 139, 660–665.
| Intravenous administration of l-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjslSkt7w%3D&md5=f3c1bc40f19ab6800023f6683a86b34eCAS | 19225132PubMed |
Lassala, A., Bazer, F. W., Cudd, T. A., Datta, S., Keisler, D. H., Satterfield, M. C., Spencer, T. E., and Wu, G. (2010). Parenteral administration of l-arginine prevents fetal growth restriction in undernourished ewes. J. Nutr. 140, 1242–1248.
| Parenteral administration of l-arginine prevents fetal growth restriction in undernourished ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXot1Cruro%3D&md5=3090a14fea7182d6e0f17afdda41c7e3CAS | 20505020PubMed |
Lassala, A., Bazer, F. W., Cudd, T. A., Datta, S., Keisler, D. H., Satterfield, M. C., Spencer, T. E., and Wu, G. (2011). Parenteral administration of l-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J. Nutr. 141, 849–855.
| Parenteral administration of l-arginine enhances fetal survival and growth in sheep carrying multiple fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltlCqtbo%3D&md5=4839207268cc9f982bbaa89df7f39510CAS | 21430253PubMed |
Lee, E. S., and Fukui, Y. (1996). Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by in vitro-produced bovine morulae and blastocysts. Biol. Reprod. 55, 1383–1389.
| Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by in vitro-produced bovine morulae and blastocysts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvFWntQ%3D%3D&md5=21fa10b382a7d4e1bbec953a8eea503cCAS | 8949897PubMed |
Lee, E. S., Fukui, Y., Lee, B. C., Lim, J. M., and Hwang, W. S. (2004). Promoting effect of amino acids added to a chemically defined medium on blastocyst formation and blastomere proliferation of bovine embryos cultured in vitro. Anim. Reprod. Sci. 84, 257–267.
| Promoting effect of amino acids added to a chemically defined medium on blastocyst formation and blastomere proliferation of bovine embryos cultured in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVCqu70%3D&md5=f391f4e163d8c8efc7fdbffc097926d0CAS | 15302369PubMed |
Leese, H. J. (1991). Metabolism of the preimplantation mammalian embryo. Oxf. Rev. Reprod. Biol. 13, 35–72.
| 1:STN:280:DyaK3szgvFyqsA%3D%3D&md5=2f69612ea82265d1b583f6468400a3c9CAS | 1845337PubMed |
McNeill, R. E., Sreenan, J. M., Diskin, M. G., Cairns, M. T., Fitzpatrick, R., Smith, T. J., and Morris, D. G. (2006). Effect of systemic progesterone concentration on the expression of progesterone-responsive genes in the bovine endometrium during the early luteal phase. Reprod. Fertil. Dev. 18, 573–583.
| Effect of systemic progesterone concentration on the expression of progesterone-responsive genes in the bovine endometrium during the early luteal phase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltVWnt7o%3D&md5=8d0ee5cd8dd3237f0acd36b85730eccaCAS | 16836964PubMed |
Menezo, Y., and Guerin, P. (1997). The mammalian oviduct: biochemistry and physiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 73, 99–104.
| The mammalian oviduct: biochemistry and physiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslemtLg%3D&md5=9c604c0ed7f94eee95d5246b043bf06eCAS | 9175697PubMed |
Metcoff, J., Cole, T. J., and Luff, R. (1981). Fetal growth retardation induced by dietary imbalance of threonine and dispensable amino acids, with adequate energy and protein-equivalent intakes, in pregnant rats. J. Nutr. 111, 1411–1424.
| 1:CAS:528:DyaL3MXls1Onu7w%3D&md5=82b79fcef1fdb61276038ecdf08b33c9CAS | 6790682PubMed |
Miller, J. G., and Schultz, G. A. (1987). Amino acid content of preimplantation rabbit embryos and fluids of the reproductive tract. Biol. Reprod. 36, 125–129.
| Amino acid content of preimplantation rabbit embryos and fluids of the reproductive tract.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXitFWmtLo%3D&md5=8b5a234ba555932279dcc76fb50be6bcCAS | 3567272PubMed |
Mito, T., Yoshioka, K., Yamashita, S., Suzuki, C., Noguchi, M., and Hoshi, H. (2012). Glucose and glycine synergistically enhance the in vitro development of porcine blastocysts in a chemically defined medium. Reprod. Fertil. Dev. 24, 443–450.
