344 ICSI-MEDIATED GENE TRANSFER SKEWS SEX RATIO AGAINST FEMALE BIRTHS IN MICE
P.N. Moreira A , B. Pintado A , L. Montoliu B and A. Gutiérrez-Adán AA Dpto. de Reproducción Animal y Conservación de Recursos Zoogenéticos, Instituto Nacional de Investigaciones Agrarias, Madrid, Spain. email: pmoreira@inia.es;
B Dpto. de Biología Molecular y Celular, Centro Nacional de Biotecnología, Madrid, Spain.
Reproduction, Fertility and Development 16(2) 292-292 https://doi.org/10.1071/RDv16n1Ab344
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
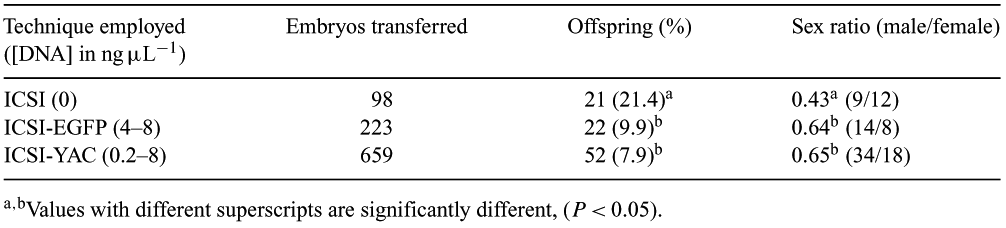
ICSI-mediated gene transfer has been used as an alternative method to pronuclear microinjection for the genomic modification of many species. With this method, transgenic embryos are produced by the microinjection of metaphase II oocytes with spermatozoa previously incubated with foreign DNA. Recently, it was shown in mice that the low percentage of transgenic animals produced from injected oocytes, results from the fact that the expression of foreign DNA is associated with paternal chromosome degradation (Szczygiel M.A. et al., 2003 Biol. Reprod. 68, 1903–1910). It is also known that sex chromosomes localize preferentially, at least in humans, on the periphery of the sperm nucleus on sub-acrosomal regions (Sbracia M. et al., 2002 Hum. Reprod. 17, 320–324), suggesting a high level of interaction with foreign DNA molecules with possible impact on the sex ratio of the offspring. In order to test this hypothesis we have compared ICSI (no DNA), and with ICSI-mediated EGFP (5 Kb plasmid DNA from Clonetech, Spain) transfer, with ICSI-mediated YRT3 (a mouse tyrosinase gene derivative YAC-DNA with 100 Kb; Montoliu L. et al., 1996 EMBO) transfer. Gametes were from 6–8 weeks old CD1 mice. ICSI-mediated gene transfer with post-thawed immotile spermatozoa, extended in M2 medium in the absence of ion chelators (EDTA and EGTA), was done as previously described (Szczygiel M.A. et al., 2003 Biol. Reprod. 68, 1903–1910). Table 1 below summarizes the data collected. Relative to our control, sex ratio deviation was a consequence of the coinjection of DNA. Forty-three percent of males were obtained with regular ICSI, whereas 64% and 65% were the respective percentages when EGFP or YRT3 DNA was coinjected with spermatozoa. This statistically significant (P < 0.05, z-test, Sigma Stat, Jandel Scientific, USA) sex ratio deviation, favoring male ICSI offspring when foreign DNA is coinjected, may result from a higher female embryo susceptibility to parental sex chromosome fragmentation induced by the interaction with foreign DNA molecules. Possible impairment of X chromosome inactivation and dosage compensation resulting from the fragmentation of the sex chromosome on X-carrying spermatozoa could explain this female embryo degeneration. Supporting this view, it was recently shown in mice that sex ratio can be skewed against female births by a mutation in a single gene of the X chromosome (Tsix) involved in such mechanisms (Lee J.T., 2002 Nat. Genet.). In conclusion, mouse ICSI-mediated gene transfer induces sex ratio deviation favoring male offspring.
|


