91 PRELIMINARY RESULTS OF SURGICAL EQUINE EMBRYO TRANSFER AFTER OPEN PULLED STRAW VITRIFICATION
G. Duchamp A , F. Guignot A , J. Grizelj A , M. Vidament A and P. Mermillod AINRA Unité de Physiologie de la Reproduction et des Comportements, 37380 Nouzillly, France
Reproduction, Fertility and Development 18(2) 154-154 https://doi.org/10.1071/RDv18n2Ab91
Published: 14 December 2005
Abstract
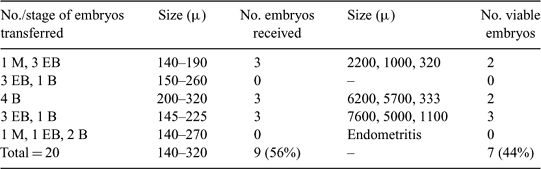
In equine species, embryo cryopreservation is not as widely developed as in some other species. Slow freezing has been applied to equine embryos but with relatively low success rates. This higher sensitivity to conventional freezing procedures may be explained by the presence of a capsule surrounding the equine embryo that may impair cryoprotectant penetration. Recently, good in vitro embryo survival rate was obtained after open pulled straw (OPS) vitrification (Moussa et al. 2005 Theriogenology 64, 1619–1632). The aim of the present study was to evaluate in vivo survival of vitrified embryos five days after surgical transfer into Welsh pony mares. Morulae (M), early blastocysts (EB), and blastocysts (B) ranging from 140 to 320 μm in diameter were collected (n = 20) in a Ringer lactate solution on Day 6.75 after ovulation. Before vitrification, embryos were assessed morphologically and their size was measured (McKinnon and Squires 1988 J. Am. Vet. Med. Assoc. 192, 401–406). Then, embryos were vitrified using the OPS method described by Berthelot et al. (2001 Reprod. Nutr. Dev. 41, 267–272). Briefly, embryos were washed twice in HEMES-TCM-199 + 20% newborn calf serum (NBCS) for 1 min, equilibrated in HEPES-TCM-199 + 20% NBCS with 7.5% dimethyl sulfoxide (DMSO) + 7.5% ethylene glycol (EG) for 3 min, and then with 18% DMSO + 18% EG + 0.4 M sucrose for 45 s. One embryo was then loaded per straw. For transfer, four straws were quickly thawed (5 s in air) and the narrow end of the straw containing the embryo was immersed in HEPES-TCM-199 + 20% NBCS + PBS + 0.2 M sucrose. Five to 8 min after thawing, four embryos were surgically transferred into the cranial portion of the uterine horn in each of five pony mare recipients. Five days after transfer, embryos recovered by transcervical flushing of the uterus were classified as viable if morphology was normal, no dark inner cells were present, the capsule was intact, and the diameter was at least 1000 μm. The results are shown in the table. One recipient of vitrified embryos had an endometritis and no embryo was recovered. From the four other recipients, nine embryos were recovered out of 16 (56%) transferred, seven of which were viable (44%). The results of the present preliminary study demonstrating survival of equine embryos transferred after OPS vitrification is very encouraging. However, the results should be confirmed by birth of foals after transfer of OPS-vitrified embryos to recipients.
|


