77 CLONED EMBRYO DEVELOPMENT IN VITRO: COMPARISON OF CONVENTIONAL AND INDUCED ENUCLEATION PROCEDURES FOR BOVINE CYTOPLAST PREPARATION
M.-K. Wang A and E.W. Overstrom BA Department of Biomedical Sciences, Tufts University, School of Veterinary Medicine, N. Grafton, MA, USA;
B Department of Biomedical Sciences, and Program in Cell, Molecular & Developmental Biology, Tufts University, School of Veterinary Medicine, N. Grafton, MA, USA. email: eric.overstrom@tufts.edu
Reproduction, Fertility and Development 16(2) 160-161 https://doi.org/10.1071/RDv16n1Ab77
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
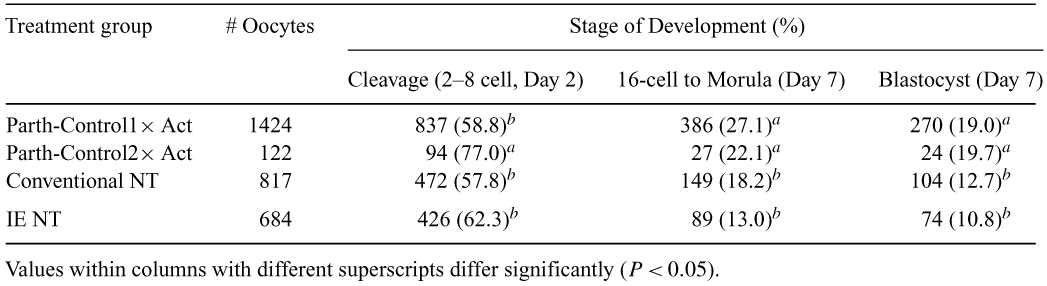
Induced enucleation (IE) of oocytes with demecolcine produces competent ooplasts for SCNT as demonstrated previously in mouse, goat, cow and pig. Whether bovine IE cytoplasts are more or less competent than conventionally enucleated MII oocytes to support nuclear reprogramming of somatic chromatin and embryo development in vitro is not known. This study compared in vitro development of cloned bovine embryos produced by conventional and IE enucleation methods. Three experimental groups were: (1) Parthenogenetic controls. In vitro-matured, MII-arrested bovine oocytes were activated by a single (1 × Act, 10 μM ionomycin in Tyrodes-HEPES, 5 min) or double activation (2 × Act; 1 × Act, wash 5 min, 10 μg mL−1 cycloheximide [CHX] 20 min, repeat 1 × Act) followed by incubation in CHX and 5 μg mL−1 cytochalasin B (CB) for 6 h, and then culture (BARC medium) for 7 days. (2) Conventional SCNT. MII oocytes were enucleated by micromanipulation in HEPES-buffered enucleation medium (BARC containing 7.5 μg mL−1 CB, 5 μg mL−1 Hoechst 33342, 10% FBS) under UV illumination (3–5 s). Donor cells (fibroblasts, passage 7–9) were inserted into the perivitelline space, and the reconstructed couplets activated (1 × Act). Reconstructed couplets were then electrofused, placed in BARC medium containing 10 μg mL−1 CHX and 5 μg mL−1 CB (6 h), and then cultured for 7 days. (3) IE SCNT. MII oocytes were activated (1 × Act), placed into BARC-5% FBS containing 0.4 μg mL−1 demecolcine (DEME), 10 μg mL−1 CHX, 2 μg mL−1 cytochalasin D for 20 min, then 20 min without DEME, then returned to DEME. At 1–1.5 h post-activation, the extruding second polar body (PB2) containing nuclear chromatin was removed by micromanipulation, couplets were reconstructed and fused as above, and additionally activated (two pulses, 20–30 V/mm, 20 μs). Embryos were cultured in 10 μg mL−1 CHX and 5 μg mL−1 CB medium for 4–5 hour, then BARC for 7 days. The results (Table 1) reveal that 2 × Act increases embryo development at Day 2, but not Day 7. Further, there are no significant differences in embryo development rates between conventional and IE SCNT protocols. Respectively, 46%, 32% and 21% of cleaved control (1 × Act), conventional and IE embryos developed to 16 cells on Day 7. In vitro development of cleavage embryos to the blastocyst stage was greater in controls (25–32%) than in conventional (22%) and IE (17%) SCNT groups on Day 7. Further comparisons of in vivo development between conventional and IE SCNT methods following embryo transfer are warranted. Supported by ACT, Cyagra and USDA NRI \#2001-35205-09966.
|