| Glucose and glycine synergistically enhance the in vitro development of porcine blastocysts in a chemically defined medium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1Wqsb8%3D&md5=c7fec57ec297dccbe96f5ef714db19e8CAS | 22401276PubMed |
Moore, K., and Bondioli, K. R. (1993). Glycine and alanine supplementation of culture medium enhances development of in vitro matured and fertilized cattle embryos. Biol. Reprod. 48, 833–840.
| Glycine and alanine supplementation of culture medium enhances development of in vitro matured and fertilized cattle embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXks1Oisb8%3D&md5=7ddc9b0d8946f1c3ced96dd2d2900564CAS | 8485249PubMed |
Moravek, M., Fisseha, S., and Swain, J. (2012). Dipeptide forms of glycine support mouse preimplantation embryo development in vitro and provide protection against high media osmolality. J. Assist. Reprod. Genet. 29, 283–290.
| Dipeptide forms of glycine support mouse preimplantation embryo development in vitro and provide protection against high media osmolality.Crossref | GoogleScholarGoogle Scholar | 22246224PubMed |
Moses, D. F., Matkovic, M., Fisher, E. C., and Martinez, A. G. (1997). Amino acid contents on sheep oviductal and uterine fluids. Theriogenology 47, 336.
| Amino acid contents on sheep oviductal and uterine fluids.Crossref | GoogleScholarGoogle Scholar |
Mullen, M. P., Elia, G., Hilliard, M., Parr, M. H., Diskin, M. G., Evans, A. C., and Crowe, M. A. (2012a). Proteomic characterization of histotroph during the preimplantation phase of the estrous cycle in cattle. J. Proteome Res. 11, 3004–3018.
| Proteomic characterization of histotroph during the preimplantation phase of the estrous cycle in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkslWju7c%3D&md5=83e015d5a124076f48e3ee2d0d4950adCAS | 22463384PubMed |
Mullen, M. P., Forde, N., Parr, M. H., Diskin, M. G., Morris, D. G., Nally, J. E., Evans, A. C., and Crowe, M. A. (2012b). Alterations in systemic concentrations of progesterone during the early luteal phase affect RBP4 expression in the bovine uterus. Reprod. Fertil. Dev. 24, 715–722.
| Alterations in systemic concentrations of progesterone during the early luteal phase affect RBP4 expression in the bovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvFehurc%3D&md5=350631dc3b0fa4f2c03980f1e9c7703aCAS | 22697121PubMed |
Murakami, S., Miyamoto, Y., Skarzynski, D. J., and Okuda, K. (2001). Effects of tumor necrosis factor-alpha on secretion of prostaglandins E2 and F2alpha in bovine endometrium throughout the estrous cycle. Theriogenology 55, 1667–1678.
| Effects of tumor necrosis factor-alpha on secretion of prostaglandins E2 and F2alpha in bovine endometrium throughout the estrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlClsbY%3D&md5=16322a9d9d98ddb8bebf546e35215744CAS | 11393218PubMed |
Nasr-Esfahani, M. H., Winston, N. J., and Johnson, M. H. (1992). Effects of glucose, glutamine, ethylenediaminetetraacetic acid and oxygen tension on the concentration of reactive oxygen species and on development of the mouse preimplantation embryo in vitro. J. Reprod. Fertil. 96, 219–231.
| Effects of glucose, glutamine, ethylenediaminetetraacetic acid and oxygen tension on the concentration of reactive oxygen species and on development of the mouse preimplantation embryo in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVCk&md5=f3a44c2bfb2e96a6ffa1d1e464f0c157CAS | 1432953PubMed |
Northey, D. L., and French, L. R. (1980). Effect of embryo removal and intrauterine infusion of embryonic homogenates on the lifespan of the bovine corpus luteum. J. Anim. Sci. 50, 298–302.
| 1:STN:280:DyaL3c7jslansQ%3D%3D&md5=35d5d8219c3d89a40709d914309040e5CAS | 7358600PubMed |
Parr, M. H., Mullen, M. P., Crowe, M. A., Roche, J. F., Lonergan, P., Evans, A. C., and Diskin, M. G. (2012). Relationship between pregnancy per artificial insemination and early luteal concentrations of progesterone and establishment of repeatability estimates for these traits in Holstein–Friesian heifers. J. Dairy Sci. 95, 2390–2396.
