328 SOMATIC CELL ISOLATION FROM SEMEN BY PERCOLL GRADIENTS
L. Nel-Themaat A B , M.C. Gomez B A , G. Wirtu B C , A. Cole B , K.R. Bondioli A , B.L. Dresser B D , R.A. Godke A and C.E. Pope BA Department of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA
B Audubon Center for Research of Endangered Species, New Orleans, LA 70178, USA
C Department of Veterinary Clinical Sciences, Louisiana State University School of Veterinary Medicine, Baton Rouge, LA 70803, USA
D Department of Biological Sciences, University of New Orleans, New Orleans, LA 70148, USA. Email: lnel1@lsu.edu
Reproduction, Fertility and Development 17(2) 314-315 https://doi.org/10.1071/RDv17n2Ab328
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
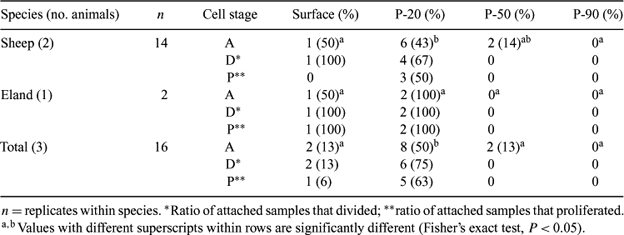
We previously isolated epithelial-like cells (ELC) from sheep (Ovis aries) and eland (Taurotragus oryx) ejaculates (Nel-Themaat et al. 2004 Reprod. Fertil. Dev. 16, 152). Success rates were low, and the presence of live sperm during initial culture may have altered medium properties, physically prevented contact between cells and the substrate, and/or damaged somatic cell membranes. Using the complete semen sample requires the sacrifice of valuable live spermatozoa without the certainty of obtaining a somatic cell population. Therefore, the purpose of the present study was to develop a method for isolating somatic cells from semen before culture. Separation by discontinuous Percoll gradient centrifugation was performed. Ejaculates obtained by electro-ejaculation from two Louisiana Native rams and rectal massage of one eland bull were washed, layered on columns of 2.5 mL each of 20% (P-20), 50% (P-50), and 90% (P-90) Percoll in 15-mL conical tubes, and centrifuged for 20 min at 400g. Bands were obtained at the three interfaces, as well as a pellet in the P-90 fraction. Each Percoll layer plus the interface band immediately below it was collected by aspiration. Cells from each fraction were re-suspended in serum-supplemented Minimum Essential Medium Alpha containing 500 IU/mL of penicillin, 0.5 mg/mL of streptomycin and 250 g/mL of gentamycin, and were plated on collagen-1 coated dishes. Trypan blue staining was used to determine cell viability in each fraction. After 24 h, dishes were washed and the culture continued until cells attached and proliferated. For both species, the surface fraction yielded primarily dead ELC that were morphologically flat and angular in shape. In the P-20 band, more viable cells were isolated compared with other fractions, although dead cells were also present. In the P-50 and P-90 fractions, few ELC were observed compared with the surface and P-20 fractions. A negligible number of sperm was observed in the surface and P-20 fractions. In contrast, mostly dead sperm were found at the P-50 and P-90 interface band, whereas live spermatozoa were detected in the P-90 pellet. Epithelial-like cells isolated from semen of both species attached (A, initial 24 h of culture), divided (D, within 3 days after plating), and proliferated (P, after 1 week of culture) (see Table). Ram ELC derived from the surface and from the P-20 and P-50 layers attached, but proliferation was detected only with cells collected in the P-20 fraction. In contrast, eland cells collected from the surface and P-20 layers readily attached and proliferated. In summary, a technique has been developed for the isolation of somatic cells from semen using a three-layer Percoll gradient. This method also allows the isolation of motile spermatozoa, hich are available for further use.
|


