161 LIPID CONTENT, RESISTANCE TO CRYOPRESERVATION, AND SEX RATIO OF IN VITRO BOVINE BLASTOCYSTS PRODUCED IN A SERUM-FREE SYSTEM
F. George A , C. Daniaux A , G. Genicot A , F. Focant A , B. Verhaeghe A , P. Lambert A and I. Donnay AUniversité Catholique de Louvain, Institut des Sciences de la Vie, Louvain-la-Neuve, Belgium
Reproduction, Fertility and Development 18(2) 188-189 https://doi.org/10.1071/RDv18n2Ab161
Published: 14 December 2005
Abstract
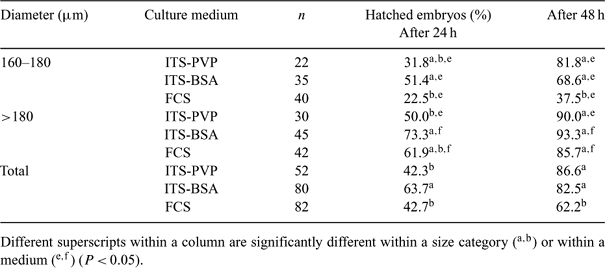
In vitro-produced (IVP) bovine blastocysts are known to be more sensitive to cryopreservation than their in vivo counterparts. Removing serum from the culture medium decreases sanitary risk and could improve embryo resistance to cryopreservation by preventing the accumulation of intracellular lipids. Our objectives were to evaluate the lipid content, resistance to cryopreservation, and sex ratio of IVP embryos cultured in a serum-free system. Oocytes from slaughterhouse ovaries were matured in a serum-free enriched medium (Donnay et al. 2004 Reprod. Fertil. Dev. 16, 274) and cultured in 5% O2 in modified SOF supplemented with 5% FCS (FCS) or with insulin-transferrin-selenium (ITS) and 0.1 mg/mL polyvinylpyrrolidone (PVP) (ITS-PVP) or 4 mg/mL BSA (ITS-BSA) (Daniaux et al. 2005 Reprod. Fertil. Dev. 17, 217). Day 5 morulae were stained with the fluorescent dye Nile Red in order to evaluate their lipid content (Genicot et al. 2005 Theriogenology 63, 1181). Day 7 blastocysts (diameter ≥160 µm) were selected, classified according to their size, and frozen in HEPES-SOF containing 1.5 M ethylene glycol, 0.1 M sucrose, and 1.8 mg/mL wheat peptones (George et al. 2002 Reproduction 29, 51). The lipid content was significantly lower in morulae cultured in ITS-BSA compared with the two other media (320 ± 10 arbitrary fluorescence units vs. 383 ± 12 in FCS and 406 ± 10 in ITS-PVP; n = 271; ANOVA2: P < 0.01). After cryopreservation, a higher total hatching rate was found 24 h post-thawing in blastocysts cultured in ITS-BSA and for both serum-free conditions at 48 h (Table 1). In particular, embryos ≤180 µm cultured in FCS were less resistant to cryopreservation than embryos of the same size produced without serum. Expanded blastocysts cultured in ITS-BSA were sexed by PCR (Grisart et al. 1995 Theriogenology 43, 1097) and a higher proportion of male embryos was found (62.7%; n = 51). In conclusion, a complete serum-free system was set up from oocyte maturation to embryo cryopreservation that gave high quality embryos resistant to cryo-preservation. Embryos produced in ITS-BSA presented a lower lipid content, but a shift of the expanded blastocyst sex ratio toward males was observed.
|