| Relationship between pregnancy per artificial insemination and early luteal concentrations of progesterone and establishment of repeatability estimates for these traits in Holstein–Friesian heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFyksr0%3D&md5=145c1e9d0b7b0f0635d850940dd52063CAS | 22541467PubMed |
Partridge, R. J., and Leese, H. J. (1996). Consumption of amino acids by bovine preimplantation embryos. Reprod. Fertil. Dev. 8, 945–950.
| Consumption of amino acids by bovine preimplantation embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmtlGgtL4%3D&md5=6b5ae63a527d1e358cce966106d11b38CAS | 8896028PubMed |
Petters, R. M., Johnson, B. H., Reed, M. L., and Archibong, A. E. (1990). Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. J. Reprod. Fertil. 89, 269–275.
| Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXksFGgsbs%3D&md5=707aab224693acf39aafe66037c5a58eCAS | 2374120PubMed |
Rieger, D., Loskutoff, N. M., and Betteridge, K. J. (1992). Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro. Reprod. Fertil. Dev. 4, 547–557.
| Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhsVCitrc%3D&md5=2c5bcc3e6d02d5eba1eb1eb59ae544f8CAS | 1299829PubMed |
Russell, D., and Snyder, S. H. (1968). Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl Acad. Sci. USA 60, 1420–1427.
| Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXltVCltL8%3D&md5=0f692cc32c4f8ecf8ce3d4c4ebde6f7dCAS | 4299947PubMed |
Satterfield, M. C., Gao, H., Li, X., Wu, G., Johnson, G. A., Spencer, T. E., and Bazer, F. W. (2010). Select nutrients and their associated transporters are increased in the ovine uterus following early progesterone administration. Biol. Reprod. 82, 224–231.
| Select nutrients and their associated transporters are increased in the ovine uterus following early progesterone administration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1WgsrjM&md5=cae4d88a408f41260a42cbb9541ad188CAS | 19696016PubMed |
Schini, S. A., and Bavister, B. D. (1988). Two-cell block to development of cultured hamster embryos is caused by phosphate and glucose. Biol. Reprod. 39, 1183–1192.
| Two-cell block to development of cultured hamster embryos is caused by phosphate and glucose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtFahurw%3D&md5=aa146ecfc05e6c347cd3766d2362a18eCAS | 3219389PubMed |
Simmons, R. M., Erikson, D. W., Kim, J., Burghardt, R. C., Bazer, F. W., Johnson, G. A., and Spencer, T. E. (2009). Insulin-like growth factor binding protein-1 in the ruminant uterus: potential endometrial marker and regulator of conceptus elongation. Endocrinology 150, 4295–4305.
| Insulin-like growth factor binding protein-1 in the ruminant uterus: potential endometrial marker and regulator of conceptus elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyrtLfP&md5=df70f8f43f8c249fc2c535a35a248e20CAS | 19497977PubMed |
Spencer, T. E., Johnson, G. A., Bazer, F. W., Burghardt, R. C., and Palmarini, M. (2007). Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod. Fertil. Dev. 19, 65–78.
| Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhtleitr%2FN&md5=fb6f7e2ba4fab5d2de3fb7ca30496dafCAS | 17389136PubMed |
Starbuck, G. R., Darwash, A. O., Mann, G. E., and Lamming, G. E. (2001). The detection and treatment of post insemination progesterone insufficiency in dairy cows. In ‘Fertility in the High Producing Dairy Cow. BSAS Occasional Publication No. 2’. (Ed. M. G. Diskin.) pp. 447–450. (BSAS: Edinburgh, UK.)
