195 CHANGES OF BLESBOK AND BLUE WILDEBEEST EPIDIDYMAL SPERM AFTER INCUBATION AT 37°C
F. Martinez-Pastor A B , F. Olivier C B , T. Spies C B , L. Anel D and P. Bartels BA Cell Biology and Anatomy, University of León, León, Spain
B Wildlife Biological Resource Center of The Endangered Wildlife Trust, Pretoria, South Africa
C Department of Animal Sciences, University of Stellenbosch, Stellenbosch, South Africa
D Animal Reproduction and Obstetrics, University of León, León, Spain. Email: felipe@unileon.es
Reproduction, Fertility and Development 17(2) 248-248 https://doi.org/10.1071/RDv17n2Ab195
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
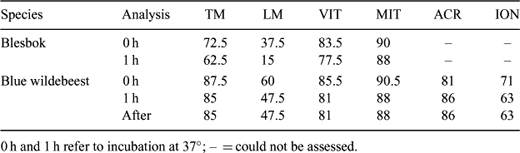
Postmortem recovery of epididymal spermatozoa and their preservation in Biological Resource Banks is a convenient source of germplasm, providing a possible future conservation resource for selected endangered wildlife species. It is necessary to gain knowledge of the biology of the gametes of the different species, in order to define effective protocols for cryopreservation and future assisted reproductive technology application. A pilot study on the changes in blue wildebeest (Connochaetes taurinus) and blesbok (Damaliscus dorcas phillipsi) epididymal sperm was carried out in order to provide some insight into the effects of incubation at 37°C. Chemicals were aquired from Sigma (South Africa), except JC-1 (Molecular Probes, Leiden, The Netherlands). Sperm was obtained by flushing the vas deferens and cauda epididymis of 6 adult blue wildebeests and 4 adult blesbok after the breeding season using 1 mL of Biladyl (fraction A; Minitüb, Tiefenbach, Germany). Cells were washed and resuspended in buffered medium (20 mM HEPES, 197 mM NaCl, 10 mM glucose, 2.5 mM KOH). Part of each sample was analyzed and part was incubated for 1 h at 37°C, and then analyzed. Analysis consisted of: motility (% of motile sperm, TM; and % of linear sperm, LM), vitality (fluorescent dye propidium iodide, 7 μM; % of unstained cells noted after 10 min at RT: vital, VIT), mitochondrial status (fluorescent dye JC-1, 7.5 μM; % of cells with orange midpiece noted after 30 min at 37°C: active mitochondria, MIT), and induction of acrosome reaction (15 min at 37°C in buffered medium complemented with 3 mM CaCl; % of intact acrosomes noted in control: splits no ionophore, ACR, and test: splits 1 μM calcimycin, ION). Samples were assessed using phase contrast microscopy (×400; ×200 for motility). Results are showed in Table 1. No significant differences (Wilcoxon Rank Sign test) were detected, possibly due to the low number of samples. However, LM appeared to decrease after incubation. Incubaton may increase the sensitivity of blue wildebeest sperm to ionophore (ION). Motility was least for blesbok, and the decrease of LM after incubation was more apparent. This treatment may induce different physiologycal changes between the species (different LM variation). The rest of the parameters suggest that the treatment did not induce extensive cell damage. Further research must be carried out to confirm these findings.
|
Sponsors of this project include Vodacom, Joan St. Leger Lindburgh Charitable Trust, Tony and Lizette Lewis Foundation, Department Science and Technology (South Africa), British Airways, IMV Technologies/CBS (France), NECSA, Zeiss Microscopes, AEC-Amersham, CryoLogic (Australia), Cook Veterinary (Australia), Mazda Wildlife Fund, The Scientific Group, Genaust (Australia), and SCI – Chesapeake Chapter.