Steeves, T. E., and Gardner, D. K. (1999). Temporal and differential effects of amino acids on bovine embryo development in culture. Biol. Reprod. 61, 731–740.
| Temporal and differential effects of amino acids on bovine embryo development in culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsFCqtr4%3D&md5=0af1434e20026952cfaa0941eb090407CAS | 10456851PubMed |
Steeves, C. L., Hammer, M. A., Walker, G. B., Rae, D., Stewart, N. A., and Baltz, J. M. (2003). The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos. Proc. Natl Acad. Sci. USA 100, 13 982–13 987.
| The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsFGitr8%3D&md5=13f61dbc513f301d8fe007d151e6f596CAS |
Stronge, A. J. H., Sreenan, J. M., Diskin, M. G., Mee, J. F., Kenny, D. A., and Morris, D. G. (2005). Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology 64, 1212–1224.
| Post-insemination milk progesterone concentration and embryo survival in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsVCgt7o%3D&md5=050fa34e24ad7530890f3a05957e6561CAS |
Takahashi, Y., and First, N. L. (1992). In vitro development of bovine one-cell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37, 963–978.
| In vitro development of bovine one-cell embryos: influence of glucose, lactate, pyruvate, amino acids and vitamins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXpvVOmtg%3D%3D&md5=2e75cafe1e37db9a99ff32d405842fc7CAS | 16727096PubMed |
Takahashi, Y., and Kanagawa, H. (1998). Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J. Vet. Med. Sci. 60, 433–437.
| Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtFWjtr8%3D&md5=bb164b59748eee6f23a2ff171ce7a5b7CAS | 9592714PubMed |
Tartia, A. P., Rudraraju, N., Richards, T., Hammer, M. A., Talbot, P., and Baltz, J. M. (2009). Cell volume regulation is initiated in mouse oocytes after ovulation. Development 136, 2247–2254.
| Cell volume regulation is initiated in mouse oocytes after ovulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXps1ehsL0%3D&md5=a85ca680e966dead96fe86c10c6b48b9CAS | 19502485PubMed |
Tiffin, G. J., Rieger, D., Betteridge, K. J., Yadav, B. R., and King, W. A. (1991). Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J. Reprod. Fertil. 93, 125–132.
| Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmt12is7c%3D&md5=3d06e87eeef7850c9f753d0895261fa4CAS | 1920281PubMed |
Van Winkle, L. J., Haghighat, N., and Campione, A. L. (1990). Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid. J. Exp. Zool. 253, 215–219.
| Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhsFGkurs%3D&md5=93a35e2fe6d3bbc193c9e50dc77a431dCAS | 2313249PubMed |
Wu, G. (1995). Nitric oxide synthesis and the effect of aminoguanidine and NG-monomethyl-l-arginine on the onset of diabetes in the spontaneously diabetic BB rat. Diabetes 44, 360–364.
| Nitric oxide synthesis and the effect of aminoguanidine and NG-monomethyl-l-arginine on the onset of diabetes in the spontaneously diabetic BB rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkt1yhs7w%3D&md5=81c01453482861189edb498be60af9f2CAS | 7533735PubMed |
Wu, G. (2009). Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17.
| Amino acids: metabolism, functions, and nutrition.Crossref | GoogleScholarGoogle Scholar | 19301095PubMed |
Wu, G., Davis, P. K., Flynn, N. E., Knabe, D. A., and Davidson, J. T. (1997). Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J. Nutr. 127, 2342–2349.
| 1:CAS:528:DyaK1cXisleitA%3D%3D&md5=f6fca842190c32cc9b5bdf9c18225e5fCAS | 9405584PubMed |
Wu, G., Ott, T. L., Knabe, D. A., and Bazer, F. W. (1999). Amino acid composition of the fetal pig. J. Nutr. 129, 1031–1038.
| 1:CAS:528:DyaK1MXivVynsr8%3D&md5=288124efcbeb391b89afab4e61475f2bCAS | 10222396PubMed |
Wu, G., Jaeger, L. A., Bazer, F. W., and Rhoads, J. M. (2004). Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J. Nutr. Biochem. 15, 442–451.
| Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVGgsr4%3D&md5=696addafaa0ee2e4557a2488b51a88a6CAS | 15302078PubMed |
Wu, G., Bazer, F. W., Davis, T. A., Kim, S. W., Li, P., Rhoads, J. M., Satterfield, M. C., Smith, S. B., Spencer, T. E., and Yin, Y. (2009). Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153–168.
| Arginine metabolism and nutrition in growth, health and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltlemtL0%3D&md5=dda8074dc67c6b1d57ada85653cf336cCAS | 19030957PubMed |



